AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Leonore Cloet, Department of Gynaecology and Obstetrics, University Hospitals Leuven Herestraat 49 3000 Leuven.
Citation: Leonore Cloet, Kobe Dewilde, Thierry Van den Bosch. (2022). Usefulness of Antibiotic Prophylaxis in Miscarriage Surgery for Induced Abortion and Retained Products of Conception: a Narrative Review. J. Women Health Care and Issues. 5(6); DOI:10.31579/2642-9756/126
Copyright: © 2022 Leonore Cloet, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 05 August 2022 | Accepted: 13 September 2022 | Published: 10 October 2022
Keywords: retained products of conception; induced abortion; miscarriage surgery; antibiotic prophylaxis; pelvic infection
Background: Miscarriage surgery is one of the most performed surgeries worldwide. Prophylactic antibiotics aim to avoid postoperative pelvic infection. Its use and benefit are well-known in induced surgical abortion, but far more ambiguous for miscarriage surgery for retained products of conception. Objective: To investigate the usefulness of prophylactic antibiotics in miscarriage surgery for retained products of conception and induced abortion and evaluate the antibiotic regimen of preference. Material and Methods: A comprehensive electronic literature search was conducted using PubMed, MEDLINE and Google Scholar. There were no specific inclusion criteria concerning study design, publication year, language, or study population. Results: When evaluating the effectiveness of antibiotic prophylaxis for surgical intervention in induced abortion, 12 out of 19 studies showed a significant reduction on pelvic infection compared to the control group. There was no consensus regarding type and regimen of antibiotics. Five studies investigated prophylaxis in interventions for retained products of conception, of which 2 could show a significant effect. Conclusion: There is evidence that antibiotic prophylaxis reduces the risk of pelvic infection. Single dose preoperatively is favoured, for its effectiveness and patient compliance. Doxycycline and metronidazole are preferred, as for the type of antibiotics. There is limited evidence that antibiotic prophylaxis for surgical removal of retained products of conception or non-viable pregnancies might reduce the risk of pelvic infection.
Globally there are an estimated 208 million pregnancies each year of which 10 to 20% end in spontaneous abortion [1]. Since miscarriage is often incomplete, surgical intervention may be required, making miscarriage surgery one of the most performed gynecological procedures worldwide [2]. When discussing miscarriage surgery, we need to acknowledge the difference between induced abortion and retained products of conception or non-viable pregnancies (previously defined as incomplete and missed abortion respectively). Spontaneous complete abortion does not require surgery in general, hence will not be discussed in this article.
A potential risk of miscarriage surgery is infection. Pelvic infection reportedly occurs in 1-4% of cases after surgical intervention for retained products of conception and may have long term consequences ranging from pelvic pain to dyspareunia, ectopic pregnancy, synechiae, and secondary infertility [3,4]. Finally, 13% (47 000) of maternal deaths are due to unsafe induced abortion practices, and this mainly because of infection [5].
The aim of prophylactic antibiotics in miscarriage surgery is to avoid pelvic infection, thereby preventing acute morbidity and mortality, as well as reducing the risk of infertility and extra-uterine pregnancies [2, 6].
Whilst antibiotic prophylaxis is often used in surgical interventions for induced abortion, data about its benefit in retained products of conception surgery, are less consistent [7]. Some guidelines do not recommend antibiotics based on a lack of data [8, 9], whilst others support its use based on hypothetical ground [10]. Also, the optimal antibiotic regimen remains uncertain [11].
An alternative strategy to prophylactic antibiotics, is the ‘screen-and-treatstrategy’. In the latter, only patients with proven infection are treated, therefore avoiding unnecessary use of antibiotics [6, 12].
In this review, we investigate the usefulness of prophylactic antibiotics in miscarriage surgery for retained products of conception and induced abortion and evaluate the eventual antibiotic regimen of choice.
A comprehensive electronic literature search was conducted using PubMed, MEDLINE and Google Scholar. The search terms ‘antibiotic prophylaxis’, spontaneous abortion’, ‘retained products of conception’, ‘missed abortion’, ‘pelvic inflammatory disease’, ‘pelvic infection’, ‘post-abortal infection’ and ‘suction curettage’ were used. Additional MeSH terms used in this search were the following: ‘abortion, spontaneous’, ‘anti-bacterial agents’, ‘antibiotic prophylaxis’, ‘pelvic infection, prevention and control’. There were no specific inclusion criteria concerning study design, publication year, language or study population.
Only few papers evaluated antibiotic prophylaxis for surgical intervention in retained products of conception (RPOC), while most studies cover antibiotic prophylaxis for induced surgical abortion. Some studies included both RPOC and induced abortion. Other studies compared different type of antibiotics regimens to prophylaxis while other studies compared one type of antibiotic prophylaxis versus placebo.
Diagnostic criteria of pelvic infection were defined in the most articles as two or more of the following symptoms: temperature > 38 degrees, adnexal masses or tenderness on pelvic examination, purulent discharge or heavy bleeding, and/or leukocytosis [3,4,11,13-22].

Table 1: Effectiveness of prophylactic antibiotics for induced surgical abortion
A.Effectiveness of prophylactic antibiotics for induced surgical abortion (cfr Table 1)
In a randomized controlled trial using a single dose of 500 mg oral doxycycline pre-operatively, Brewer et al. found a significant reduction in pelvic infection, with 1/1519 (0,07%) patients being affected in the treatment group, compared to 8/1431 (0,66%) in the placebo group (X2=4,37) [6].
Darj et al. showed a significant reducing effect on pelvic infection rates of a single dose of 400 mg of oral doxycycline 10-12 hours pre-operatively, with a RR of 0,33 (95% CI 0,15-0,73) [13]. Of the 800 women included in the study, 32 were diagnosed with pelvic infection. Eight of them (2,1%; 8/380) had received prophylactic antibiotics, whereas the 24 (6,2%; 24/387) others did not.
A randomized controlled trial by Levallois et al. compared prophylactic 300 mg doxycycline with placebo in 999 patients undergoing induced surgical abortion. It confirmed a significant effect with 2/502 (0,44%) patients in the antibiotic group developing pelvic infection versus 15/497 (3,0%) in the placebo arm (p=0,001) [14].
A meta-analysis by Sawaya et al. showed an overall relative risk of 0,58 (95% CI 0,47-0,71) in developing pelvic infection in women receiving antibiotic therapy after induced surgical abortion (155/2587 (66%)), compared to placebo (267/2601 (10,33%)) [3]. In those with a history of PID, considered a high-risk-population, a summary RR of 0,56 (95% CI 0.37- 0.84) was seen. In a low-risk population, without history of PID, there was a summary RR of 0,65 (95% CI 0,47-0,90).
In a double-blind randomized controlled trial, Heisterberg et al. compared the risk of pelvic infection in a group of patients receiving prophylactic metronidazole, in a regimen of 400 mg 1 hour pre-operatively, and 4 hours and 8 hours postoperatively, to a placebo group. A significant effect was shown with 2/51 (3,9%) infections in the prophylactic group in comparison to 10/49 (20,4%) infections in the placebo group (p<0>
A double-blind randomized trial focused on women with bacterial vaginosis and compared the risk of pelvic infection after induced abortion when treated with metronidazole or placebo [16]. Metronidazole 3x500 mg was used for 10 days. In the treatment group 3/87 patients (3,45%) were diagnosed with pelvic infection, whereas infection was diagnosed in 11/87 (12.6%) patients of the placebo group (p<0>
A multi-centered randomized controlled trial by Crowley et al. (2001) compared the risk of pelvic infection after a single peri-operative dose of 2- gram metronidazole rectally to placebo, in women undergoing induced abortion and who were positive for bacterial vaginosis. Twelve out of 142 (8,5%) of the treatment group developed a pelvic infection, compared to 21/131 (16%) with a RR of 0,53 (95 CI 0,27-1,03) [16].
In a randomized trial, a group of 1672 women in Scotland undergoing induced abortion were allocated to either prophylaxis (metronidazole 1 gram rectally pre-operatively and doxycycline 100 mg twice daily for seven days) or a screen-and-treat-strategy [12]. Pelvic infection was seen in 4,6% of the prophylaxis group versus 6,8% of the screen-and-treat-strategy, not reaching a significant difference between the two strategies (RR 1,53; CI 0,99-2,36).
Henriques et al. investigated the effect of single dose ceftriaxone on pelvic infection, compared to a standard treatment in high- and low-risk patients [17]. Patients were considered high-risk when having a history of PID or STD, and their standard treatment was peroperative intramuscular ampicillin 1 gram and metronidazole 500 mg, followed by oral metronidazole 500 mg and pivampicillin 500 mg three times daily for four days. No significant effect was shown with 3,7% infections in the ceftriaxone-group and 4,7% in the standard treatment group.
On the other hand, the standard management for low-risk patients was no antibiotics. In the ceftriaxone-group 0,7% infections were diagnosed versus 3,6% in those who did not receive any antibiotics (p<0>
When comparing 500 mg of erythromycin twice daily for 7 days to placebo in a double-blind randomized trial, there was no significant difference in infection rates [28]. Pelvic infections occurred in 20/189 (10,6%) patients in the treatment group versus 30/180 (16%) in the placebo group (p= 0.13).
Nielsen et al. compared the risk of pelvic infection in women receiving ofloxacin 400 mg 90 min prior to intervention, to placebo [29]. Patients were divided in two groups: those with (N=308) and those without (N=765) a history of PID. In the former group, 20 out of the 149 (13,4%) patients receiving ofloxacin developed pelvic infection, compared to 27/159 (17%) in the placebo group (p=0.39). In the group without a history of PID, 35/376 (9,3%) of the antibiotics group were diagnosed with pelvic infection, versus 46/389 (11,9%) patients in the placebo group (p=0.26). The differences did not reach significance in any of the groups.
A double-blind randomized study by Larsson et al. assessed the use of vaginal clindamycin on pelvic infection rates after induced surgical abortion, compared to placebo [18]. The first group had a pre-operative treatment for three days with clindamycin cream 2%. Of them, 29/650 (4,5%) were diagnosed with pelvic infection, versus 30/626 (4,8%) in the placebo group (p=0,68). In the subgroup with abnormal vaginal flora, using Nugent’s criteria defined as bacterial vaginosis and intermediate flora, there was a significant lower risk of pelvic infection in the treatment group (RR: 4.2 (95% C.I. 1.2–15.9)).
A Cochrane systematic review including 19 randomized controlled trials showed that antibiotic prophylaxis in women undergoing induced surgical abortion is effective in preventing pelvic infection [11]. Fourteen of these 19 RCTs have already been aforementioned [12]–[16], [18], [27]–[33]. The five other studies will be discussed here. Krohn et al. conducted a double-blind randomized trial to investigate the effect of a single pre-operative dose of oral tinidazole 2 grams compared to placebo in preventing infections in patients undergoing first trimester abortion with vacuum aspiration [23]. Six out of 104 (5,8%) patients in the treatment group, and 11 out of 106 (10,4%) patients in the control group were eventually diagnosed with postoperative pelvic infection and needed antibiotic treatment. Results were not significant (p = 0,23).A single-blind randomized trial by Krohn et al. investigated the effect of a pre-operative single intravenous dose of sulbactam 0,5 gram and ampicillin 1 gram compared to placebo in preventing postoperative endometritis after first trimester abortion with vacuum aspiration [26]. Four of 145 (2,75%) patients in the treatment group and 11/140 (7,86%) in the control group developed endometritis. Results were not significant (p = 0,08).
Sonne-Holm et al. assessed the effect of antibiotic therapy on pelvic infection compared to placebo in a double-blind study in patients undergoing induced first trimester abortion [24]. Patients in the treatment group were given two million IU penicillin G, one hour pre- and three hours postoperatively, and additionally 350 mg pivampicillin three times daily for four days. Pelvic infection occurred in 14 out of 254 (5.5%) patients in the treatment group, and 26 out of 239 (10.9%) patients in the placebo group (p= 0,05). In a double-blind study by Westrom et al., 212 patients undergoing induced first trimester abortion were allocated to either single pre-operative dose of 2 grams tinidazole, or placebo [25]. Patients who were positive for gonorrhea were excluded. Ten out of 102 (9,8%) patients in the treatment group, and 17 out of 110 (15,4%) in the control group developed febrile reactions with rectal temperatures above 38 degrees. There was no significant effect (p = 0,22).
Caruso et al. conducted a randomized controlled trial in 466 patients undergoing induced first trimester abortion to assess the effect of prulifloxacin in preventing postoperative pelvic infection [19]. Patients were randomized in three groups: one receiving 600 mg prulifloxacin for 5 days after surgery, another group receiving 600 mg prulifloxacin for 3 days postoperatively and the last group receiving 600 mg prulifloxacin one day pre-operatively and two days postoperatively. Respectively 16 (10,4%), 11 (7,10%) and four (2,53%) patients developed symptoms of pelvic infection. Results were significant when comparing the first and last group (p=0,01).
In the Cochrane review, 203 out of a total of 3525 (5,875%) patients developed an infection in the study group, as compared to 330/3500 (9,42%) patients in the control group (RR 0.59 (95%CI 0.46 to 0.75)).
No recommendations were made regarding the most effective type of antibiotics and dosage regimen.
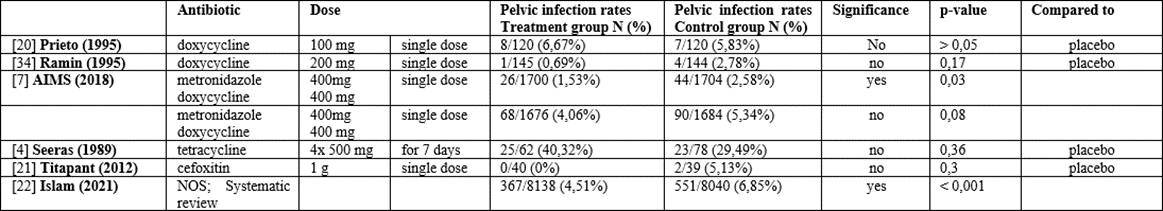
Table 2: Effectiveness of prophylactic antibiotics for retained products of conception
B. Effectiveness of prophylactic antibiotics for retained products of conception (RPOC) or non-viable pregnancies (cfr Table 2)
A randomized open-label trial by Prieto et al. failed to show a decrease in postoperative pelvic infection in the prophylactic intravenous 100 mg doxycycline-group. Eight out of 120 (6,6%) patients in the prophylactic group developed postoperative infectious morbidity, versus 7/120 (5,8%) controls (p>0,05) [20].
A randomized double-blinded prospective study by Ramin et al. compared the effectivity of a single dose of 200 mg doxycycline 30-60 minutes prior to curettage to placebo in preventing endometritis [34]. Endometritis was diagnosed in 1/145 (0,6%) in the study group and 4/144 (2,8%) in the control group (p=0,22). It was calculated that 700 patients would be needed to be able to reach statistical significance.
Morrill et al. reviewed eight randomized controlled trials on prophylactic antibiotics for induced abortion and RPOC, and one randomized trial including second-trimester dilation and curettage [35]. Results of pelvic infection rates when comparing prophylaxis to placebo, were ambiguous. Seven of the 9 studies have already been discussed in this paper [14], [17], [18], [20], [28], [32], [36]. One study was left out, since it focused on doxycycline serum levels and side-effects, but also because it included second-trimester dilation and curettage (Reeves et al. 2009).
The other study, by Lichtenberg et al., compared two different regimens of doxycycline after suction curettage, namely 100 mg twice daily for three or seven days [33]. There was no significant difference between these two regimens, but the requirement for sample size was not reached.
A Cochrane Review by May et al. about antibiotics for incomplete abortion could only include 1 RCT: by Seeras et al [38]. The latter did not show any statistically significant effect on sepsis rate, using 500 mg of tetracycline four times daily for a week [4]. Twenty-five out of 62 (40,32%) patients were diagnosed with pelvic infection despite antibiotic prophylaxis, versus 23/78 (29,5%) patients in the placebo group. Since the vast majority of the treatment group (82,6%) did not take the prescribed medication, the lack of significant reduction may be attributed to poor compliance. Therefore, the authors suggest a single-dose regimen, e.g., doxycycline, to overcome the issue of poor compliance.
In 2012, a randomized controlled trial by Titapant et al., investigated the effectiveness of cefoxitin in reducing the risk of endometritis in curettage for incomplete abortion [21]. One gram of cefoxitin was given 20 minutes preoperatively in the study group, and 0,1 ml of vitamin B in the control group. Two out of the 79 cases (2,53%) developed endometritis, both belonging to the control group. The difference did not reach statistical significance, with a p-value of 0,24.
The AIMS-trial was a randomized controlled trial investigating whether prophylactic antibiotics, defined as single dose oral doxycycline and metronidazole (400 mg each), used in low-resource countries effectively reduced the risk of pelvic infection [7]. Diagnostic criteria were defined as purulent or foul-smelling discharge, pyrexia, adnexal tenderness and leukocytosis more than 12x109 per liter. The strict definition of PID required at least two of these characteristics, while the broad definition of PID required just one of these features and the clinical judgement for the necessity of antibiotics. When considering the broad criteria of PID, the results were not significant, with a risk of infection of 4,1% and 5,3% in the prophylactic group and placebo group respectively. However, when using strict criteria, there was a significantly lower rate of pelvic infection in the prophylactic group: 26/1700 (1,5%) versus 44/1704 (2,6%) patients respectively (RR 0,60 (95% CI, 0.37 - 0.96)).
A systematic review by Islam et al. studied prophylactic antibiotics in preventing pelvic infection in women with incomplete abortion [22]. In the overall group, 367/8138 (4,5%) patients receiving prophylactic antibiotics were diagnosed with pelvic infection, compared to 551/8040 (6,8%) in the control group (RR 0,72 (95% CI 0.58–0.90)). However, three studies focusing on the subgroup of women of low- and middle-income countries (N= 3579), did not show any significant effect. (RR 0,90 (95% CI 0,50-1,62). In 21 studies on women of high-income countries (N= 12599), there was a statistically significant effect of prophylactic antibiotics (RR 0,67 (95% CI 0,53-0,84). No recommendations concerning antibiotic dosage and regimen were made.
C. Choice of antibiotics
Several studies focused more specifically on the dosage regimen and type of antibiotics. Only studies comparing antibiotic regimens are included in this paragraph.
As discussed above, both oral doxycycline and metronidazole have proven to be efficient in miscarriage surgery for induced abortion [14], [36], [39]. A few studies focused on other regimens. In a meta-analysis by Heisterberg et al., based on 5 controlled clinical trials, penicillin and ampicillin reduced pelvic infection in women with a history of a pelvic inflammatory disease history [32], [36]. However, in women without a history of PID, imidazoles are more efficient in reducing PID.
As aforementioned, the Cochrane Review by Low did not make any recommendations regarding the most effective type of antibiotics and dosage regimen [11].
Herawati et al. conducted a retrospective study and evaluated the effects on the infection rates of 2 grams cefazoline pre-operatively and amoxicillin three times 500 mg postoperatively, administered in three regimens [40]. There were no significant differences between the three antibiotic regimens (p>0,05).
The systematic review by Islam et al. did not make any recommendations concerning dosage or regimen [38].
D. Screen-and-treat versus prophylaxis
An estimated 1% and 10% of patients attending a family planning clinic, are positive for N. Gonorrhea and C. Trachomatis respectively [41]. The isolation of Neisseria gonorrhea is associated with a three times elevated risk of post-abortal infection [42]. Several studies indicated that Chlamydiapositive patients have a significantly increased risk of pelvic infection after induced abortion with rates from 10-20% in comparison to 1-8% in a Chlamydia-negative population [41]. An Australian study showed that there was a significant higher risk of pelvic infection after induced abortion in patients positive for bacterial vaginosis (10,8%) then in patients without anaerobic flora (4,5%) [43].
Osser et al. compared the rates of pelvic infection following induced abortion in Chlamydia-positive and -negative women [44]. Of the 1101 women undergoing curettage, 69/1101 (6,3%) were Chlamydia positive. Of these women, 16 (23,2%) and 10 (14,5%) were diagnosed with endometritis and salpingitis respectively in the first four weeks. In comparison, in chlamydianegative women these rates were 59 (5.7%) and 5 (0.6%) respectively. These differencePenney et al. randomized women to either prophylactic antibiotics (metronidazole 1g prior to intervention and doxycycline 100 mg twice daily for seven days) or to the screen-and-treat-strategy. In the screen-and-treat group, adequate antibiotics was prescribed only if the culture came back positive [12]. Patients in the prophylaxis group had lower rates of pelvic infection (38/826 (4,6%)) than those in the screen-and-treat-group (58/846 (6,8%)), but no significance was reached.
There is sufficient data supporting the use of prophylactic antibiotics for induced surgical abortion [3], [11], [13], [39], [41]. However, data on prophylactic antibiotics in surgery for retained products of conception, are far more ambiguous [2], [20].
Some studies suggest a significant reduction in infection rates in retained products of conception, when using oral doxycycline or metronidazole [14], [22], whilst others fail to show a significant effect of antibiotics [20], [21], [34]. The inconsistency in these trials is mostly due to a lack of sufficient sample size and of rigorous study design [2].
The most important and largest randomized controlled trial on this topic is the AIMS-trial and could only demonstrate a significant reduction in infections using strict diagnostic criteria for PID [2]. The participating countries in this trial included Malawi, Tanzania, Uganda and Pakistan. These specific countries were selected, since the researchers considered this clinical problem to be of particular importance in low- and middle-income countries [7]. Given the absence of similar studies in high-income countries, the issue whether these data can be extrapolated to other clinical populations has to be explored. In 2020, a study protocol for a systematic review and meta-analysis by Yu et al. was published. The researchers plan to investigate the effects of prophylactic antibiotics on the risk of pelvic infection in women undergoing surgery for incomplete spontaneous abortion [45]. This data could possibly lead to new insights.
The inconsistency amongst trials is also reflected in international guidelines, publishing different recommendations on the topic. RCOG and SCOG for example, do not recommend prophylaxis for retained products of conception due to the lack of robust data [8], [9]. ACOG on the other hand, extrapolating the findings on prophylaxis in induced surgical abortion, does advise antibiotics for this indication [10].
As for the type of antibiotics of choice for prophylaxis in miscarriage surgery, there is more consistency [14], [39]. Since infections after obstetrical surgical interventions are usually caused by endogenous flora or STD, the antibiotic prophylaxis should cover gram-negative, -positive and anaerobic agents. Therefore, the combination of doxycycline and metronidazole tend to be the preferred. Moreover, both antibiotic agents are relatively inexpensive, easily accessible, and allergies are infrequent [2], [46].
When comparing multiple versus single dose, the latter is to be preferred as to patient compliance [47]Given the plasma half-life of both doxycycline and metronidazole is over 10 hours, and given a curettage takes less than 30 minutes, a single dose may be considered sufficient [48], [49]. Therefore,antibiotic prophylaxis administered as a single dose pre-operatively is to be favored [46].
Another issue that remains uncertain, is whether a screen-and-treat-strategy should be preferred over universal prophylaxis. An advantage of a screenand-treat-strategy is that only women who were tested positive would be treated, thereby avoiding unnecessary administration of antibiotics [41], [50]. Also, in screen positive women, the partner could be immediately treated as well, hence preventing re-infection [12]. A key study on this topic by Penney et al. failed to demonstrate a significant difference between prophylaxis and screen-and-treat [12]. The prophylactic regimen consisted of a ten-day course, and therefore should be considered therapeutic instead of prophylactic.
One of the downsides to the screen-and-treat-strategy is that more patients are at risk to be lost in follow-up, due to diagnostic delay, and hence won’t be treated adequately. Also, to be successful, this strategy requires a vast organizational structure and thorough multidisciplinary communication [50]. Lastly, there may be a difference in population between women undergoing induced surgical abortion and those undergoing miscarriage surgery for retained products of conception. Women attending an abortion clinic may be more likely to match the high-risk profile for STD of being young with a lower socio-economic status and more likely to have a higher sexual risk behavior [38], [51]. Thus, for those specific patients a screen-and-treatstrategy could be considered more useful and cost-effective.
Importantly, antibiotic prophylaxis is applicable in asymptomatic patients. Clinical examination remains of utmost importance, to rule out infection already present at the time of intervention. In that case the patient should be treated according to local therapeutic protocol.
It is remarkable that there are few high-quality studies on one of the most performed surgeries worldwide. There is evidence that antibiotic prophylaxis for an induced abortion reduces the risk of pelvic infection. Single dose pre-operatively is favored, not only for its effectiveness, but also for patient compliance. As for the type of antibiotics, doxycycline and metronidazole are preferred. The role for the alternative screen-and-treatstrategy needs to be validated.
There is limited evidence that antibiotic prophylaxis for surgical removal of retained products of conception or non-viable pregnancies might reduce the risk of pelvic infection.
Further research should validate the benefit of prophylaxis in retained products of conception and clarify whether management should depend on the population profile.
RPOC (retained products of conception)
The authors declare no conflicts of interest. They received no specific funding for this work.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
