AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2637-8914/304
University of Life Sciences "King Mihai I" from Timisoara, 300645, Calea Aradului 119, Timis, Romania.
*Corresponding Author: Monica Butnariu, Chemistry & Biochemistry Discipline, University of Life Sciences
Citation: Bianca- Valentina Recsky, Ioana Grozea, Monica Butnariu, (2025), Analytical Techniques for Obtaining Volatile oils (Odorants and Flavorings), J. Nutrition and Food Processing, 8(4); DOI:10.31579/2637-8914/304
Copyright: © 2025, Monica Butnariu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 March 2025 | Accepted: 24 March 2025 | Published: 27 March 2025
Keywords: volatile oils; complex mixtures; entrainable liquids; volatilize
Volatile oils are complex mixtures of aliphatic, aromatic and hydroaromatic hydrocarbons, aldehydes, alcohols, esters and other constituents in which compounds from the terpenoid class predominate. They have a characteristic, aromatic and pleasant smell. They are liquids that can be carried by water vapor. The name volatile oils is appropriate because it expresses the characteristic property of these compounds: high vapor pressure and the fact that they volatilize at ordinary temperature. Volatile oils are relatively widespread in the plant kingdom, some families being very rich in such substances, both in number of species and in quantity. Several variants and systems are used to obtain volatile oils, which are based on their property of being carried by water vapor, as a result of the high vapor pressure that characterizes them.
The process used to obtain these natural substances depends on the way they are contained in the plant raw material, their chemical nature and their properties. Most of the production processes are based on two physical properties of odorants and flavorings, namely: the property of being entrained with water vapor and their solubility in certain solvents (hydrocarbons, alcohols, halogenated organic compounds, fats, carbon dioxide, etc.). The main processes applied in practice are the following: entrained with water vapor, extraction with animal fats, extraction with organic solvents, extraction with liquefied gases, extraction by pressing, adsorption on an adsorbent material. Among these, the most representative families are: Pinaceae, Labiatae, Umbelliferae, Myrtaceae, Lauraceae, Rutaceae, Cariophylaceae, Compositae, Zingiberaceae, etc., which are considered aromatic plants. The name aromatic plants is assigned to those species that contain at least 0.1-0.2% volatile oil and have a sufficiently perceptible odor. In addition to these, there are other species that, although they do not have a characteristic odor, still contain terpene substances that are part of the composition of vegetable oils (Monteiro, et al, 2010).
There is almost no organ in the composition of aromatic plants, in which, in various species, there are no larger or smaller amounts of volatile oils. Such are valerian roots, ginger rhizomes, lobelia leaves, rose flowers, citrus fruits, almond seeds (Batish, et al, 2006).
A. Chemical methods
a) Distillation with water (hydrodistillation)
In this process, the raw material is brought together with water into the distillation flask, the bottom of the container is heated directly at the heat source, and the mixture of oil vapor and water is collected by condensation in a cooling system. The distillate is then passed into a collecting vessel.
The method, although simple and easy to perform, nevertheless presents a number of disadvantages:
b) Water vapor entrainment
The raw material is brought into a metal sieve basket, which may or may not be previously closed in sackcloth, so that it does not come into contact with the water in the lower part of the blaza. The water vapor formed penetrates the vegetable material, entraining at the same time the volatile oils. This eliminates a number of disadvantages of the first process.
However, this method has some disadvantages:
To overcome these disadvantages, a separate steam generator can be used. The method has the advantage that a single steam generator can serve a battery of stills. This generator can be placed in a different building than the one where the distillation flasks are installed. An advantage of this system is that the extraction is no longer interrupted to replenish the distilled water. In addition, when the contents of a distillation vessel have been exhausted, it can be replaced without interrupting the other stills (Ge, et al, 2000).
c) Extraction with volatile solvents
This process is applied to obtain fine volatile oils, for those with labile components or existing in minute quantities in the raw material. The method has the following advantages: extraction takes place at low temperatures; the possibility of resenification is avoided; the fine scent is protected; a series of resins are extracted, not evaporable with water vapor, but fixative of the scent.
The disadvantages of this procedure are: high cost; complexity of the installation; toxicity and explosion hazard of solvents.The most commonly used solvent is petroleum ether. This meets most of the required conditions, but preliminary purification is necessary to remove the upper fractions with an unpleasant odor. Extraction gasoline has a high boiling point and contains less volatile fractions. It is used mainly for obtaining fatty oils and to a lesser extent for volatile ones. Benzene is sufficiently pure, but dissolves too many ballast substances, and when mixed with air it is detonating. Ethyl ether is quite often used, but it has a high cost price, dissolves ballast substances and is flammable. Liquefied gases, such as carbon dioxide or liquid nitrogen, are considered ideal solvents, because they leave no residue on evaporation, have no odor, are not explosive and have a high dissolving capacity. Although it is flammable, the use of liquefied butane as an extraction solvent has recently been gaining more and more ground. Purification of the crude volatile oil is achieved by trituration with alcohol, followed by filtration of the insoluble parts. The filtrate is cooled to a temperature of -10...-15 °C, when most of the ballast leaves the system. It is filtered cold. The alcoholic solution is concentrated under vacuum, at a temperature as low as possible, after which, by entrainment with water vapor, the volatile oil is obtained (Piras, et al, 2022).
d) Extraction with non-volatile solvents
It can be done by several methods, namely:
• Maceration
The solvents used are usually mixtures of various fats, depending on the extraction process and the nature of the material treated. Thus, the following are used: animal fats (pork, tallow, or their mixture called corpus), vegetable fats (olive, almond, apricot or peach oil), synthetic substances (benzyl benzoate). The fats used as non-volatile solvents must be very pure, not containing residues of protein substances or water. In the extraction process, the flowers packed in rare cloth bags are brought in several batches into tinned iron boilers, which contain the corpus melted at a maximum of 60-70°C. A time is left for maceration, after which the bags are removed from the fat and a new batch of fresh flowers is brought. The operation is repeated approximately 25 times. The product obtained with the help of animal fats is called pomade, and the one with vegetable oils is called antique oil. The separation of volatile oils from the fat mixture is achieved by draining the melted fat from the maceration container and drying it on anhydrous sodium sulfate. Next, after filtration, it is cooled and stirred for a long time with alcohol. It is then cooled to freezing temperature, when the alcohol that has absorbed the volatile oil is separated from the fat. The product obtained is called flower extract. By distilling the alcohol under reduced pressure, the specific flower oil is obtained. The recovered alcohol is used for a new extraction (Hafez Ghoran, et al, 2021).
• Enfleurage
It is an expensive process through which, however, volatile oils of the best quality are obtained. In this case, the solvent, made up only of solid animal fats, retains only the most volatile fractions that are released from the flowers. The system in which the extraction of the volatile oil is carried out is made up of the so-called chassis, made up of a wooden frame, 3-5 cm high, which has a thick glass bottom. On the glass bottom of the chassis, a layer of solvent is spread about. 1 cm thick over which petals or whole fresh flowers are laid, as much as they can fit.
The maceration of the flowers takes place in the chassis placed in cool rooms where they are kept for 12-72 hours. During maceration, the volatile fractions contained in the flower petals saturate the space available to them. At the same time, some of these volatile compounds are absorbed by the solid fat, which causes the saturation of the space to decrease. In this way, a new amount of volatile oil is released from the flowers, and the process continues until an equilibrium is established that corresponds to a certain depletion of the flowers in volatile components. As the depletion progresses, batches of flowers are replaced with fresh material, the operation being repeated 30-50 times, to ensure saturation of the corpus with volatile oil.
Sometimes, solid absorbents can be used instead of the corpus. It is recommended that the flowers be harvested before they are pollinated. After pollination, biochemical processes take place in the plant tissues that can change the composition of the volatile oil (Paibon, et al, 2011).
• Solid solvent extraction
In this case, the phenomenon of sorption is used. Two distinct processes can occur: capillary condensation, when volatile oil vapors agglomerate and liquefy in the pores of the absorbent, and chemisorption, when a chemical reaction takes place between the absorbed and the absorbent. Obtaining the volatile oil or desorption is done by extraction with alcohol. The following absorbents are used: activated carbon or silica gel (Won, et al, 2009).
B. Mechanical methods
These methods are particularly applicable to the extraction of volatile oils contained in the pericarp of citrus fruits. Several variants of this method are known:
a) By squeezing
The fresh fruit, cut into two or three parts, is cleaned of the core. The remaining peel is squeezed into a collecting vessel.Sometimes, before squeezing, the peels are kept in piles for one or two days, when a slight fermentation takes place, after which they are soaked for a few hours in water and then squeezed. Along with the volatile oil, part of the cell juice is also squeezed. After collection, the aqueous liquid is allowed to separate and then the oil is decanted (Perez-Cacho, & Rouseff, 2008).
b) By shaving
The surface of the fruit is scraped with a needle grater or one or two fruits are rotated in a spiked funnel or in a cylindrical vessel whose bottom is covered with short spikes arranged in concentric circles. The spikes must be sized so as to perforate only the oil glands, since, penetrating too deeply into the pericarp of the fruit, they increase the amount of cellular fluid, which will decrease the quality of the volatile oil (Uter, et al, 2007).
c) By pressing
Hand presses, screw presses and hydraulic presses are used for this purpose. The latter method gives the highest yields, but the best quality is provided by the manual method (Li, et al, 2021).
C. Fermentative methods
It is applied to obtain unformed volatile oils, which are found in plants in the form of glycosides.
First, the glycosides are cleaved, by fermentation, with the help of enzymes that are usually found in the same plant. Some products are processed immediately, others are stored from a few hours to a few months, during which the enzymatic cleavage of the glycosides takes place.
In order to carry out this method, the plant material is crushed and mixed with water.
A small fraction is set aside, and the rest is heated to 70-80°C to dissolve the glycosides, when, at the same time, the enzymes are inactivated. To cause fermentation, the unheated part (which contains the enzymes in the active state) is added, then left for a while for fermentation.
The volatile oil, set free, by this enzymatic pathway is carried away by distillation of the mixture. The volatile oil, obtained by the described processes, is collected in collectors, from where it is sent to the settling vessels, where it remains for a variable time depending on the characteristics of the oil and the dispersion of impurities. Usually, settling batteries are used (Zhang, et al, 2019).
After settling, the oil is dehydrated with anhydrous sodium sulfate, sometimes with calcined sodium chloride. The dehydrated oil is filtered through a pleated paper filter.
From the sodium sulfate used for dehydration, as well as from the used filters, the oil is recovered by entraining them with water vapor or by introducing them into the entraining flasks, together with the plant. All purification and recovery operations are carried out in the laboratory, in glass apparatus of reduced capacity and protected from the harmful action of some physico-chemical agents (oxidants, heavy metals, etc.). To obtain high-quality volatile oils, by removing the fractions that disqualify the product, fractional distillation is used. In this way, those components that give an unpleasant odor are removed, such as amines or furfural and some irritating compounds, mainly made up of aldehydes. Volatile oils are liquid substances, very volatile, with an aromatic and pleasant odor. Due to their high vapor pressure, they form azeotropic mixtures with water vapor and distill at much lower temperatures.They boil at temperatures between 150-300°C and are lighter than water. The exceptions are cinnamon, bitter almond and clove oils, which are heavier than water (Zhang, et al, 2018).
They are substances with optical activity, with characteristic refractive indices, with low viscosity, some being very fluid. They are soluble in organic solvents, insoluble in water, soluble in 60% chloral hydrate solution, themselves being very good organic solvents.
Volatile oils are flammable substances. Their odor can sometimes be irritating, although aromatic, and their taste is burning.
Depending on its components, each volatile oil has specific chemical properties.
Analytical control of volatile oils
In addition to the characteristic odor, the various volatile oils can be identified by the major component, which can be recognized by characteristic identification reactions.
Thin-layer chromatography has been widely used recently for the rapid identification and overall assessment of the quality of volatile oils (Kotra, Satyabanta, & Goswami, 2019).
For a more in-depth analysis, the volatile oil is subjected to a fractionation (figure 1), which obtains the neutral components on the one hand, and the acidic components on the other.
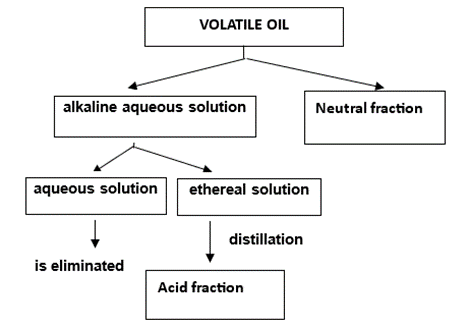
Figure 1: Fractionation of volatile oil
The fractions thus obtained are subjected to chromatographic analysis on silica gel plates. As developing solvents, mixtures based on hexane-ethyl ether, hexane-ethyl acetate, benzene-toluene-ethyl acetate, benzene-chloroform, benzene-acetic acid, etc. are used. Strong reagents are used for development, as follows:
To determine the quality of a volatile oil, a series of physical and chemical tests are performed, which highlight specific characteristics. To perform a correct analysis, the volatile oil sample must be clear (freshly filtered) and free of water (dried on anhydrous sodium sulfate) (Zhang, et al, 2021).
Among the physical characteristics necessary for the characterization of a volatile oil, the following should be mentioned: olfactory examination, density at 20°C (d2020), specific rotation angle (nD20), refractive index at 20°C (nD20), melting point, freezing point, dropping point, solubility, clarity, iodine color, evaporation residue, ultraviolet and visible spectrograms, gas chromatogram, thin layer chromatogram (Romanenko, Domrachev, & Tkachev, 2022).
The most important chemical characteristics that must be performed, partially or entirely, to characterize a volatile oil are: acidity index, esterification index, saponification index, acetyl index, ester index, carbonyl index, alcohol content (%), aldehyde and ketone content (%), phenol content (%), solubility in 5% sodium hydroxide solution, determination of sulfur derivatives, determination of nitrogen derivatives, special determinations.
In general, volatile oils stored in the light turn brown as a result of photochemical oxidation reactions. Their oxidizability is explained by the existence of double bonds as well as oxydryl and carbonyl functions grafted onto the chain or the basic nucleus. As a result, some of the oil components easily pass into macromolecular condensation compounds.
With concentrated sulfuric acid they give intense colors.
Volatile oils are used in various medications, in the form of crude plant products or extracted oils, as well as as semi-synthetic material, for obtaining other medicinal substances (for example: obtaining synthetic camphor, hydrated terpine, etc.).
In pharmacy, volatile oils are used as flavorings and taste correctors.
They are widely used in cosmetics and perfumery, when used in the form of extracts, hydrolates, alcoholates, tinctures, concretes, resinoids and oleoresins, essences, perfumes, etc. From these, beauty and maintenance cosmetics are prepared in the form of creams, emulsions, lotions, gels, oils, masks, make-up removers, soaps, bath and shower preparations, deodorants and antiperspirants, moisturizers, sunscreens, as well as numerous shaving preparations. Special attention is paid to preparations for dental and hair care, as well as products with a preventive and curative role in dermopharmacy.
Also, volatile oils are successfully used in the maintenance of public premises (fir oil), in microscopy (cedar oil), in the lens manufacturing technique (Canada balsam), in chromatographic techniques, in the varnish and paint industry, as insecticides and as flotation oil (pine oil) in the mining industry.