AUCTORES
Globalize your Research
Research article
*Corresponding Author: Tom Langenaeken, Department of Cardiac Surgery, University Hospitals Leuven Herestraat 49, 3000 Leuven, Belgium.
Citation: Tom Langenaeken, Filip Rega, Bart Meuris, (2024), Trileaflet Mechanical Valves: What Is Already Known? A Systematic Review, Journal of Clinical Surgery and Research, 5(3); DOI:10.31579/2768-2757/116
Copyright: © 2024, Tom Langenaeken. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 26 February 2024 | Accepted: 05 March 2024 | Published: 30 April 2024
Keywords: trileaflet mechanical valve; mechanical valve; hemodynamics; animal trials; valve testing
Background: Trileaflet mechanical valves were introduced in the late 90’s. Despite promising results, none have made it past preclinical testing. Ample in vitro, in vivo and computational testing have already been done.
Methods: This systematic review is intended to summarize all available preclinical and clinical testing regarding trileaflet mechanical valves. A literature search of Pubmed, Embase and Web of Science (Core Collection) (from inception to August 2023) was performed using a search string that was well defined and not modified during the study. An extensive overview of the search terms used in each database can be found in the appendix. 24 publications were included in this review.
Results: This systematic review serves as an overview evaluating the rationale behind a trileaflet mechanical valve as well as the computational, in vitro and in vivo testing. We aim to summarize all available knowledge on trileaflet mechanical valves.
Conclusions: A trileaflet mechanical valve is a promising concept, with equal or even superior hemodynamics compared to conventional bileaflet valves based on computational, in vivo and in vitro data. First in men trials are warranted to elude the true potential of this design.
The history of mechanical heart valves spans several decades and represents a remarkable journey of medical innovation and technological advancement. The quest to develop artificial heart valves emerged in the mid-20th century as a response to valvular heart diseases, which often led to severe health complications and inevitable death.
The first successful implantation of a mechanical heart valve occurred in 1952 when Dr. Hufnagel implanted for the first time a ball-and-cage valve [1]. This design consisted of a metal ball that would pivot in a cage, allowing blood to flow in one direction. Over 200 patients received the Hufnagel prosthesis, with some patients living for another 30 years.
In the late 1960s, the tilting-disc valve was introduced, featuring a single disc that opened and closed to control blood flow [2]. This design was more efficient and reduced the risk of clot formation. However, complications such as valve thrombosis and mechanical wear persisted.
A major leap in mechanical heart valve technology came with the introduction of the bileaflet valve in 1977 [3]. This design imitated the natural flow of blood through the heart more closely, leading to improved hemodynamics and reduced chances of clotting. Bileaflet valves became the gold standard for mechanical heart valves due to their durability and biocompatibility and remain the only available mechanical valves to this day.
Despite these advancements, mechanical heart valves still pose the risk of blood clot formation. This drawback prompted researchers to develop valve designs that reduced clotting risks and are safe under lower doses of anticoagulation [4, 5]. Despite these advancements, lifelong anticoagulation with vitamin K antagonists (VKAs) remains necessary. Recent trials with direct oral anticoagulants (DOACs) as a better alternative to VKAs failed [6–8].
Since their introduction, the mechanical valve as a bileaflet concept has largely remained untouched. In 1996, the first studies of a trileaflet mechanical valve were published. The idea is based on mimicking the native trileaflet aortic valve, while maintaining the durability of a mechanical valve. This review aims to provide a detailed overview of the broad literature of trileaflet mechanical heart valves and an update to the current status of preclinical development.
Study selection:
This systematic review is intended to summarize the most recent data on the topics "trileaflet" and "mechanical valve". It gives an overview of the development and preclinical testing of the valve, as well as current state of affairs and future directions.
We searched all possible databases: Pubmed, Embase and Web of Science (Core Collection) (from inception to August 2023) with no language limitations, using the following search string: “trileaflet” AND “mechanical” AND “valve”. This systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [9].
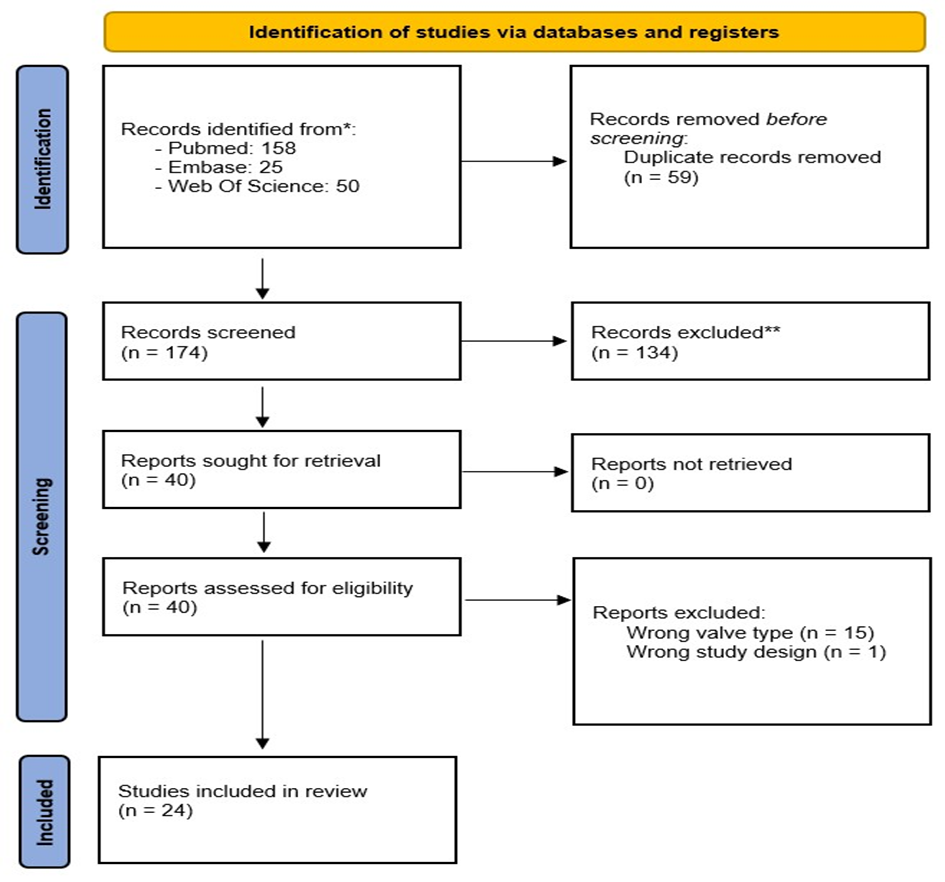
Figure 1: PRISMA flow diagram
Search outcome
Our search strategy generated a total of 852 results from all the different databases listed above (see fig. 1). After removing the duplicates, 174 remained. We exported these articles to Rayyan, in which 2 people independently screened the studies by title and abstract [10]. There were 40 articles selected for full-text eligibility, from which 24 articles finally remained to be included in our review. Articles are divided between in vitro, computational and in vivo models and discussed in order of publication date. A separate section is dedicated to a new type of trileaflet
valve with the hinges placed centrally. Out of our 24 articles, 18 were in vitro or computational studies and the remaining 6 were animal models.
In-vitro studies
In vitro studies were summarized in table 1. The first in vitro study of a trileaflet mechanical valve was published in 1996 [11]. The JCL-trileaflet mechanical heart valve prosthesis, called ‘Tricusp’, consisted of 3 leaflets designed as separate segments of a circle in a titanium housing. A hinge was in the center of each leaflet, with the hinge itself consisting of a single stud. The leaflet opening angle was 86°C. The valve was tested in an electrohydraulic, computer-controlled pulse duplicator simulating the left side of the human circulatory system and testing conditions set according to a Food and Drug Administration interlaboratory comparison protocol with cardiac outputs of 3.0, 4.5, 6.5, and 8.0 L/min at a constant heart rate of 70 beats/min[12]. Compared to other commercially available bileaflet valves (Saint Jude Medical (SJM), Carbomedics (CM) and Duromedics (DM)), the trileaflet valve had less pressure drop over the valve and less regurgitation. The mean energy losses for all cardiac outputs closely resembled the SJM valve. Peak flow velocities over the valve were in line with the bileaflet control valves. In the trileaflet valve, the velocity at the aortic wall between the sinuses was very close to zero. Only transient recirculation at the right wall and a washout vortex in the sinus cavity were present. Turbulent shear stress occurs only during systolic ejection phase and is generated within the sinus cavity at both jet boundaries. In all measurable aspects, the trileaflet ‘Tricusp’ valve performed better than its bileaflet counterpart.
A study analyzing closing mechanisms of mono-, bi- and trileaflet valves was conducted in 2004 [13]. The valves were mounted in a circulatory mock loop system, closely mimicking native physiology [14]. The aortic root of 25mm with 3 sinuses was made of plastic glass. The trileaflet valve had curved, umbrella shaped leaflets creating a single, almost completely circular orifice. The other valves were monoleaflet Medtronic Hall 27mm and 3 bileaflet valves: CM 27mm, SJM and Edwards DM 29mm. When there was a positive transvalvular pressure, all valve types opened simultaneously, but the closing mechanism differed. When transvalvular pressure approached zero, the trileaflet valve started closing; the mono- and bileaflet valve only started closing with relatively high negative transvalvular pressure and cardiac output was near zero, as these valve types rely heavily on reverse flow. The trileaflet valve needed 75ms to fully close, while the mono- and bileaflet valves took less than 35ms. Digital particle image velocimetry (DPIV) measurements were made vertically along the centerline of one sinus from which the phase average velocity fields and vorticity distributions were obtained. The trileaflet valve’s velocity profile and vector field create a uniform, smooth central flow absent of any vortices. There are however large velocity gradients in the sinus walls, leading to counterclockwise vortices. Bileaflet valves have 3 separate jet flows. Main recirculation vortices occur in the aortic sinuses and wake behind the leaflets. The smaller opening angle of the CM valve leads to a higher vorticity strength compared to the SJM. The side orifice flows in the CM are stronger than the central one, leading to increased shear gradients and aortic sinus vortices. The monoleaflet valve generates unevenly distributed jet flows, due to a larger and smaller orifice[14].
In closing, the central orifice in the trileaflet valve reduces in area but maintains forward flow. As the vortices in the aortic sinuses increase, the leaflets are pushed to close. The closing of the SJM is marked by very small forward flow compared to the trileaflet valve in the early stages of closing. Reverse flow begins to appear in the downstream regions generating weak vortices. The CM and DM exhibit reverse flow in the central orifice but stronger forward flow in the side orifice with a very nonuniform downstream flow field. The smaller opening angle of CM and curved leaflets of DM form large downstream wakes during valve closure. In the monoleaflet valve, the forward flow curves around to form a large vorticity in the aortic sinus and downstream wake. SJM has the largest amount of reverse flow because its full opening angle is 85°, meaning the leaflets require a larger force to overcome the oncoming flow when closing. The bileaflet valve also disturbs normal aortic flow physiology, as 2 leaflets need to be fitted in 3 sinuses. The trileaflet valve with its 90° opening angle, each leaflet properly positioned in each sinus, in addition to the negative transvalvular gradient in the deceleration phase, leads to slower closing velocity and less reverse flow. When the trileaflet valve starts closing, the transvalvular pressure drops to zero, and the vortices in the aortic sinus reduce the lift force. A small amount of reverse flow completes the closing process, with minimal water hammer effect and cavitation. The negative transvalvular pressure during the deceleration phase benefits the trileaflet valve but has no effect on the closing behavior of other valves[14].
A year later, a follow-up hemodynamic study was published comparing the trileaflet valve to a 27mm SJM valve in a similar flow loop pulse duplicator with glass aortic root [15]. A cardiac output of 28L/min mimicked peak systole. Using DPIV and laser-Doppler anemometers (LDA), principal Reynolds Shear stress (PRSS) vorticity and axial velocity were measured. Measurements were made in 2 axes. In the bileaflet the XZ plane perpendicular to the leaflets and the YZ plane parallel to the leaflets. In the trileaflet valve, the XZ plane was perpendicular to one leaflet and the YZ plane where it was parallel to one leaflet.
In the trileaflet valve, both DPIV and LDA data exhibit uniform central flow with reverse flow on both sides in the XZ plane. There is minimal PRSS in the central flow region but maximal PRSS in the sinuses of 300 and 600dynes/cm² measured with DPIV and LDA, respectively. In the YZ plane, there are similar findings[15].
The bileaflet valve showed symmetrical flow fields in both planes. There is a central jet flow expanding outwards as the flow progresses downwards. The two side flows generate large recirculation flows in the sinuses, creating wider and more dispersed high shear stress regions. The maximum velocity was similar to the trileaflet valve. The PRSS measured with DPIV was 300dynes/cm², but 800dynes/cm² when measured with LDA. Under these high PRSS values, blood cells suffer a high risk of rupture [16, 17].
In 2011, a more detailed flow study analyzing the impact of forces on blood cells during valve closure were examined [18]. In a pulse duplicator simulating physiological conditions, 4 valve types were tested: SJM Regent, the Edwards Perimount Model 2800 (EP), the Lapeyre Industries Triflo (experimental LT), and the complementary (non-test) valve, the Mitroflow pericardial (29 mm). In the SJM, there was prominent flow and transvalvular pressure at or near valve closure consistent with microbubble formation. The closing backflow volumes were fivefold greater for bileaflet mechanical valves compared to tissue valves, resulting in high-magnitude transient regional backflow velocities, followed by damped water-hammer oscillations. The prominent flow and transvalvular pressure at closure were consistent with microbubble formation, which were absent in the control tissue valves. The valve rebound was observed as a partial reopening after closure, driven by water-hammer power. The trileaflet valve behaved similar to biological control valves in this experiment: its leaflets followed flow deceleration towards closure with minimal closing regurgitation. Flow acceleration and rate of acceleration (jerk) approach maximum values for the bileaflet mechanical valves, while almost absent in the tissue or trileaflet valve.
A parallel study was published in 2011 where flow over the trileaflet valve was modeled with computational fluid dynamics to analyze flow patterns causing thrombus formation on valves [19]. The Thrombosis Tester Helmholtz Institute Aachen (THIA II) is a test rig for in vitro assessment of thrombogenic potential and blood damage of heart valves. Physiologic conditions were recreated with a pressure of 140/70mmHg at 75bpm. During systole, a vortex behind the trileaflet occurs and moves downwards towards diastole. The highest velocities occur in the middle of the leaflets, as well as the highest shear rates. Shear rates above 10.000s-1 appear at the edges of the leaflets and in the bearing positions. Threshold for platelet activation is 35dyn s/cm². Only 7% of volume fraction during valve opening and 0.5% during valve closure is exposed to such shear rates. However, the very high shear rates (>10000s-1) may immediately cause platelet activation. These high shear rates are in the middle of the leaflets but more importantly in the critical pivot regions.
Another DIPV study in 2011 was published comparing the trileaflet valve to a standard bileaflet (SJM) valve in aortic position in a pulsatile circulatory mock loop under physiological conditions [20]. There was virtually no difference in opening time for the 2 valve types. The trileaflet started closing sooner and took longer to complete closing. In the trileaflet, the valve closes almost solely by vortices in the aortic sinus and not on reverse flow, as does the bileaflet valve. Calculations were made for effective orifice area (EOA), PI and regurgitant volume from the flow waveforms over 30 cardiac cycles. It was concluded that the trileaflet valve has less energy loss and regurgitation compared to the bileaflet counterpart. Major principal Reynolds shear stress (PRSS-maj) and major principal Reynolds normal stress (PRNS-maj) were determined using the Baldwin method [21]. The PRSS-maj of neither valve exceeded the threshold of RBC damage of 150N/m². At valve closure, there is a sudden increase in PRNS-maj and PRSS-maj for the bileaflet valve, likely due to rapid valve closure producing a large fluctuation at this instant. The Kolmogorov length scales, the smallest scales in turbulent flow of fluids, were measured. For the trileaflet valve this was 25µm, for the bileaflet 27µm, which was approximately 3-4 times the diameter of RBCs. These measurements were similar to previous data [20]. The turbulent viscous shear stress (TVSS) could be calculated, which peaked at valve closure for the bileaflet valve however remained relatively low at <15N>
In 2012, a study was published analyzing the trileaflet valve and SJM in a numerical simulation using the Fluent software program [22]. Data obtained was compared to previous experimental data [20]. The aim was to develop a validated computer model of leaflet motion and flow fields to assist and improve future valve design. The researchers do state however that their model is limited by error messages in the coding process, requiring slight modification of the geometry of the SJM leaflet. This leads to a slight overestimation of reality, making it difficult for these simulated data to replace experimental data. The conclusion was that the trileaflet valve takes more time to open and close: 64 and 60 ms respectively. The SJM only takes 57 and 40ms to open and close. The traveling angle for the SJM is 60° compared to 45° for the trileaflet valve, meaning a slower closing velocity of the trileaflet valve. The reason is that the SJM relies on reverse flow and a pressure gradient for closing, while the trileaflet closes due to vortices in the sinus. This is similar to the closing of the native human aortic valve [23]. Closing velocity is an important parameter in valve functioning, as the higher the velocity of the leaflet tips, the greater the cavitation formation [24, 25].
In 2018, Venneman et al. tracked each leaflet of different valve types in a compliant model of the aortic root in a physiological flow loop [26]. Main parameters were leaflet kinematics, asynchronous leaflet motion and leaflet tip velocities. Valves included were Edwards Intuity (EINT), Medtronic Advantage (MADV) and Lapeyre-Triflo Furtiva (TFUR). Valve movements were recorded using a high-speed camera in an optimal optical setup and cross-correlation based image stabilization algorithm. The opening phase was similar for all 3 valve types, starting at 30 ms and completing at 50ms. However, the EINT and TFUR started closing at 270ms with completion at 330ms, but the MADV only started closing at 330ms and completion at 360ms.
Rapid valve closing time (RVCT) was measured as the time between onset of rapid valve movement and full closure divided by the duration of the heart cycle. For EINT, it started at τ = 0.3 and ended at τ = 0.39. For TFUR, it started at τ = 0.32 and was completed at τ = 0.39. Valve closure of MADV did not start until τ = 0.39 and it ended only at τ = 0.425. The rapid valve closing speed (RVCS) was measured as the square root of the rGOA over the RVCT, which can be interpreted as the average non dimensional closing speed of the valve. EINT had the lowest RVCS (9.09), followed by TFUR (11.84). MADV had a RVCS of 24, almost double that of TFUR and EINT. Researchers concluded that a strong similarity exists between EINT and TFUR, both qualitative and quantitative. Maximum leaflet tip velocities were significantly higher in the bileaflet valve[26].
In 2020, Bruecker et al. investigated the effect of a bileaflet and trileaflet design on the preservation of early helical flow in the ventricular outflow tract [27]. Using a pulse duplicator, high speed particle image velocimetry and a 25mm aorta with comparable geometry, the effect of helical flow was studied over a SJM Regent valve and Triflo T2B trileaflet mechanical valve.
The researchers conclude that the trileaflet valve preserves over 80% the swirling core flow and angular momentum compared to a biological valve[27]. Stronger retrograde flow was found in the sinuses of the trileaflet valve, supporting the early onset of leaflet closure. In comparison, the bileaflet valve works as a flow-straightener: the nearly parallel planes guide the helical flow in axial direction, similar to guide-vanes of a stator in turbomachinery. This leads to energy loss, as the kinetic energy of swirling flow is converted to higher pressure. Only 30% of the angular momentum of the swirling flow survives passage over the BMH. The left ventricle must overcome this additional hydrodynamic pressure drop.
Conservation of this helical flow can be of importance to the distribution of microbubbles, generated by MHV due to degassing during the localized pressure drop at valve closure[27]. These microbubbles can be propagated in the ascending aorta and cause gas embolism. These microbubbles migrate to the area of least pressure, being the core of the vortex in a swirling flow. As this flow is less disturbed in the trileaflet valve, one could argue that microbubbles are contained in this flow instead of ejected in the brachiocephalic arteries.
In vitro
| Reference | Valve used | Setup | Conclusion |
| Lentell et al. 1996 [11] | JCL TV mechanical heart valve ‘Tricusp’ Control valves: Carbomedics, Duromedics, SJM | Electrohydraulic, computer-controlled pulse duplicator cardiac outputs of 3.0, 4.5, 6.5, and 8.0 L/min at a constant heart rate of 70 BPM | Less regurgitation and pressure drop in Tricusp valve. Mean energy loss for all cardiac outputs comparable to SJM. Tricusp better in al measurable aspects. |
| Lu et al. 2004 [13] | Monoleaflet Medtronic Hall valve 27mm BV: Carbomedics 27mm, SJM 27mm, Duromedics 29mm | circulatory mock loop system with a glass aortic root of 25mm with 3 sinuses heart rate of 70 BPM cardiac output of 5 L/minute blood pressure 120/80 mmHg | Mono- and bileaflet valves close in much less time than TVs. TV produces a single central flow in the systolic phase. Mono and bileaflet valves rely on high negative transvalvular gradients for closure. TV relies on aortic sinus vortices for a more controlled, slower closure. |
| Liu et al. 2005 [15] | TV 27mm SJM 27mm | Steady flow loop with a glass aortic root 32mm at the sinuses. Cardiac output of 28 l/min | Strong turbulent flow in the BV resulting in higher shear stresses. TV creates central flow with sufficient pressure to inhibit formation of separation shear layers. Principal Reynold shear stresses measured in the BV were large enough to rupture blood cells. |
| Scotten et al. 2011 [18] | SJM Regent 25mm Edwards Perimount Model 2800 25mm Lapeyre Industries Triflo 25mm Mitroflow pericardial 29mm | ViVitro Systems Inc. pulse duplicator with prototype adaptation (LeonardoVSI) pulse rate 70 BPM pressures ~120/80 mmHg cardiac output 5 l/min | SJM remains open during flow deceleration phase. Rapid leaflet closure leads to elevated regional backflow velocities that promote cavitation, microbubble formation and inflicts shear damage to passing blood cells. Results for the TV were similar to those obtained for tissue control valves. |
| Kaufman et al. 2011 [19] | TV mechanical valve ‘Triflo’ | Thrombosis Tester Helmholtz Institute Aachen (THIA II) 75 BPM Blood pressure 140/70 mm Hg | Very high shear rates (enough for platelet activation) occur in the middle of the leaflet but also in the critical pivot regions. |
| Li et al. 2011 [20] | SJM 27mm TV 27mm | Pulsatile mock circulatory loop system 70 BPM, cardiac output of 5 L/min Aorta: 120/80mmHg Left ventricle: 120/0mmHg Left atrium: 7/5mm Hg | No difference in opening time for both valve types. The TV closes solely on vortices in the aortic sinus, while the BV depends on reverse flow. The TV has less energy loss and regurgitation. Shear stresses were higher in TV, but did not exceed RBC damage threshold. Shear stress spike occurred at the instant of SJM leaflet impact. Closing velocity is a more important factor in evaluating valve efficiency and contribution to cavitation. Turbulent Viscous Shear Stress and Principal Reynold Shear Stress higher in TV. |
| Venneman et al. 2018 [26] | Biological valve: Edwards Intuity BV: Medtronic Advantage TV: Lapeyre-Triflo Furtiva | Compliant model of the aortic root in a physiological flow loop HR = 72 BPM, CO = 5 L/min, blood pressure 120/80 mmHg | Opening phase is similar for all 3 valve types. Biological and TV earlier and longer closing phase, starting at 270ms and completing at 330ms. BV starts closing at 330ms and completes at 360ms. Opening leaflet velocities highest in biological valves (2.03m/s), followed by TV (0.77m/s) and BV (0.66m/s). Maximum closing leaflet tip velocities are significantly higher in BV (0.83m/s) compared to biological (0.37m/s) and TV (0.39m/s). |
| Bruecker et al. 2020 [27] | SJM Regent TV: Triflo T2B | Pulse duplicator and a 25mm aorta with comparable geometry combined with an inlet swirl generator. | Helical flow originates from a swirl in the ventricular outflow tract. TV conserves 80% of swirling core flow and angular momentum compared to only 30% in the BV. Hydrodynamic pressure drops in BVs, converting the helical to axial flow in a ‘flow-straightener effect. |
Table 1: Overview of all in vitro testing. TV: Trileaflet mechanical Valve. BV: Bileaflet mechanical Valve. SJM: Saint Jude Medical valve. JCL: JCL Technic Limited, company name. BPM: Beats Per Minute
Computational models
Computational studies were summarized in table 2. The first numerical study was by Li et al. in 2012 [22]. The computational domain was a 25mm aorta, with a 36mm symmetrical aortic sinus. The opening and closing angles for the trileaflet valve were 45° and 90°, for the control SJM valve 25° and 85°. Only half of the computational domain was simulated for the SJM valve, and only one-third for the trileaflet valve to save cost and time. The faces in the connecting planes were set to symmetric conditions. This assumes synchronized leaflet motion, which is not the case in real flow. Calculations were made with the software Fluent 6.3. The gaps between the leaflet and valve housing could not be zero, so they were set to 0.25mm despite the real gap of 1.118µm. A heart rate of 70 bpm was simulated. Results showed that opening and closing for the SJM were 57 and 40ms, compared to 64 and 60 ms for the trileaflet valve. Due to lesser traveling angle, the trileaflet valve closed slower at an angular velocity of 18.4 rad/s compared to 146.3 rad/s for the SJM. Tip velocities of the SJM were higher at 1.46m/s compared to 0.24m/s for the trileaflet valve. These results were compared to experimental data [20, 28]. The numerical calculations were a slight overestimation of experimental data but were nonetheless very accurate. As the angle between the leaflets and the direction of axial flow is almost zero, reverse flow does not contribute to leaflet closure as is the case for the SJM valve. The trileaflet valve closes due to vortices in the aortic sinus, similar to a biological valve. The native aortic valve’s closure is mostly during forward flow, with a vortex in each sinus pushing the leaflet inward [29]. The slower and more physiological opening and closing of the trileaflet valve may reduce cavitation and RBC damage.
In 2013, a computational study designed a trileaflet model and compared it to a bileaflet model [30]. The trileaflet valve was designed as a hemisphere equally divided in three parts. The hinges were placed centrally in each leaflet, not at the commissures as more common trileaflet designs. The housing was 26mm in diameter with a 2mm thick ring. The leaflets had one 1mm uniform thickness. The bileaflet valve was also modeled, however with a clear distinction from conventional bileaflet valve in that there was virtually no central gap. Both leaflets seemed to have the same central hinge, which is not the case in currently available bileaflet valves. Using Solidworks Simulation, two stages of valve operation were analyzed: fully opened and fully closed. Hemodynamic parameters were set to 120/80mmHg blood pressure. Results showed that during valve opening, maximum stress was higher in the bileaflet valve at 14 MN/m² compared to only 7.83 NM/m² for the trileaflet valve. Overall, researchers conclude that stress on the leaflets and hinges was less in the trileaflet valve compared to the bileaflet valve. As this computational model assumes a trileaflet and bileaflet valve design that is not linked to real life models and this in a static manner, the results of this study should be used with caution.
Another computational study was published in 2015 by Kuan et al. [31]. Computational models of a 29 mm SJM and a 29mm trileaflet valve were used in a debranched aortic model for simplified geometry. Blood was modeled as an incompressible viscous flow over a prespecified domain. The SJM was fully open at 85°, while the trileaflet valve was fully open at 90°. Results were presented as velocity plots at three different positions at three different time points: mid-acceleration, peak systole and mid-deceleration.
Interestingly, the velocity plots of both valve types were completely different: the vortices created by the bileaflet valve were more towards the outer arch with regions of low velocity and recirculation at the inner wall. The vortices of the trileaflet valve were on the inner arch. A flat velocity profile is visible for the majority of the plane for the trileaflet valve, while only a small portion of the velocity profile is flat for the bileaflet valve. The flow through the bileaflet valve was not in a streamline pattern, hitting and reflecting the aortic wall, compared to streamlined central flow of the trileaflet valve[31].
With regards to shear stress, the trileaflet valve only showed 10% (0.27 kPa) of the shear stress seen in the bileaflet valve (0.255 kPa). The trileaflet valve had an evenly distributed wall shear stress on the peripheral gap between leaflets and valve holder and lower shear stress in the sinus region and downstream aortic wall. For both valves, high wall shear stress at the hinge regions confirms these are weak spots for thrombosis formation[31].
A computational study published in 2020 focuses on the influence of leaflet curvature and opening angle of trileaflet heart valves [32]. 8 different leaflet types were tested, with varying inner radius and opening angles. Computational models were made using the Fluent software program, while the 3D models of the valves were made using Solidworks software program. Researchers concluded that a flat leaflet with an opening angle of 85° had the lowest risk of blood clotting.
The limitations of this study are not to be ignored. Firstly, it is not found in any peer reviewed journal to our knowledge. Secondly, the grammar is poor and hard to comprehend. Thirdly, there is no numerical data given. Only low-quality figures can be found. Lastly, the valve is situated far below the aortic sinus, which is not representative for normal clinical practice. We only included this study because of its unique setup, truly analyzing the curvature of leaflets, but we do stress that any conclusions drawn from this should be used with caution.
The final computational study was by Pawlikowski et al. in 2022 [33]. The researchers used an existing geometrical model of the left ventricle and a fragment of the aorta. The valves were created using Solidworks 2019 CAD software. The dynamics of blood circulation were determined using ANSYS 2020 R2 software. The modeled valves had an internal diameter of 21mm for an outer diameter of 27mm and a profile height of 16.5mm. The aorta had an internal diameter of 27mm and a height of 40mm.
In the assumption of non-Newtonian fluid, the von Mises stress at the pivots, ring and center of the disc at any opening angle is higher for the bileaflet valve. There are, however, higher velocities in the trileaflet valve at both Newtonian and non-Newtonian fluid assumptions. The wall shear stress for the trileaflet valve is lower at 49.64 Pa compared to 151.5 for the bileaflet valve. The highest shear stress occurs at the hinges and places of leaflet attachment. One must note that the leaflet thickness in this model varies from 0.6 to 2.6mm, with the latter being a vast exaggeration. Unlike many others, this study is unique in that it does not assume blood as a Newtonian fluid. Blood flow is highly inhomogeneous, with a non-Newtonian model definitely worth including in the experiments [34].
Computational
| Reference | Valve used | Setup | Conclusion |
| Li et al. 2012 [22] | One-third of the computational domain of Triflo TV 27mm and half of the computational domain of a SJM 27mm. | FLUENT 6.3 software 25mm aorta, 36mm aortic sinus, 130mm calculating domain trileaflet valve opening angle 45°-90° SJM opening angle 25°-85° | Longer opening and closing times for TV. Leaflet closure in TV by vortices in the aortic sinus. Slower leaflet motion might reduce cavitation and RBC damage. |
| Kiang-la et al. 2013 [30] | 3 identical leaflets forming a hemispherical shape of 1mm thickness of titanium alloy simple hinge joint connecting the leaflet centrally to housing ring diameter of 26mm, housing ring thickness 2mm Computer designed BV: 2 flat leaflets with central hinge joint, 1mm thickness of titanium alloy housing ring of 2mm thickness | Solidworks software 2 stages of valve operation: fully opened and fully closed. Blood pressure of 120/80mmHg | Stress levels on BV double that of TV during opening. Stress levels on TV 2 double that of BV during closure. Maximum stress at hinges for both valve types. Only static analysis, not a dynamic model |
| Kuan et al. 2015 [31] | TV 29mm SJM 29mm | Dynamic Studio software Debranched aortic model | TV creates vortices at inner aortic curvature, BV at outer aortic curvature Flat velocity profile is seen for the TV aortic valve. Streamlined flow is only seen in the TV Shear stress of the TV is only 10% of the shear stress of a BV. |
| chatpon et al. 2020 [32] | 3 flat TVs with fully opening angles of 85°, 87° and 90° 3 curved TVs with inner radius of 8.672 at the opening angles of 85°, 87°, and 90° 1 curved TV with an inner radius of 9.328 at opening angle of 85° 1 curved TV with an inner radius of 8 at opening angle of 85° 27mm diameter for all models | Solidworks software ANSYS fluent software
| Maximum shear stress at peak systole. Of the flat TVs, 90° opening angle had highest shear stress. Of the curved valves, the 85° opening angle had the highest shear stress. For all valves, highest shear stresses were found in the commissures. |
| pawlikowski et al. 2022 [33] | Geometrical model of left ventricle and fragment of aorta available from GrabCAD platform. Internal diameter of ascending aorta of 27mm and length of 40mm. Computer model based on known TV and BV: internal diameter of 21mm, external diameter of 27mm, profile height of 16.5mm. New leaflet curvature in the TV model, additional discs cover to protect leaflets in BV model. | Solidworks 2019 CAD software 3 valve positions analyzed: 40°, 20° and 0° (fully opened). 2 blood models (Newtonian and non-Newtonian) over TV, only non-Newtonian over BV. | TV showed more physiological blood flow mainly by a central jet, less shear stress and thus risk of hemolysis. Lower stress extends valve durability and minimizes leaflet dislocation. TV ensures similar blood flow regardless of valve implantation angle. Reduced leaflet curvature increases flow area, having a positive effect on pressure gradient. BV causes different flow patterns under various implantation angles. |
Table 2: Overview of all computational testing. TV: Trileaflet mechanical Valve. BV: Bileaflet mechanical Valve. SJM: Saint Jude Medical valve.
In-vivo studies
In vivo studies are summarized in table 3. The first clinical study of a trileaflet mechanical valve was in 1994 by Lapeyre et al, who invented the valve [35]. The trileaflet valve, named ‘Lapeyre-Dassault’ prosthetic valve due to its development by the French aviation company, meant to duplicate the hemodynamic performance of the natural valve with the surplus of mechanical durability. Its main difference with conventional mechanical valves would be no anticoagulation needed. A total of 6 calves were implanted with the trileaflet valve in mitral position. The first 2 received no anticoagulation. The 3th and 5th received no anticoagulation after 30 days and 1 week, respectively. The 4th and 6th were terminated prematurely due to paravalvular leakage and a broken leg, impeding study completion.
The other 4 lived for 165, 158, 219 and 281 days. Hematology and transthoracic echocardiography values were all within range. Transvalvular gradients were acceptable (11.57+-1.26mmHg). At necropsy, all valves appeared normal. The hinges were free and the leaflets were mobile. The sewing cuffs were covered with dense fibrous and connective tissue, without compromising leaflet movement. No thromboemboli were found in distant organs. The positive results of this pilot study encouraged further development of the trileaflet valve.
A follow up in vivo study occurred 7 years later by Sato et al. in 2003 [36]. In this study, blood compatibility in the absence of any anticoagulation was the main focus. 8 calves had mitral valve replacement by a new design of the trileaflet valve (type IIA). The major differences were the windows in the downstream commissure region and curved leaflets instead of flat ones. Importantly, neither anticoagulant nor antiplatelet was used postoperatively. One calf died from valve thrombosis at day 25 attributed to prolonged post implant fibrillation. The other calf was killed at day 105 due to downer calf syndrome. 6 animals completed the designated 5 month follow up period. There were no signs of thromboembolism. All hematology, liver, renal function or serum electrolytes were absent major changes, except for a hematocrit level that were lower than preoperatively but consistent in the chronic phase (3-5 months). However, there were no concrete signs of hemolysis: serum hemoglobin and LDH levels remained constant and there was no hemosiderin deposition in the reticuloendothelial system.
With regards to blood compatibility, collagen- and ADP induced platelet aggregation were increased however not significantly[36]. Clot signature analyzer results showed no difference throughout the entire experiment. Platelet mediated hemostasis time showed no significant shortening. Collagen induced thrombus formation remained unchanged. Clotting time, as an indicator for the entire coagulation system, was not shortened. While their pannus overgrowth in 26%, there was only one leaflet in one animal that had movement restriction. Scanning electron microscopy revealed small (<15>
The results suggest that the trileaflet valve does not noticeably activate the platelet or coagulation system. However, calf platelets are less responsive to shear stress but equally responsive to collagen. This leads to longer PHT and shorter CT times in calves. The overall clotting ability of blood is more accentuated in calves compared to humans [37].
A year later in 2004, Gregoric et al. published a follow-up calf study of the trileaflet mechanical valve in mitral position compared to standard bileaflet valves [38]. 26 calves were divided into 2 groups. 17 calves in group 1 received either the T1 type trileaflet valve (n=12) or control bileaflet valve (n=5). 9 calves in group 2 received either T2 type trileaflet valve (n=7) or control bileaflet valve (n=2). The main difference between both types is that the T1 type has flat leaflets, compared to concave leaflets in the T2 type. Both groups received IV heparin for 10 days, however in group 1 PTT was monitored at 1.5-2.0 times baseline. In group 2, an equal amount of heparin was given, however there was no monitoring of PTT. Follow up in group 1 was 3 months and in group 2 5 months.
All but 2 calves survived the immediate postoperative period. 5 out of 12 experimental and 5 out of 5 control calves survived until study termination in group 1[38]. In group 2, 6 out of 7 experimental and 2 out of 2 control calves survived. Echocardiography at implantation showed normal valve function for both T1 and T2 types, with transvalvular pressure lower than control valves. Hemodynamics assessed by catheterization at explanation showed no difference between control and T1 valves. Regurgitation objectivated by Seller’s grade was less in group 2 compared to group 1 or control valves[38].
Laboratory values were all within normal limits and comparable to baseline in all groups except for platelet counts, which dropped in both groups. Necropsy revealed normal sewing ring and valve function in all calves that survived until study termination. In group 1, equal amounts of valvular thrombi were found in T1 and control valves, but renal infarcts were found more often in T1 calves. In group 2, valvular thrombosis and renal infarcts were found less often in T2 valves compared to control valves. In 2 T1 and 1 T2 calves, thrombus impaired leaflet motion was noted, but not in any control valves. 3 calves were kept alive after study termination to examine wear and tear on the valve, with the longest surviving for 502 days with a T1 valve[38].
In conclusion, it appeared that in this calf model, the valve functioned similarly to control valves. Left ventriculography showed excellent valve function regardless of valve type in all 3 contractility states. Both types were hematologically and biologically compatible. The slight decrease in platelets was to be expected due to the increased affinity of bovine platelets for foreign material. The curved leaflets in the T2 design as well as the addition to windows in the commissures appeared to have little beneficial effect. It is known that the calve as a species is less thrombogenic than humans, thus the results of this calf study may very well be translatable to humans[38].
In a follow-up study in the same year, Gregoric et al. assessed the trileaflet valve in aortic position compared to a standard bileaflet valve (SJM) in calves[39]. 6 trileaflet and 3 bileaflet valves were implanted. All valves survived the immediate postoperative period. However, after 2 months, two bileaflet calves showed signs of functional aortic stenosis at transthoracic echocardiography. This was attributed to rapid calf growth (16-18 kg/month), leading to their premature sacrifice. Total study duration in the trileaflet group was 159+-55 days and 102+-67 days in the bileaflet group. The mean and peak gradients in the trileaflet group were 35+-14 and 24+-9mHg, significantly lower than in the bileaflet group (100+-72 and 59+38mmHg). All explanted valves were free from any thrombi with normal leaflet motion. In 2 of the 3 bileaflet calves, pathological left ventricle hypertrophy and patchy areas of transmural infarction were visible. Only one trileaflet calf had mild ventricular hypertrophy combined with a subaortic muscular band, the other 5 had no remarkable histological changes. The researcher concluded that the trileaflet valve has superior hemodynamics, as the data obtained at explantation were under a high cardiac output (11+-3 L/min). As it is known, small aortic roots and hypertrophic heart are related to early mortality, so a valve that can minimize cardiac workload would be vital in the long-term survival of patients.
Another study of Gregoric et al. in 2004 compared the trileaflet valve to control bileaflet valves in 27 cases[40]. In a complex scheme, a prototype TV1 trileaflet valve was implanted in 4 calves in aortic position after successful results in the mitral position in 7 calves. However, all 4 calves died from thrombogenic complications after 18 +- 12 days. The valve was redesigned and the commissural windows, which had no apparent downsides in the mitral position, were left out. The redesigned valve, named TV2, was tested in mitral (n=4) and aortic (n=5) position. This redesign greatly reduced thrombus grade and significantly increased survival time in the aortic group. In the control bileaflet group, 2 out of the 3 calves in the aortic group were killed prematurely due to shortness of breath and pulmonary edema, suggesting heart failure due to an effective orifice area too small for the animal’s size. In mitral position, all 4 control animals survived until study completion.
The main conclusion in this study is that site specific testing is crucial. Computerized models do not cover all parameters of valve functioning. Where computerized models predicted reduced thrombogenicity in the TV1 valve, the opposite was true in aortic position. The aortic orifice area is smaller, meaning a much higher velocity over the valve. This creates severe turbulence and flow separation behind small openings such as the windows in the commissures of the TV1 type. Without this in situ testing of preclinical valves, poor designs may reach clinical trial stages. A robust, worst case scenario animal model that pushes the physiological extremes is important.
In 2006, the first sheep study of the trileaflet valve was done by Gallegos et al.[41]. 26 sheep received either mitral (n = 8) or aortic (n = 18) valve replacement. Survival cohorts were 150 or 365 days. In aortic position, transvalvular gradients at 150 and 365 days were 31.2 ± 23.2 and 27.5 ± 2.1, respectively. In both mitral and aortic position, regurgitation was only mild. Laboratory values were all within range. Haptoglobin was elevated at 150 days in mitral sheep, however absent at 365 days. In aortic position, haptoglobin levels were highest preoperatively.
There was pannus overgrowth in all mitral valves at 150 days, along with mild vegetations on the inflow side of the hinges and thrombotic deposits on the outflow side of the stent posts of the valve[41]. One Triflo in aortic position had a leaflet stuck in the open position due to deposits. 3 sheep with the valve in aortic position showed small renal infarcts. In 2 aortic and 2 mitral Triflo’s, thrombi were formed on the hinges. Overall, pathology findings between mitral and aortic position were very comparable. Without anticoagulation, the risk of thromboembolism with the trileaflet design implanted between 150 and 365 days ranged from 4 to 20%. The main reason for opting for sheep as a model was the limited somatic growth of these animals. This limited ‘patient-prosthesis mismatch’ justifies the sheep as a model[41].
The last in vivo preclinical study was published in 2023 by Langenaeken et al. [42]. 21 female sheep were implanted with a trileaflet 21mm prosthesis in either aortic (n = 8) or pulmonary position. 7 female sheep were implanted with a control bileaflet prosthesis (On-X) in either aortic (n = 1) or pulmonary (n = 6) position. Follow-up was 3 or 5 months in the aortic group and 10 or 20 weeks in the pulmonary group. Follow-up consisted of serial cardiac ultrasounds combined with blood samples and, which is unique in this study, acoustic measurements of valve noise.
All sheep survived their designated follow-up period. No signs of macro- or microscopic thromboembolic. Gradients were significantly better for the trileaflet valve in both aortic and pulmonary position. Interestingly, this is the first study that concluded that the trileaflet’s closing noise is significantly lower compared to the bileaflet control valve. Due to the slower and more physiological closing and Poly-Ether-Ether-Ketone (PEEK) leaflets, the ‘slam door’ effect responsible for the audible closing tick of bileaflet valves is avoided. This may be of clinical significance, as up 14.3% of mechanical valve patients suffer from valve sound-related complaints [43].
In vivo
| Reference | Lapeyre et al. (1994) [35] | Sato et al. (2003) [36] | Gregoric et al. (2004) [38] | Gregoric et al. (2004) [39] | Gregoric et al. (2004) [40] | Gallegos et al. (2006) [41] | Langenaeken et al. (2023) [42] |
| Animal | 6 calves | 8 calves | 26 calves | 9 calves | 27 calves | 26 sheep | 28 sheep |
| Valves used | TV | TV IIA | Group 1: TV T1 (12) SJM (5) Group 2: TV T2 (7) SJM (2) | TV (6) SJM (3) | TV 21mm: - TV1 (20) - TV2 (7) SJM (7)
| TV 29mm and 21 mm | TV 21mm On-X 21mm |
| Position | Mitral | Mitral | Mitral | Aorta | Aorta (12) - TV1 (4) - TV2 (5) SJM (3) Mitral (15) - TV1 (7) - TV2 (4) SJM (4) | Aorta (18) Mitral (8) | Aorta: - TV: 8 - On-X: 1 Pulmonary: - TV: 13 - On-X: 6 |
| Duration | 165, 158, 219 and 281 days. Two prematurely at day 37 and 39 due to valve-unrelated complications. | 5 months | Group 1: 3 months Group 2: 5 months | TV: 159 ± 55 days BV: 102 ± 67 days | Aortic TV1: 18 ± 12 days TV2: 159 ± 61 days SJM: 108 ± 62 days Mitral TV1: 215±112 days TV2: 140±62 days SJM: 159±89 days | 150 days: Aortic (11) Mitral (7) 365 days: Aortic (7) Mitral (2) | Aorta: 3 and 5 months. Pulmonary: 10 and 20 weeks. |
| Survivors | 4/6: 1 POD37 perivalvular leakage and untreatable gastrointestinal bloating 1 POD39 broken leg. | 6/8 1 valve thrombosis at day 25, probably due to postoperative ventricular fibrillation 1 downer calf syndrome at day 105 | Group 1 TV 7/12: - 1 perivalvular leak (POD 0) - 1 misplaced left arterial pressure line obstructing valve movement (POD 1) - 1 misplaced valve suture obstructing leaflet movement (POD 14) - 1 heart failure due to free floating free leaflet (POD 25) - 1 vegetative endocarditis (POD 56) Control: 5/5 Group 2: TV: 6/7 - 1 systemic infection (POD 104) control: 2/2 | TV 6/6 BV 1/3: 2 animals sacrificed prematurely at day 67 and 60 due to recipient-prosthesis size mismatch and functional aortic stenosis. | 2 aortic BV killed prematurely due to cardiac failure caused by too small effective orifice area. 3/4 TV1 aortic calves dies on POD 11, 13 and 15 due to valve thrombosis. All TV2 valves survived until study completion. All BVs survived until study completion. | All animals survived until study completion. | 28/28 |
| Anticoagulation | 1 & 2: warfarin at PT 1.5-2 for duration of study 3: warfarin until 30 days no anticoagulation past 30 days 4: no anticoagulation past 1 week. | None | Group 1: continuous heparin infusion (500-1000 mg/d) at PTT 1.5-2.0 x baseline for 10 days. Group 2: IV heparin 100mg/d 10 days | Low dose heparin 8-10U/kg/h first 7 days. | Continuous heparin infusion (50mg/d) for 7 days. | Heparin sulfate 2000U/d SC first 2 days. | Enoxaparin 40mg/d for 1 week. |
| Thromboemboli | None | 1 embolus in renal artery in the calf with valve thrombosis. No thromboembolic events in the remaining 7 cases. | Group 1: Valve thrombi: T1 4/7, BV: 3/5 Renal infarcts: T1 2/7, BV 1/5. Group 2: Valve thrombi: T2 4/7, BV 2/2 Renal infarcts: T2 2/7, BV 1/2. Thrombus-impaired leaflet motion: 2 T1 calves, 1 T2 calve, BV 0/7 | None | Aortic*: - TV1: 2.75 ± 1.00 - TV2: 0.50 ± 0.58 SJM: 0.67 ± 0.58 Mitral*: - TV1: 0.71 ± 0.76 - TV2: 0.33 ± 0.58 - SJM: 1.50 ± 0.58 3/4 TV1 aortic valves partial or full thrombosis. Grade 2 thrombus with mobile leaflets in 1 TV1 aortic calve. | 150 days: 96% free from renal thrombi. 365 days: 81% free from renal thrombi. Mitral: thrombotic deposits on stent posts on outflow side (n = 3). Aortic: 3 renal infarcts. | None |
| Necropsy | All valves free of pannus/thrombi. Normal function. Normal endothelialization. Normal peripheral organs. | 26% tissue ingrowth, no interference with leaflet mobility except in one case. | Normal function of all tri- and bileaflet valves with normal endothelialization of sewing ring in all calves that survived until planned study termination.
| Normal leaflet motion and free of thrombi. Significant left ventricular concentric hypertrophy and patchy areas of transmural infarction. | TV1 valves severely thrombosed in aortic position, control BVs minimally thrombosed. TV2 valves thrombus free in both mitral and aortic position. | 150 day: mitral: 2-16% pannus overgrowth. Endocardial scars due to turbulent flow (n = 4). 1 fixed aortic leaflet. All valves are structurally intact. | All valves free of pannus/thrombi. No signs of thrombo-embolic disease. |
| Hematology | Normal hemolysis. Stable RBC and platelet count. | Hematocrit significantly lower than preoperatively at day 7 and day 14. No difference at 3 and 5 months. | Normal hematology. Platelet count is reduced in both groups. | Normal. | No values reported. | Normal. | Normal. |
| Hemodynamics | TV: 11.57 + 1.26mmHg | No measurements made. | Peak gradient explantation: T1: 9 ± 2 mmHg T2: 10 ± 6 mmHg Control: 10 ± 3 mmHg | TV: - PG: 35 ± 14 mmHg - MG: 24 ± 9 mmHg BV: PG: 100 ± 72 mmHg MG: 59 ± 38 mmHg | No measurements made. | Aortic TV gradient 150 days: 31.2 ± 23.2mmHg 365 days: 27.5 ± 2.1mmHg Mitral position hemodynamics not available.
| Aortic (mmHg): - MG: TV 5.1 (4.2–7.7) vs. On-X 10.7 (8.7–12.9) - PG: TV 8.7 (7.5–12.5) vs. On-X 16.5 (14.2–19.6) Pulmonary (mmHg): - MG: TV 4,30 (3.70–5.73) vs. On-X 6.80 (4.63–7.96) - PG: TV 8.05 (6.75–10.23) vs. On-X 13.15 (9.20–14.76) |
| Conclusion | TV design offers potential to compete with bioprosthetic valves. | No platelet or anticoagulation activation in a calf mitral TV model. Only one (renal) embolus observed. | TV may be equivalent to BV control valve. | Favorable trend towards TV hemodynamics. | Strict in situ orthotopic valve testing mimics clinical setting. Calf is a superior model allowing exaggerated physiological conditions. | TV performs to safety levels comparable with standard BV SJM valves. | Gradients and valve noise are significantly better in TV. Safe long-term function. No thromboembolic events. |
Table 3: Summary of all in vivo preclinical testing. SJM: Saint Jude Medical valve. TV: trileaflet valve. BV: Bileaflet valve. TV1: prototype trileaflet valve. TV2: redesigned trileaflet valve. PG: Peak Gradient. MG: Mean Gradient. * The explanted valves were graded according to a semiquantitative scale ranging from 0– 4: grade 0, no visible thrombi; grade 1, thrombi less than 5 mm; grade 2, thrombi greater than 5 mm and/or one leaflet obstructed; grade 3, thrombi greater than 5 mm and/or 2 leaflets obstructed; and grade 4, thrombi greater than 5 mm and/or 3 leaflets obstructed.
Sievers Trileaflet Valve
H. H. Sievers et al. first published the computational study with regards to a new trileaflet valve design in 2018 (see table 4) [44]. As H.H. Sievers is the patent holder, we will refer to it as the Sievers Valve (SV). The striking difference is the positioning of the hinges centrally in the flow, rather than in the commissures like conventional bileaflet and trileaflet designs. The main goal would be freedom of anticoagulation, both by material improvements as well as fluid dynamical optimizations. Rather than utilizing vortex generation in the aortic sinus, the design was such that these turbulent areas were avoided as much as possible.
In this study, a 3D model was created using the NX software (Siemens Industry Software GmbH, Köln, Germany). A mildly dilated porcine heart was used as the basis for the 3D model, mimicking the often-dilated left ventricle in severe valve disease. Conical left ventricle outflow tract simulations, from 22 to 48mm were used, as the left ventricle is conical in shape instead of a tube-like structure as in most other simulations.
With regards to velocity profiles, the highest velocities were in the center of the valve orifice and the edge area behind the leaflets[44]. Pressure decreased for increasing inflow diameters at the center of the valve, but increased in the periphery. At the leading edge of the leaflet, a spot of high pressure was observed. With regard to flow characteristics, the more conical configuration resulted in a reduced peak flow velocity, with the velocity profile becoming more homogeneous in the valve orifice. A more homogeneous velocity distribution could indicate lower shear stress and thus positive effect on thrombus generation. The main limitation of this study is that simulations were only done in steady state[44].
In 2018, Sievers et al. published the first in vitro results of his valve [45]. This design was tested in a pulse duplicator and compared to SJM Regent (Abbott, Chicago, IL, USA) and On-X heart valve (CryoLife Inc., Kennesaw, GA, USA) [46]. Valves were tested in three flow values: 9 – 11 L/min, 11 – 13 L/min and >13 L/min. The SV showed lower gradients and larger EOA in all flow rates compared to the On-X control valve. Compared to the SJM, only in the high flow group the SV was better at a statistically significant level. Closing times of the On-X, SV and SJM were 38.8 ± 16.8ms, 43.5 ± 16.4ms and 63.0 ± 49.8ms, respectively. The closing velocities for the On-X, SV and SJM were 84.7 ± 40.4 cm/s, 78.4 ± 32.2 cm/s and 75.2 ± 23.6 cm/s, respectively. The least leakage volume was measured for the SJM valve, followed by the trileaflet and the On-X valve.
Interestingly, the valve was also tested for possible clot formation using an in vitro setup [47]. As expected, deposits formed at the hinges of the bileaflet valves on the downstream side and between the leaflet edge and orifice ring. In the SV, the deposits only formed in the vicinity of the hinges. These deposits did not lead to valve dysfunction.
After these promising results, the group of Sievers continued with in vitro 4D flow MRI testing of this valve in 2019[48]. Two BV (Perimount MagnaEase [Carpentier‐Edwards], Trifecta [Abbott]) and two MV (On‐X [CryoLife], SV) were placed in a silicone aortic phantom with preserved distensibility and normal diameters. Biological valves were placed supra-annular, mechanical valves were placed annularly. A blood mimicking fluid was pumped through the model with a typical triphasic aortic flow profile at 58 bpm using a MRI-compatible pump[48].
In biological valves, the ejection jet at the sinotubular junction only filled 27% of the vessel area compared to 53% for the mechanical valves. Peak velocities were higher in the biological valves. Sinus vortices were more pronounced in mechanical valves while absent in the biological valves. The biological valves did however form secondary flow patterns with large vortices and helices in the ascending aorta. Flow in the ascending aorta after passing the mechanical valves was more physiological, which is in line with other studies confirming abnormal flow patterns after biological valve implantation[49]. The reason may be the supra-annular position of the biological valves, with an ejection jet disintegrating downstream in the ascending aorta compared to at the sinotubular junction as in the mechanical valves.
The results of the first in-vivo testing of the SV were published in 2021 [50]. Four female sheep were implanted with the SV. No postoperative anticoagulation was given. Survival was set at 90 days for 2 sheep, while the other 2 sheep had a follow-up of 1 year. All animals survived their designated follow-up period. These are promising. It is unique not only as a trileaflet mechanical valve, but the first and only valve to place the hinges and pivot points centrally. Future studies will undoubtedly reveal the valve’s true potential.
Sievers Trileaflet Valve
H. H. Sievers et al. first published the computational study with regards to a new trileaflet valve design in 2018 (see table 4) [44]. As H.H. Sievers is the patent holder, we will refer to it as the Sievers Valve (SV). The striking difference is the positioning of the hinges centrally in the flow, rather than in the commissures like conventional bileaflet and trileaflet designs. The main goal would be freedom of anticoagulation, both by material improvements as well as fluid dynamical optimizations. Rather than utilizing vortex generation in the aortic sinus, the design was such that these turbulent areas were avoided as much as possible.
In this study, a 3D model was created using the NX software (Siemens Industry Software GmbH, Köln, Germany). A mildly dilated porcine heart was used as the basis for the 3D model, mimicking the often-dilated left ventricle in severe valve disease. Conical left ventricle outflow tract simulations, from 22 to 48mm were used, as the left ventricle is conical in shape instead of a tube-like structure as in most other simulations.
With regards to velocity profiles, the highest velocities were in the center of the valve orifice and the edge area behind the leaflets[44]. Pressure decreased for increasing inflow diameters at the center of the valve, but increased in the periphery. At the leading edge of the leaflet, a spot of high pressure was observed. With regard to flow characteristics, the more conical configuration resulted in a reduced peak flow velocity, with the velocity profile becoming more homogeneous in the valve orifice. A more homogeneous velocity distribution could indicate lower shear stress and thus positive effect on thrombus generation. The main limitation of this study is that simulations were only done in steady state[44].
In 2018, Sievers et al. published the first in vitro results of his valve [45]. This design was tested in a pulse duplicator and compared to SJM Regent (Abbott, Chicago, IL, USA) and On-X heart valve (CryoLife Inc., Kennesaw, GA, USA) [46]. Valves were tested in three flow values: 9 – 11 L/min, 11 – 13 L/min and >13 L/min. The SV showed lower gradients and larger EOA in all flow rates compared to the On-X control valve. Compared to the SJM, only in the high flow group the SV was better at a statistically significant level. Closing times of the On-X, SV and SJM were 38.8 ± 16.8ms, 43.5 ± 16.4ms and 63.0 ± 49.8ms, respectively. The closing velocities for the On-X, SV and SJM were 84.7 ± 40.4 cm/s, 78.4 ± 32.2 cm/s and 75.2 ± 23.6 cm/s, respectively. The least leakage volume was measured for the SJM valve, followed by the trileaflet and the On-X valve.
Interestingly, the valve was also tested for possible clot formation using an in vitro setup [47]. As expected, deposits formed at the hinges of the bileaflet valves on the downstream side and between the leaflet edge and orifice ring. In the SV, the deposits only formed in the vicinity of the hinges. These deposits did not lead to valve dysfunction.
After these promising results, the group of Sievers continued with in vitro 4D flow MRI testing of this valve in 2019[48]. Two BV (Perimount MagnaEase [Carpentier‐Edwards], Trifecta [Abbott]) and two MV (On‐X [CryoLife], SV) were placed in a silicone aortic phantom with preserved distensibility and normal diameters. Biological valves were placed supra-annular, mechanical valves were placed annularly. A blood mimicking fluid was pumped through the model with a typical triphasic aortic flow profile at 58 bpm using a MRI-compatible pump[48].
In biological valves, the ejection jet at the sinotubular junction only filled 27% of the vessel area compared to 53% for the mechanical valves. Peak velocities were higher in the biological valves. Sinus vortices were more pronounced in mechanical valves while absent in the biological valves. The biological valves did however form secondary flow patterns with large vortices and helices in the ascending aorta. Flow in the ascending aorta after passing the mechanical valves was more physiological, which is in line with other studies confirming abnormal flow patterns after biological valve implantation[49]. The reason may be the supra-annular position of the biological valves, with an ejection jet disintegrating downstream in the ascending aorta compared to at the sinotubular junction as in the mechanical valves.
The results of the first in-vivo testing of the SV were published in 2021 [50]. Four female sheep were implanted with the SV. No postoperative anticoagulation was given. Survival was set at 90 days for 2 sheep, while the other 2 sheep had a follow-up of 1 year. All animals survived their designated follow-up period. These are promising. It is unique not only as a trileaflet mechanical valve, but the first and only valve to place the hinges and pivot points centrally. Future studies will undoubtedly reveal the valve’s true potential.
Sievers valve
| REFERENCE | background | methods | results | Conclusion |
| 2018: A novel Trileaflet mechanical heart valve first in vitro results [45] | Hinges of novel SV were placed centrally to avoid adverse flow areas near the housing of the valve. Hemodynamic and deposition behavior, in vitro testing and comparison to 2 conventional BV mechanical valves (SJM, On-X). | Valves were mounted in a pulse duplicator with systemic pressure of 125/80 mmHg, frequency of 64 BPM, flow rates differing from 9,5 l/min to 15,8 l/min.
High-speed camera was used to record leaflet motion. Flow values were assigned to 3 groups: group 1 (range 9l/min to <11l>13l/min).
Simulation of clot formation was performed using an in vitro test setup described by Scharfschwerdt et al. using fresh unpasteurized milk [60]. | SV showed the largest EOAs and the lowest gradients: - On-X vs SV: significant difference in pressure gradient and EOA in all groups - SJM vs SV: only significant pressure difference in group 3, significant difference in EOAs in group 1 and 2 Leaflets of the On-X valve started to close first, followed by the SV and the SJM.
Closing velocity highest in the On-X valve, followed by SV and SJM.
The least leakage volume was measured for the SJM valve, followed by the SV and the On-X valve.
SV had some deposits around the hinges as well as below the struts. SJM and On-X valves had comparable results. | The results of the first in vitro test results of hemodynamics and clot formation of the novel SV were promising, with a large EOA and minor deposits.
Further optimization of the design, as well as in vivo experiments will provide further knowledge about the real performance of the novel design |
2018: The influence of different inflow configurations on computational fluid dynamics in a novel three-leaflet mechanical heart valve prosthesis [44]
| Aim of this study: investigate a model for different anatomical inflow characteristics within a novel 3-leaflet mechanical heart valve with CFD.
| A 3D computer-aided model of the novel valve was created + used for CFD simulations.
Blood was modelled to be a Newtonian, incompressible and steady fluid.
Flow rates at inflow: 18l/min and 3l/min, representing the maximum systolic and near end-systole flow. Aortic pressure was 125 mmHg, with regard to the peak pressure in mid-systole.
Different inflow configurations were used, in order to investigate the impact of the varying LVOT anatomy. Outflow geometry was identical in all simulations.
The ascending aorta had a length of about 100mm, so fully steady state flow could be attained
The simulations were analyzed in 3 planes: through the center of the valve, at the leading and at the trailing edge of the leaflet
| The area with the highest velocities was in the center of the valve orifice and in the edge area behind the leaflets. With increasing diameter, the velocity profile became more homogenous.
Velocity at the leading edge of the leaflet decreased with increasing inflow diameter of the valve for 18 l/min
Velocity at the trailing edge of the leaflet increased with an increasing inflow diameter. This increase in velocity forces the leaflet open and might compensate for the increased forces at the leading edge.
Conical configuration resulted in a reduced peak flow velocity. Moreover, the velocity profile became more homogenous in the valve orifice. This could indicate lower shear stress, which may have a positive effect on thrombus generation
Total pressure increased at the valve entrance for increased inflow diameters, while pressure at the center of the valve orifice decreased. | Differences for the novel 3-leaflet mechanical heart valve were observed in cylindrical and conical configurations in CFD models, using different anatomical inflow geometries
Increasing inflow diameters led to decreased flow velocity and maximal pressure. This may have positive effect on shear stress and the thrombogenicity of the prosthesis |
| 2019: In vitro 4D Flow MRI evaluation of aortic valve replacements reveals disturbed flow distal to biological but not to mechanical valves [48] | Aortic valve replacement has an impact on hemodynamics, but only sparse data from 4D Flow MRI studies are available. The contribution of the replacement valve itself remains hardly known.
This study proposes in vitro 4D Flow MRI to test various aortic valve prostheses under standardized conditions. | The valves were placed in a silicone aortic model with a typical triphasic aortic flow profile. BVs (Perimount Magna Ease and Trifecta) were placed supra-annular, mechanical valves (On-X, SV) annular.
Hemodynamic parameters, such as stroke volume, velocity, flow and regurgitant volume were evaluated in the inflow tube, ascending aorta at the level of the pulmonary trunk, sinotubular junction and aortic bulb. | Mechanical valves showed a broader ejection jet, consisting of 3 or 4 peaks.
Peak velocities were higher in biological valves.
Net flow in ascending aorta at peak systole was comparable between both valve types.
All valves showed regurgitation.
Sinus vortices were more pronounced in mechanical valves. BVs showed no systolic vortices. | The results of this study showed near-physiological hemodynamics distal to mechanical valves.
Supra-annular position for the BVs decreased systolic sinus flow, because the valve leaflets reach further into the aortic bulb.
Narrower and faster ejection jet in BVs, which disintegrates further downstream in the ascending aorta, leading to vortex formation in the ascending aorta, reduced sinus flow and no sinus vortices. |
| 2021: Aortic valve replacement in sheep with a novel trileaflet mechanical heart valve prosthesis without anticoagulation [50] | First in vivo study in an aortic model in 4 sheep. Follow-up periods of 90 days and 1 year. | 4 animals (78 – 79,5 kg). Aortic position and valve size of 21 mm. | No clinical complications. Biochemistry, hematology and hemolysis were normal.
MG was low (< 6>
Aortic and mitral regurgitation were trivial.
Pannus integration was normal. No valve thrombosis. No tissue ingrowth, fibrin deposits or endothelial cells on the inflow or outflow surfaces of the leaflets at 90 days or 1 year. | Low transvalvular gradient, no regurgitation, a low rate of thrombotic events and no hemolysis were observed in the first in vivo study.
Pressure gradients were comparable to currently used commercial valves which is favorable, particularly because the valve size was only 21 mm and the weight of the animals increased. |
Table 4: Overview of preclinical testing of Sievers' valve. TV: Trileaflet mechanical valve. BV: Bileaflet mechanical Valve. SV: Sievers Valve. SJM: Saint Jude Medical valve. MG: Mean Gradient. PG: Peak Gradient. CFD: Computational Fluid Dynamics. MRI: Magnetic Resonance Imaging.
This paper reviews almost 30 years of preclinical trileaflet mechanical valve testing. The in vitro, computational and in vivo testing runs parallel. There is, to the extent of our knowledge, no standardized testing model for the computational, in vitro or in vivo testing of (mechanical) heart valves. This leads to a wide variety of testing set ups as well as different parameters analyzed.
The computational testing is the most diverse. Different setups and different software programs are used. In some cases, there is a clear computational render of existing trileaflet valves compared to existing bileaflet models [22]. This provides data that can be used in designing the valve for optimal in vitro and in vivo testing. However, some papers artificially render their idea of a trileaflet and bileaflet mechanical valve, which has limited value aside from a purely scientific research effort [30]. For the completeness of this review these papers were included, but should be interpreted with scrutiny. The main takeaways from the computational modeling are a longer opening and closing time, a more physiological flow, lesser shear stress and less disturbed flow patterns. As stated before, the results of computational models only approach reality, but are in no way a replacement of formal in vitro and in vivo testing.
One could imagine that the logical order of testing is first computational, followed by in vitro to end with in vivo. However, the first in vivo paper we found is from 1994, the first in vitro is from 1996 and the first computational paper we found is from 2012. This is probably due to the fact that a lot of computational and in vitro testing must have been done to design the valve, but did not make it to a formal publication. Publication criteria combined with publication fees have increased substantially in recent years, discouraging researchers to publish less important or less interesting work[51–53]. It is commonly known that papers of preclinical (valve) testing are sometimes published years after the actual testing was done.
The in vitro testing is done mostly using pulse duplicators and some form of blood analogue in an aortic root and arch substitute. Testing conditions mimic human parameters with regards to blood pressure and heart rate. The in vitro testing confirmed the slower opening and closing of the trileaflet valve. Actual measurements with regards to shear stress can be made, and these surpass the lysis limit of red blood cells. However, platelet activation occurs at as low as 10Pa shear stress, which virtually all mechanical valves generate[54]. The main downside of in vitro testing is the use of blood analogues, absent of clotting ability. This impedes making statements about thrombogenicity.
In vivo testing is marked by mostly calf testing. The main upside is the possibility of testing the valve in ‘extreme’ conditions, as the calf generates suprahuman cardiac outputs. The main downside of this model is the growth of the calf leading to handling difficulties and ‘patient’-prosthesis mismatch. This did not stop researchers however from achieving follow-up of up to 7 months [38]. The longest study was by Gallegos et al. in 2006, with 9 sheep reaching up to one year of follow-up[41].
Reassuring from all animal trials is that hematology values were almost always normal. The valve had minimal to no impact on circulating blood elements. Lysis was virtually absent, testimony of near physiological flow over the valve. With regards to hemodynamics, the valve performed equally if not better in all animal trials. In most cases, no valve thrombosis was noted even in the absence of anticoagulation. This could confirm the in vitro and computational findings of lower shear stress. It is however known that the sheep and calf are less thrombogenic than their human counterparts [55]. Statements about thrombogenicity solely based on sheep testing are precarious, since sheep platelets do not spread nor attach to nearly to the same extent as human platelets [56]. This was made painfully clear after the failed clinical launch of a mechanical valve, despite flawlessly passing preclinical sheep testing [57].
Overall, one can state that the preclinical testing of a trileaflet mechanical valve prosthesis has been completed. It is known that the final preclinical step is a successful long-term sheep study, which has been performed by 2 independent research groups (University of Minnesota and University of Leuven) [42, 58]. First-in-men (FIM) trials are expected to start in the beginning of 2024 [59]. The expected hypothetical clinical benefits are avoidance of long-term anticoagulation and related bleeding problems, elimination of iterative valve replacements due to the durability of the trileaflet valve and better quality of life due to avoidance of long-term anticoagulation [58]. Especially in developing countries with challenges of INR monitoring, a durable anticoagulant-free valve would be very welcome. For safety reasons, the clinical trial will incorporate standard mechanical valve anticoagulation with KVAs, at least for the first months. Switching to either low-INR regimes, alternative anticoagulation or antiplatelets has to be decided on.
A remarkable development in the concept of a trileaflet mechanical valve is the Siever’s valve[45]. The valve is truly unique by placing the hinges in the center of the systolic flow. It is known that the hinges of a mechanical valve represent an area of low flow, where thrombogenic material is drawn to in the closing phase. Mechanical valve thrombosis virtually always starts at these hinges, with a clot growing outwards from there on. With the hinges centrally, this may not be the case. The sheep testing was successful; however, this does not guarantee success in human trials as stated before. One could argue that the pig as a model is better for mechanical valve testing, as these tend to be more closely related to the human coagulation system [60–62].
A trileaflet mechanical valve behaves differently compared to bileaflet mechanical valves in a number of ways. The trileaflet valve’s hemodynamic, hematological and in-vivo test results are equal or better compared to current bileaflet valves. Computational modeling and in vitro testing give valuable insight in valve behavior, but animal testing followed by FIM trials remains paramount.
Conceptualization, T.L. and B.M.; methodology, T.L.; writing—original draft preparation, T.L.; writing—review and editing, B.M. and F.R. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
No new data was generated.
The authors declare no conflict of interest.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
