AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Abdul M Gbaj, Professor of Genetics and Biochemistry, Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya.
Citation: Nisreen H Meiqal, Sofian S. Mohamed, Shahrazad Ali Eteer, Jamal Ali M Elbakay, Inass A Sadawe, et al, (2024), Symmetrical 1,2-Phenylenediamine Schiff’s Base Derivatives as New Potential Dna Intercalators, J, Surgical Case Reports and Images 7(7); DOI: 10.31579/2690-1897/208
Copyright: © 2024, Abdul M Gbaj. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 02 August 2024 | Accepted: 13 August 2024 | Published: 20 August 2024
Keywords: schiff’s base; dna intercalator; anticancer; ortho-phenylenediamine; molecular docking
Fifteen symmetrical 1,2-phenylenediamine Schiff’s base derivatives were designed as DNA intercalators. Adsorption, distribution, metabolism, elimination, and toxicity (ADMET) properties and drug-likeliness of the designed compounds were predicted by Swiss-ADME software, and the molecular docking study was performed in the PyRx tool. Four Compounds NHM01, NHM04, NHM06 and NMH11 were synthesized and the structure of each compound was analyzed using Fourier transform infrared spectroscopy (FTIR), 1H NMRand13C NMR. Moreover, the four compounds were in vitro biologically screened for their interactions with the genomic DNA. The binding properties of these compounds to genomic DNA (G-DNA) were investigated by UV-visible absorption and fluorescence spectroscopy. The molecular docking studies of compounds revealed that all the compounds are bioactive, however, compound NHM01 shows significant DNA binding affinity over standard drugs. The biological results indicate that the four synthesized compounds can interact with G-DNA by intercalation binding. Compound NHM01 showed the highest key selection vector (KSV) value, followed by NHM04, which suggests that compound NHM01 binds most tightly to G-DNA. Our results demonstrate that NHM01 and NHM04may serve as novel lead compounds for the discovery of more 1,2-phenylenediamine Schiff’s base derivatives with improved anticancer potency and selectivity.
Deoxyribonucleic acid (DNA) is the storage unit of genetic information and considered as major biological target for numerous anticancer agents. The anticancer agents that target DNA generally interact with DNA through reversible binding by three main modes: static electronic interactions, groove binding, or intercalation. The static electronic interactions (external surface binding) could be related to the cationic molecules that bind non-specifically with the anionic sugar phosphate backbone of DNA through outer edge stacking (Serec et al., 2016). The second mode of interaction is groove binding which comprises the insertion of the molecule into the base edges of the minor or major groove (usually G•C sites in the major groove, A•T sites in the minor groove).The major groove has multiple sites of interaction, its dimensions are 8.5Å in depth and 11.6Å in width, where, bulky molecules can easily access the major groove. Conversely, the minor groove, which has a depth of 8.2 Å, is narrower and has fewer binding sites, however, binding to the minor groove is the most common. The third mode of interaction is intercalation which is closely related to most anticancer drugs which are currently in clinical use. In essence, small molecules can interact with DNA involving a single or mixed binding mode(Bhaduri et al., 2018). In this paper, our focus will be on DNA intercalation. DNA intercalators area class of compounds that can reversibly bind to DNA through insertion between adjacent base pairs perpendicularly to the axis of the helix. These interactions canlead to conformational changes involving an increase in the vertical separation between base pairs, unwinding and lengthening of the DNA helix. It may also interfere with the detection and function of associated proteins or enzymes leading to the failure of DNA repair systems, transcription processes or replication of the DNA (Biebricher et al., 2015). Numerous clinical intercalating agents are available as anticanceragents, for example daunomycin, doxorubicin, mitoxantrone or dactinomycin. Ethidium bromide is a phenanthridine monomer dye frequently used as a fluorescent marker (nucleic acid stain) in molecular biology for many procedures such as visualization of nucleic acid. Ethidium bromide intercalates between adjacent base pairs in the double-stranded DNA molecule and leads to deformation of the DNA. The deformation of DNA will possibly affect DNA biological processes, such as DNA transcription or replication (Binaschi et al., 2001). DNA intercalators have three main structural features: i) Chromophores, which are planar polyaromatic rings that bind to DNA, ii) cationic moieties, such as protonated amino groups which may interact with the phosphate backbone and iii) side chains that can interact with DNA minor grooves. In general, DNA intercalators are classified into two major classes: monofunctional and bifunctional (El-Adl et al., 2020).Monofunctional intercalators also referred to as simple intercalators. They consist of one intercalating unit, often carrying a positive charge on their ring system (Wadler et al., 1994).Secondly, bifunctional intercalators, also called bis-intercalators orthreading intercalation, have two typically cationic intercalating units separated by flexible linker groups being long enough to permit double intercalation. Since bis-intercalators have two different intercalating moieties, they can cause stronger binding with DNA than simple mono-intercalators (Wadler et al., 1994). Schiff bases are ranked among the most privileged synthetic organic intermediates extensively employed in industrial, pharmaceutical, and medicalconcerns. These compounds are also known as imines or azomethines with a general formula R-CH=N-R’, where R and R’ are an alkyl or aryl group(Peng et al., 2014). Schiff bases have been reported to exhibit a wide spectrum of biological properties and can also interactwith other ligands like ortho-phenylenediamines which leads to diverse and multipurpose applications(Koll, 2003).Bis-Schiff bases are usually synthesized from the condensation reaction of diamines with two equivalents of aldehyde or ketone in 1:2 molar ratio. The bases can be either symmetric or asymmetric structures, depending on the reacting aldehydes or ketones. (Vernekar & Sawant, 2023). Literature review revealed a great interest on ortho-phenylenediamines Schiff complexes and their applications. In contrast, less attention has been focused on the uncoordinated Schiff bases of ortho-phenylenediamines. In this regard, the search for other uncoordinated anticancer agents with improved safety profiles has become an attractive area of study for cancer management(Lucaciu et al., 2022). The main aimof this work is to design and synthesize some symmetrical Schiff’s base derivatives of o-phenylenediamine and study their DNA intercalating ability using an ethidium bromide competition fluorescent assay and UV-visible spectroscopic method. In addition, there is increasing interest to explore how different structural features of 1, 2-Phenylenediamine Schiff’s base derivatives can affect the DNA binding capability using molecular modeling tools, which may serve as a basis for understanding the molecular mechanism of action of the 1, 2-Phenylenediamine Schiff’s base derivatives and can help to design new chemotherapeutic molecules.
2.1Molecular docking
Molecular docking is an in silico method for recognizing the correct binding pose of a DNA-ligand complex and evaluating its strength using various scoring functions for selecting the best pose generated by each molecule to a rank-order. The molecular docking studies of all compounds with DNA were performed using PyRx software to generate grid, calculate dock scores and evaluate conformers. The steps of molecular docking include DNA structure optimization, in silico preparation for standard and designed compounds, and process of docking calculations (Adelusi et al., 2022).
2.1.1 Opimization of DNA molecule
The crystal structures of B-DNA fragments (PDB ID: 1BNA,1D60, 1ZEW, 2DES and 1DCO) were downloaded from the protein data bank(http://www.rcsb.org/pdb), and analyzed for its active site by Biovia Discovery Studio Visualizer (http://accelrys.com). Auto Dock 4.2 software was used to correct the imported DNA coordinates (The corrections involved deleting unwanted water molecules, adding hydrogen atoms (polar only) followed by adding kolloman charge, and saved as pdpqt format used as input in PyRx software.
2.1.2 Designing and in silico preparation of NHM compounds
The starting geometry of all investigated compounds was constructed using Chem3D Ultra (version 12.0, Cambridge soft Com., USA). The optimized geometry of tested compounds with the lowest energy was used in further processes of molecular docking. Discovery studio visualizer was used to all tested compounds in which the compounds were converted to PDB files that will be used as input in PyRx software.
2.1.3 Docking process and analysis
B-DNA crystal structures (PDB ID: 1BNA, 1D60, 1ZEW, 2DES and 1DCO) were loaded in PyRx virtual screening tools as pdbqt format. Standard drug and designed compounds pdb files were loaded and automatically converted to pdbqt format. Docking was performed using Autodock Vina incorporated in Pyrx software. The centers of the grid box were assigned automatically and the dimensions of the box were set to 25 × 25 × 25 Å. After docking, the complexes with the lowest binding energy (kcal/mol) were selected and their interaction modes were visualized by Discovery Studio.
2.2 Prediction of ADMET properties and drug-likeliness
The in silico procedure has become a very important tool in drug identification and screening. Ligand absorption, distribution, metabolism and excretion (ADME) pharmacokinetic properties of the designed Schiff’s base derivatives of o-phenylenediamine, are evaluated to determine their activity within the human body and prediction of these properties in advance will save the cost of drug discovery substantially. To verify drug-likeliness, canonical SMILES format of the designed NHM compounds were used in swissADME (http://www.swissadme.ch/index.php). The main bioavailability parameters were molecular weight (g/mol), lipophilicity (XlogP3), solubility (log S), polarity (Topological Polar Surface Area - TPSA in Å2), saturation (fraction of carbon atoms in Sp3 hybridization – Csp3) and flexibility (No of rotatable bonds). Consequently, absorption parameters such as Human Intestinal Absorption, Blood Brain Barrier, P-glycoprotein interaction (substrate or inhibitor) and metabolism (inhibitor or substrate) of the bio-active molecules with different cytochrome P450 enzymes were also estimated using admetSAR, which is based on QSAR data for prediction of absorption, distribution, metabolism, excretion and toxicity (ADMET). Drug-like molecules considering Lipinski rules and good ADMET properties were chosen as ligands in consequent molecular docking procedure.
2.2.1 SwissADME
Swiss ADME is a free web tool developed by the Swiss Institute of Bioinformatics (SIB), it was employed to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules and freely available at www.swissadme.ch. It calculates six physicochemical properties: lipophilicity, size, polarity, solubility, flexibility, and saturation. In addition, it promotes the assessment of ADME parameters (absorption, distribution, metabolism and excretion) of drug candidates and molecules and provides information to determine Lipinski’s rule of five for drug likeness of oral bioavailability (Daina et al., 2017). The drug-likeness assessment is based on the following factors: molar mass smaller amount than 500 g mol-1, l Log P is a smaller amount than five, number of acceptors of hydrogen bonds is a smaller amount than 10; accounted for in the molecule function of N or O atoms, and number of donors of hydrogen bonds is a smaller amount than 5, accounted for in the molecule function of NH or OH groups. Hence, the molecules outside this range will be unlikely to become orally bioavailable as a drug (Lipinski et al., 2001).
2.3 Chemistry
2.3.1 General procedure for Synthesis of Schiff base (NHM Compounds):
Schiff’s bases were prepared by reacting of one mole of phenylenediamine and two moles of substituted aromatic aldehydes or ketones in 100 ml ethanol. The solution was refluxed for 7hrs under constant stirring. The condensation reaction was carried out by using glacial acetic acid as a catalyst and monitored by thin layer chromatography (TLC). The mixture was left for cooling and thesolid product (crude) was gathered by filtration and washed four times with ethanol and then dried utilizing vacuum. The gained product was re-dissolved in ethanol for recrystallization and after that dried to give a clean pure product (Scheme 1). The crystalline products obtained were characterized by Fourier-Transform Infrared (FTIR) spectra (Cary 600 FTIR, USA), which was operated inawavenumber range of 4000–400 cm−1, and 1H and 13C nuclear magnetic resonance (NMR) spectroscopies were performed with dimethyl disulfide (DMDS) used as solvent. Tetramethylsilane (TMS) was the standard reference, and the probe temperature was 25°C (Hamil et al., 2009).


Scheme 1: General scheme for the synthesis of Schiff base NHM Compounds.
((4Z,4'E)-4,4'-(1, 2-phenylenebis (azanylylidene)bis (2-hydroxynaphthalene-1(4H)-one) (NHM01)
NMH01 has been synthesized according to the general procedure, by reacting of one mole of phenylenediamine and two moles of 2-hydroxynaphthalene-1,4-dione in 100 ml ethanol.
Spectral data
Orange crystals, mp 240-241ºC, yield: 62%; %; FTIR (cm-1):3570 (O-H), 1619(C=O) 1586(C=N), 1445(C-N),1216(C-O). 1H NMR (400 MHz, DMSO-d6, δ ppm) 7.84-8.29(m, 12H, C-H aromatic), 7.19 (S, 1H, C-H aromatic), 15(S, 2H, OH). 13CNMR (400 MHz, DMSO-d6):189.10, 157.04, 145.42, 142.65, 139.36, 139.62, 131.07, 130.29,130.16, 129.38, 128.84, 128.60, 128.23, 124.90, 123.00,103.00 .Anal Calcd for C26H16N2O4: C, 74.28; H, /3.84; N, 6.66; O, 15.22.
(4Z,4'Z)-4,4'-(1,2-phenylenebis (azanylylidene)) bis(2-methylnaphthalene-1(4H)-one)NHM04compound:
NMH04 has been synthesized according to the general procedure, by reacting of one mole of phenylenediamine and two moles of 2-methylnaphthalene-1,4-dione in 100 ml ethanol.
 Spectral data
Spectral data
Green crystals, mp 241-242ºC, yield: 75%; FTIR (cm-1):3053 (C-H, Aromatic), 2965 C-H (CH3)3570 (O-H), 1600(C=O) 1550(C=N), 1526 (C=C), 1440(C-N),1220(C-O). 1H NMR (400 MHz, DMSO-d6, δ ppm) 7.30-8.05 (m, 12H, C-H aromatic), 6.9 (s, 1H, CH=C aromatic), 2.09 (s, 6H, CH3). 13CNMR (400 MHz, DMSO-d6); 184.81, 147.82,135.02,133.77, 133.74, 131.59, 131.56, 125.82, 125.13, 16.50. Anal Calcd for C28H20N2O2: C, 80.75; H, 4.84; N, 6.73; O, 7.68.
(N1E,N2E)-N1,N2-bis (naphthalen-1ylmethylene)benzene-1,2-diamine) NHM06 compound:
NMH06has been synthesized according to the general procedure, by reacting of one mole of phenylenediamine and two moles of 1-naphthaldehyde in 100 ml ethanol.
Spectral data
White crystals, mp 239-240ºC, yield: 65%; FTIR (cm-1): 3045(C-H aromatic), 1529(C=N),1446(C=C), 1397(C-N) 1H NMR (400 MHz, DMSO-d6, δ ppm):7.32-8.05 (m, 18H,C-H aromatics),9.1(s, 2H, CH=N). 13CNMR (400 MHz, DMSO-d6); 156.00,140.41, 135.18,134.84,133.42, 133.36,130.42, 130.31, 127.88,127.68,126.63,124.13,116.39. Anal Calcd for C28H20N2: C, 87.47; H, 5.24; N, 7.29.
(N1,N2-di(anthracen-9(10H)-ylidene)benzene-1,2-diamine) NMH11 compound:
NMH11 has been synthesized by the described procedure, by reacting of one mole of phenylenediamine and two moles of anthracen-9(10H)-one in 100 ml ethanol.
Spectral data
Light green crystals, mp 291-292ºC, yield: 80%; FTIR (cm-1):3287(C-H aromatic), 3060(C-H SP3), 1655 (C=N), 1577(C=C), 1308(C-N). 1H NMR (400 MHz, DMSO-d6, δ ppm) 3.81(s, 4H,CH2), 7.30-7.72 (m, 20H, C-H aromatics). 13CNMR (400 MHz, DMSO-d6); 185.01,148.62, 140.85, 139.86, 134.35, 132,81, 131.94, 128.91, 127.68, 127.59, 126.55, 125.37, 125.34, 123.44, 122.35 119.74, 116.43, 50.01. Anal Calcd for C34H24N2: C, 88.67; H, 5.25; N, 6.08.
2.4 DNA binding studies
To study how competently the synthesized compounds interact with G-DNA, the synthesized compounds were investigated for their DNA binding ability using fluorescence emission spectra. All experiments were conducted in Tris (Hydroxymethyl)aminomethane (Tris buffer) (0.01M Tris, 0.1M NaCl, at pH 7.4), glass-distilled deionized water and analytical grade reagents were used throughout experiments. The pH values of all solutions were measured with a calibrated Jenway pH-meter model 3510 (Staffordshire, UK). All buffer solutions were filtered through Millipore filters (Millipore, UK) of 0.45 mm pore diameter.
2.4.1 Absorbance spectra
Absorbance spectra were measured on a Jenway UV-visible spectrophotometer, model 6505 (London, UK) using quartz cells of 1.00cm path length. The UV-Vis absorbance spectra were recorded in the 200-500nm range, and spectral bandwidth of 3.0nm. For the final spectrum of each solution analyzed baseline subtraction of the buffer solution was performed. Genomic DNA was used in a concentration of 75 μg/ml. DNA was extracted from peripheral lymphocytes of anticoagulated blood (EDTA) samples by Proteinase K digestion and phenol/chloroform extraction.Purity was determined by measuring the absorbance at 260/280nm indicating that the sample is free from protein contamination. The concentration was assayed spectrophotometrically using 6600M-1cm-1 as a molar extinction coefficient at 260nm.
2.4.2 Fluorescence spectra and DNA-binding studies
Fluorescence emission and excitation spectra were measured using a Jasco FP-6200 spectrofluorometer (Tokyo, Japan) using fluorescence 4-sided quartz cuvettes of 1.00 cm path length. The automatic shutter-on function was used to minimize photo bleaching of the sample. The selected excitation wavelength for ethidium bromide was 480nm. The emission spectrum was corrected for background fluorescence of the buffer. The ethidium bromide (EB) fluorescence displacement experiment was performed by sequential addition of aliquots of 1790 μlTris buffer, 10 μl EB (final concentration of 72μM), 100μl G-DNA from stock solutions (1.5 mg/ml) and finally 10 μl of compounds (NHM compounds, final concentration of 30 μM). Emission spectra were recorded for each system using excitation wavelengths of maximum fluorescence intensity determined for the systems to be 480nm using a slit width of 5nm to examine alterations in emission spectra resulting from the complex construction of both systems. On construction of the full systems, the system was allowed to equilibrate for 30minutes at room a temperature and emission spectra (500-730nm) were recorded to monitor changes in EB intensity.
3.1 Design strategy of ortho-phenylenediamineSchiff’s base derivatives
Based on the increased role and importance of phenylenediamineSchiff’s base derivatives as DNA intercalators as described in the introduction part, and because of their versatile route of chemical synthesis, ortho-phenylenediamine were used as key scaffold to develop a series of aromatic imino analogues. Fifteen compounds named with NHM symbol were designed, the strategy which applied to design these compounds was based on the symmetrical distribution of the amino groups of ortho-phenylenediamine with different carbonyl compounds including naphthaldehyde, naphathquinone and anthraquinone derivatives, where the aldehydes or ketones differ in their lipophilicity, hydrogen bond ability as donor or acceptors, and ionizability. The applied strategy yielded the scaffold which contains three parts: 1) A planar polycyclic pattern to intercalate between DNA base-pairs and to provide π-π interactions. 2) Some polar functions providing hydrogen bond interactions with nucleic acids. 3) Variety of substituents to improve the interactions and promote the physicochemical properties. To the best of our knowledge, there have been no literature reports regarding Schiff’s base of ortho-phenylenediamine with naphthoquinones and anthraquinone derivatives as DNA intercalating agent so far. The designed compounds were investigated for their docking energies. In addition, the effect of replacing of the naphthyl ring with a three ring system (anthracene) or aromatic rings with flexible linkage was also investigated. The chemical structures are listed in Table 1.
Table 1: The chemical structures of designed compound
3.2. Molecular docking analysis
In this part of the current study, the molecular dockings of NHM compounds with five B-DNA fragments were performed using Auto Dock vina in PyRx Virtual Screening Tool to understand the possible binding mode. The molecular docking study was employed to identify the binding interactions and estimate the binding affinity of the most active derivatives to the DNA duplexby combining and optimizing variables such as hydrophobic, steric, and electrostatic complementation. Two well-known biologically active DNA intercalating agents were used as references namely doxorubicin and daunorubicin. Considering the values of free energies of binding mentioned in Table 2, it could be deduced that the compounds were properly accommodated between the DNA base pairs. The molecular docking analysis revealed that the compounds have contributed in hydrophobic and Pi-Pi interactions, and hydrogen bonding with the DNA base pairs.
| Names of compound | Docking Energy(kcal/mol) | ||||
| 1D60 | 1ZEW | 2DES | 1BNA | 1DC0 | |
| Daunorubicin | -8.9 | -10.0 | -8.8 | -8.9 | -9.8 |
| Doxorubicin | -8.6 | -9.8 | -8.4 | -8.8 | -9.6 |
| NHM01 | -9.2 | -10.9 | -9.1 | -8.9 | -8.8 |
| NHM02 | -7.7 | -9.4 | -9.1 | -8.6 | -8.8 |
| NHM03 | -6.8 | -8.5 | -8.4 | -7.3 | -8.1 |
| NHM04 | -7.3 | -8.1 | -8.7 | -6.9 | -8.0 |
| NHM05 | -6.9 | -8.2 | -9.7 | -6.5 | -8.0 |
| NHM06 | -7.3 | -8.1 | -7.7 | -8.1 | -7.2 |
| NHM07 | -7.3 | -8,3 | -8.0 | -7.3 | -7.2 |
| NHM08 | -7.0 | -8.6 | -9.0 | -7.5 | -7,3 |
| NHM09 | -8.6 | -8.3 | -8.6 | -8.1 | -7.3 |
| NMH10 | -7.2 | -7.8 | -7.6 | -7.7 | -7.6 |
| NMH11 | -8.9 | -9.1 | -8.4 | -8.4 | -8.5 |
| NMH12 | -8.3 | -9.4 | -9.3 | -8.5 | -8.8 |
| NMH13 | -8.9 | -9.0 | -9.4 | -8.5 | -8.8 |
| NMH14 | -6.5 | -7.2 | -6.4 | -6.3 | -7.1 |
| NMH15 | -6.0 | -7.2 | -6.3 | -6.7 | -7.4 |
Table 2: Various energies in the binding process of NHM compounds with DNAs obtained from molecular docking.
(1BNA): D(CGCGAATTCGCG)2, (1DC0): D(CATGGGCCCATG)2, ((2DES): D(CGTACG)2, (1D60) :D(CCAACNTTGG)2, (1ZEW): D(CCTCTAGAGG)2,
ΔGa is the binding free energy change in the binding process.
The more negative the numerical values for the binding affinity, the better is the predicted binding between a ligand and a macromolecule. In this particular case of screening five DNA fragments (1BNA, 1ZEW,2DES,1D60, 1BNA and 1DCO) with designed compounds. Table 2 shows that all the designed compounds have good binding energies when compared to standard drugs. Moreover, NMH01 and NMH11 are both predicted to have the best binding affinity.
The DNA fragment (1zew) was used to discuss and compare the intercalating activity of all compounds. It appears worth to note that compounds which exhibit high activity against (1zew) also showed high activity against the other DNA fragments. Simultaneously, any modification which might decrease the activity against (1zew) will produce the same effect against all other DNA fragments.
Molecular docking results were identified based on the strongest ligand binding poses, where identified using the low binding energy and the number of H-bonding and hydrophobic interactions. From the docking studies the molecular docking analysis revealed that all the docked molecules were inserted between the adjacent nucleotides at the active site. These compounds were stabilized at the active site through different interactions. The planar system was involved in hydrophobic stacking with the base pairs of different nucleotides, and the hydrophilic moieties formed several hydrogen bonds with different nucleotides.
It was observed that introduction of hydrophilic substituent's to the aromatic ring increases the intercalating activity (decrease of the docking energy) as shown with NMH01,NMH09 and NMH13. While, the introduction of hydrophobic substituents (R) to the same aromatic ring leads to a decrease of the intercalating activity (increase ofthe docking energy) as shown for NMH04 and NMH08. A substantial observation was that the highest activity was predicted to be with an introduction of 2-hydroxynaphthalene-1,4-dione at NMH01. The importance of hydroxyl substituents at meta-positions of the aromatic ring are attributed to the formation of seven hydrogen bonds at intercalating sites keeping the planarity which is needed for optimum localization at the intercalating site (Figure1). Whereas, hydroxyl substituent at position five builds three H-bonds as shown in NMH02.H-bonding characteristics of compounds rendered significantly high binding affinity with DNA.
Lipophilic substituents (Cl or Br) at position 3 of the naphthyl side chain had little effect on the intercalating activity when compared with the non-substituted derivative (NMH03, NMH07). Furthermore, introduction of a hydrogen bond acceptor substituent such as carbonyl group on the aromatic ring leads to an increase of the intercalating activity, as could be seen from the comparison of 2-hydroxynaphthalene-1,4-dione derivatives with the corresponding non-substituted 2-hydroxynaphthalene derivatives as shown with NMH01,NMH02 and NMH09. In the case of compounds NMH04, NMH08,NMH05 and NMH10 replacement of the methyl moiety on aromatic rings with the isopropyl moiety leads to a decrease of the intercalating activity, which may be caused by steric hindrance of branched groups. Furthermore, as observed in the docking study, compound NMH14 was located at the groove pocket interacting with DNA by pi-anion interaction with DGB:17and DGA:7. Compound NMH15 was located at the groove pocket and interacted with DNA by six hydrogen bonds with DGA:10, DGA:9 DTB:15 and DAB:16. Nevertheless, it was evident that NMH14 and NMH15 had the lowest binding energy scores which may be due to the lack of the planarity pattern of the polyaromatic systems.
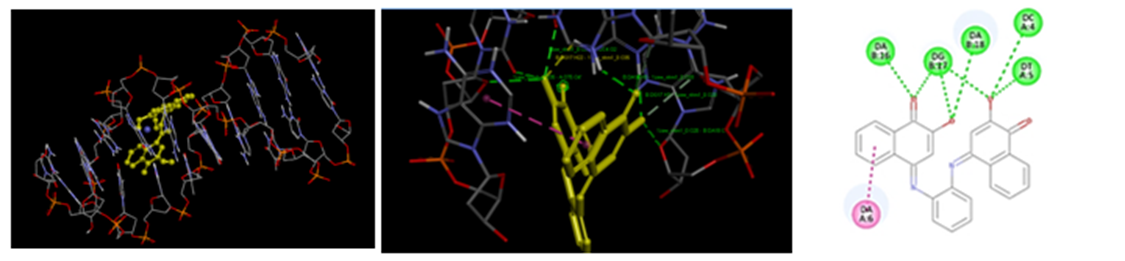
Figure 1: Localization of NHM01 (yellow) at the intercalating site of DNA. (ii)Compound NMH01 interacting with DNA strands with seven intermolecular hydrogen bonds. (iii) 2-dimensional structure of NMH01 at the active site.
It can be clearly seen that the interaction mode of most compounds consist of a classical intercalation mode except the binding of NMH03, NMH05, NMH08 and NMH10 with DNA which involves partial intercalation and groove binding. Moreover, compounds NMH14 and NMH15 bind to DNA via groove binding. It is worth mentioning that compounds NMH01, NMH04, NMH06 and NMH11 were the most active compounds for each DNA fragment (Table 2). For this reason they have been selected for further synthesis and analysis.
3.3 ADMET properties
3.3.1 Analysis of Physicochemical Properties.
General characteristics of designed Schiff’s base derivatives of o-phenylenediamine revealed that all these compounds have a molecular weight less than 500 g/mol, except NMH03, NMH07, and NMH13 with molecular weights of 546.21, 542.26 and 552.53g/mol, respectively. This appears a prime property to be called as drug likeness of the small molecules. It is evident from Table 3 topological polar surface area (TPSA) values of all compounds were found to be within the limit of ≤ 140 Å. The prediction of TPSA parameters helps to understand the passive molecular transport of drug molecules. TPSA is an important tool in drug discovery and development. By analyzing a drug candidate's TPSA, we can predict its potential for oral bioavailability and ability to reach target sites within the body. This prediction hinges on a drug's ability to permeate biological barriers. The Molar refractivity (MR) of all compounds is within the ranges of 121.50–158.01, their number of rotatable bond (nRotb), number of H-bond donors (nHBD) and the number of hydrogen bond acceptors (NHBAs) of all o-phenylenediamine derivatives are within the recognized limits ≤10, ≤5, and ≤10, respectively.
SwissADME predictions suggest that symmetrical phenylenediamine Schiff’s base derivatives have optimum parameters for anticancer activity and can be considered as lead molecules for further modifications.
| Compound | MW (g/mol) | nHA | nRB | nHBD | nHBA | MR | TPSA (A2) |
| NHM01 | 420.42 g/mol | 32 | 2 | 2 | 6 | 121.80 | 99.32Ų |
| NHM02 | 420.42 g/mol | 32 | 2 | 2 | 6 | 122.71 | 99.32Ų |
| NHM03 | 546.21 g/mol | 32 | 2 | 0 | 4 | 134.40 | 58.86Ų |
| NHM04 | 416.47 g/mol | 32 | 2 | 0 | 4 | 128.27 | 58.86Ų |
| NHM05 | 472.58 g/mol | 36 | 4 | 0 | 4 | 147.50 | 58.86 Ų |
| NHM06 | 384.47 g/mol | 30 | 4 | 0 | 2 | 128.85 | 24.72Ų |
| NHM07 | 542.26 g/mol | 32 | 4 | 0 | 2 | 144.25 | 24.72Ų |
| NHM08 | 412.52 g/mol | 32 | 4 | 0 | 2 | 138.78 | 24.72Ų |
| NHM09 | 416.47 g/mol | 26 | 4 | 2 | 4 | 132.89 | 65.18Ų |
| NMH10 | 468.63 g/mol | 36 | 6 | 0 | 2 | 158.01 | 24.72Ų |
| NMH11 | 460.57 g/mol | 36 | 2 | 0 | 2 | 148.83 | 24.72Ų |
| NMH12 | 488.53 g/mol | 38 | 2 | 0 | 4 | 149.67 | 58.86Ų |
| NMH13 | 552.53 g/mol | 42 | 2 | 4 | 8 | 157.76 | 139.78Ų |
| NMH14 | 436.55 g/mol | 34 | 6 | 0 | 2 | 142.81 | 24.72Ų |
| NMH15 | 454.57 g/mol | 34 | 4 | 2 | 6 | 152.22 | 89.72Ų |
Table 3: Physicochemical properties of designed Schiff’s base derivatives of o-phenylenediamine calculated using swissADME database.
Molecular weight: M. W, Number of heavy atom: nHA, , Number of rotatable bonds: nRB, Number of H-bond acceptors: nHBA, Number of H-bond donors: nHBD, Molar refractivity: MR, topological polar surface area: TPSA.
3.3.2 Lipophilicity and Water Solubility.
Log Po/w values of all derivatives range from 2.85 to 8.00 reflecting its partition preferably into the lipid compartment (Table 4). This implies that
these compounds will be well absorbed through the membranes into the systemic circulation to achieve high bioavailability. On the other hand, solubility, Log Sis aqueous solubility, exhibit a defined range of -4.4to-8.72 as shown in Table 5. The compounds NMH01, NMH02, NMH03, NMH15 predicted as moderately soluble (with optimal lipophilicity and moderate water solubility) can achieve good bioavailability when administered orally. All other derivatives are poorly water soluble.
| Compound | iLOGP | XLOGP3 | WLOGP | MLOGP | SILICOS-IT | Consensus Log Po/w |
| NHM01 | 2.62 | 4.36 | 5.20 | 1.25 | 5.06 | 3.70 |
| NHM02 | 3.15 | 5.04 | 4.84 | 1.79 | 5.10 | 3.98 |
| NHM03 | 3.20 | 6.45 | 6.88 | 3.49 | 7.37 | 5.48 |
| NHM05 | 4.13 | 6.92 | 7.49 | 4.06 | 8.35 | 6.19 |
| NHM06 | 4.13 | 7.02 | 7.49 | 5.35 | 7.89 | 6.38 |
| NHM07 | 4.09 | 8.40 | 9.02 | 6.46 | 9.22 | 7.44 |
| NHM08 | 4.04 | 7.75 | 8.11 | 5.74 | 8.94 | 6.92 |
| NHM09 | 3.57 | 6.31 | 6.91 | 4.15 | 6.91 | 5.57 |
| NMH10 | 4.32 | 9.27 | 9.74 | 6.48 | 10.21 | 8.20 |
| NMH11 | 4.26 | 8.46 | 7.83 | 6.03 | 9.19 | 7.15 |
| NMH12 | 3.62 | 7.49 | 7.11 | 4.17 | 8.56 | 6.19 |
| NMH13 | 3.88 | 7.17 | 5.91 | 2.01 | 6.63 | 5.13 |
| NMH14 | 3.89 | 8.28 | 8.02 | 6.05 | 8.40 | 6.93 |
| NMH15 | 3.62 | 2.58 | 1.61 | 3.73 | 1.08 | 2.52 |
Table 4: The lipophilicity of designed Schiff’s base derivatives of o-phenylenediamine calculated with SwissADME database.
| Compound | ESOL | Ali | SILICOS- IT | |||
Log S (ESOL) | Class | Log S (Ali) | Class | Log S SILICOS-IT | Class | |
| NHM01 | -5.48 | Moderately soluble | -6.16 | Poorly soluble | -7.75 | Poorly soluble |
| NHM02 | -5.91 | Moderately soluble | -6.87 | Poorly soluble | -7.31 | Poorly soluble |
| NHM03 | -7.57 | Poorly soluble | -7.48 | Poorly soluble | -10.47 | Insoluble |
| NHM04 | -6.11 | Poorly soluble | -6.39 | Poorly soluble | -9.68 | Poorly soluble |
| NHM05 | -7.24 | Poorly soluble | -7.97 | Poorly soluble | -10.49 | Inoluble |
| NHM06 | -7.02 | Poorly soluble | -7.35 | Poorly soluble | -10.90 | Insoluble |
| NHM07 | -8.83 | Poorly soluble | -8.79 | Poorly soluble | -12.44 | Insoluble |
| NHM08 | -7.62 | Poorly soluble | -8.11 | Poorly soluble | -11.65 | Insoluble |
| NHM09 | -6.73 | Poorly soluble | -7.47 | Poorly soluble | -9.73 | Poorly soluble |
| NMH10 | -8.72 | Poorly soluble | -9.69 | Poorly soluble | -12.47 | Insoluble |
| NMH11 | -8.51 | Poorly soluble | -8.85 | Poorly soluble | -13.50 | Insoluble |
| NMH12 | -8.04 | Poorly soluble | -8.56 | Poorly soluble | -13.24 | Insoluble |
| NMH13 | -8.18 | Poorly soluble | -9.93 | Poorly soluble | -10.87 | Insoluble |
| NMH14 | -8.02 | Poorly soluble | -8.66 | Poorly soluble | -12.54 | Insoluble |
| NMH15 | -4.41 | Moderately soluble | -4.41 | Moderately soluble | -5.97 | Moderately soluble |
Table 5: Water solubility characteristics of designed Schiff’s base derivatives of o-phenylenediamine calculated with swissADME database
3.3.3 Pharmacokinetic Profile
Values of ADME properties of designed o-phenylenediamine derivatives presented in Table 6 were all within the permissible range. All of the compounds have a low gastro intestinal absorption except NMH01, NMH02, NMH04 and NMH15 show high gastrointestinal absorption. All
these compounds lack blood brain barrier (BBB) penetration. BBB is very important to limit the penetration of drugs into the central nervous system (CNS). The lack of BBB penetration ability suggests their low CNS side effects as well as their unsuitability to treat brain tumors'. In the present study, all the compounds were predicted to be non-substrate of P-glycoprotein, except compounds NMH06, NMH07, NMH08, NMH09, NMH10, NMH14 and NMH15. In the case of drug metabolism NMH01 was identified as an inhibitor of CYP1A2, CYP2C9 and CYP3A4. Compound NMH04 was predicted as an inhibitor of CYP2C9, CYP2C19 and CYP3A4 and in addition, NMH06 as CYP1A2, CYP2C19 and CYP3A4 inhibitors. However, NMH11 was predicted to show no inhibitory activity against any of the CYP isoforms. The skin permeability coefficient (Log Kp, a multiple linear regression, Potts and Guy, 1992), indicates the more negative the log Kp (withKpin cm/s), the less skin permeant is the molecule. Among the NHM compounds, NMH15 (-7.24 cm/s) is the least permeant compound and NMH10 (-2.58 cm/s) is most permeant, respectively.
| Compound | Absorption | BBB Permeant | P-gp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Log Kp (Skin Permeation) (cm/s) |
| NHM01 | High | No | No | Yes | No | Yes | No | Yes | -5.77 cm/s |
| NHM02 | High | No | No | Yes | Yes | Yes | No | Yes | -5.29 cm/s |
| NHM03 | Low | No | No | No | No | Yes | No | No | -5.05 cm/s |
| NHM04 | High | No | No | No | Yes | Yes | No | Yes | -5.01 cm/s |
| NHM05 | Low | No | No | No | No | No | No | Yes | -4.27 cm/s |
| NHM06 | Low | No | Yes | Yes | Yes | No | No | Yes | -3.66 cm/s |
| NHM07 | Low | No | Yes | Yes | No | No | No | No | -3.64 cm/s |
| NHM08 | Low | No | Yes | Yes | No | No | No | Yes | -3.31 cm/s |
| NHM09 | Low | No | Yes | Yes | Yes | No | No | No | -4.36 cm/s |
| NMH10 | Low | No | Yes | No | No | No | Yes | Yes | -2.58 cm/s |
| NMH11 | Low | No | No | No | No | No | No | No | -3.10 cm/s |
| NMH12 | Low | No | No | Yes | No | No | No | No | -3.96 cm/s |
| NMH13 | Low | No | No | No | No | No | No | No | -4.58 cm/s |
| NMH14 | Low | No | Yes | Yes | Yes | No | No | Yes | -3.08 cm/s |
| NMH15 | High | No | Yes | No | No | No | Yes | No | -7.24 cm/s |
Table 6: Pharmacokinetic parameters of designed Schiff’s base derivatives of o-phenylenediamine calculated with swissADME database.
3.3.4 Drug likeness
NMH01 do not violate any of the five drug likeness parameters, i.e., Lipinski, Muegge, Ghose, Veber, and Eganrules of drug likeness(Daina et al., 2017). The other compounds showed violation of at least one of the drug-likeness filters. As per Lipinski's rule-of-five, candidates violating none or less than one of the following four criteria are likely to be developed as a prospective oral drug. The criteria are as follows: log P (octanol-water partition coefficient) ≤5, molecular weight ≤500, number of hydrogen bond acceptors ≤10 and number of hydrogen bond donors ≤5. In this investigation, none of the designed Schiff’s base derivatives of o-phenylenediamine compounds has violated Lipinski's rule of five except NMH07 which has two violations: MW>500 and MLOGP>4.15 (Table 7) thus providing the possible utility of as eries for developing compounds with drug-like properties. All derivatives have a similar bioavailability score of 0.55. The results obtained from in silico studies using SwissADME clearly indicate that the selected compounds to be synthesized, namely NMH01, NMH04, NMH06 and NMH11 could be druggable substances. It is interesting to note that the results from the SwissADME predictor values of MW1, log P, HBA, HBD, molar refractivity and the total polar surface area of these molecules are in excellent agreement with the most important rules of drug likeness. The results show that all four compounds have zero violations of Lipinski’s rule of five. SwissADME predictions suggest that symmetrical phenylenediamine Schiff’s base derivatives have optimum parameters for anticancer activity and can be considered as lead molecules for further modifications.
| Compound | Lipinski | Hose | Veber | Egan | Muegge | BA. Score |
| NHM01 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| NHM02 | Yes | Yes | Yes | Yes | No;1violation: XLOGP3>5 | 0.55 |
| NHM03 | Yes;1violation: MW>500 | No; 3 violations: MW>480,WLOGP>5.6, MR>130 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NHM04 | Yes | No;1violation: WLOGP>5.6 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NHM05 | Yes | No; 2 violations: WLOGP>5.6,MR>130 | Yes | No; 1 violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NHM06 | Yes;1violation: MLOGP>4.15 | Yes | No;1violation: WLOGP>5.88 | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NHM07 | No;2violations: MW>500,MLOGP>4.15 | No;3violations: MW>480,WLOGP>5.6, MR>130 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.17 |
| NHM08 | Yes; 1 violation: MLOGP>4.15
| No; 2 violations: WLOGP>5.6,MR>130 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NHM09 | Yes;1violation: MLOGP>4.15 | No;2violations: WLOGP>5.6,MR>130 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NMH10 | Yes;1violation: MLOGP>4.15 | No;2violations: WLOGP>5.6,MR>130 | Yes | No;1violation: WLOGP>5.88 | No;1violation: XLOGP3>5 | 0.55 |
| NMH11 | Yes;1violation: MLOGP>4.15 | No;2 violations: WLOGP>5.6,MR>130 | Yes | No;1 violation: WLOGP>5.88 | No;1 violation: XLOGP3>5 | 0.55 |
| NMH12 | Yes;1violation: MLOGP>4.15 | No; 3 violations: MW>480,WLOGP>5.6, MR>130 | Yes | No; 1 violation: WLOGP>5.88 | No;1 violation: XLOGP3>5 | 0.55 |
| NMH13 | Yes;1violation: MW>500 | No;3violations: MW>480,WLOGP>5.6, MR>130 | Yes | No; 2 violations: WLOGP>5.88,TPSA>131.6 | No;1 violation: XLOGP3>5 | 0.55
|
Table 7: Druglikenessof designed Schiff’s base derivatives of o-phenylenediamine calculated with swissADME database
3.4 Chemical synthesis
The best four promising compounds according to the in silico studies were synthesized. The targeted compounds, symmetrical 1, 2-phenylenediamine with di-substituted aromatic carbonyl moiety, have been successfully synthesized using a previously reported synthetic procedure. The reaction order for the synthesis of selected compounds is given in general schemes 2. All the derivatives were synthesized producing good yields with characteristic color. The compounds were soluble in chloroform and dimethyl sulfoxide (DMSO) but insoluble in water. Melting points were in the range of 239–292 °C. Thin-layer chromatography (TLC) was used to monitor the progress of the reaction in methanol: dichloromethane (1:4). Their chemical structures were confirmed by means of spectroscopic analysis as described previously.

Scheme 2: Synthesis scheme of NHM compounds
Analysis of spectra
Proton 1H andCarbon 13CNMR spectra
NMR analysis is very important to confirm the structure and functional groups. Experimental H-NMR chemical shifts were expressed in ppm, dispersed through the spectrum starting form tetramethylsilane as the internal standard and recorded in DMSO-d. Importantly, the spectra lacked the signal of the amino group (NH) characteristic of the starting material. The ¹H-NMR spectrum of NHM01 displayed singlet at 15 ppm and multiplet at 7.84-8.29 ppm as signal to the O-H proton and the protons of aromatic rings, respectively. Moreover, a peak appears at 2.09 ppm attributed to the aliphatic–CH (6H) compound contained in NHM04 and multiplet signals at 7.30-8.05 ppm were ascribed to aromatic protons. Besides, the signal due to –CH=N appears at 9.1 ppm for the compound NHM06 corresponding to the azomethine group which confirms the formation of Schiff bases and the multiple peaks attributed to the aromatic protons between 7.32 and 8.05 ppm. Additionally, the ¹H-NMR spectrum of compound NMH11 exhibited as singlet signal at 3.81 ppm corresponding to the CH2 group. The multiplet signals at 7.30-7.72 ppm were assigned to the 20 H aromatic protons. Consistently13C-NMR spectra showed a chemical shift at 189.10, 184.81,156.40,185.01 ppm assigned to the imine group revealing the formation of Schiff's base derivatives of o-phenylenediamine (NHM01, NHM04, NHM06 and NMH11), respectively. In addition, the peak observed at 16.50 ppm was attributed to a methyl carbon (C20) integrated at the aromatic group of compound NHM04. The peaks observed at 157.04 ppm and 183.00 ppm corresponding to carbonyl groups of the compounds NMH01 and NMH04, respectively.The peak at 50.01ppm attributes to the methylene group of compound NMH11. The peaks at 103.11-148.62 ppm range are ascribed to aromatic carbons.
Fourier-Transformed Infrared
In order to continue the characterization of NHM compounds, the FT-IR spectrum was carried out. The FT-IR spectra of NHM compounds showed the disappearance of both carbonyl groups atthe aldehyde, keton or amino groups atthe amine, whereas NH is vanished or hidden underneath the broad bands at3450-3300cm-1 in Schiff’s base. Additionally, the presence of the azomethine CH=N stretching at about 1529−1655 cm-1 depending on the nature of R side chain substituents, indicates the formation of the Schiff base compounds. The FTIR spectrum of the compound NMH01 showed a stretched band around 3200 − 3570 (O-H) cm-1, which is attributed to the intramolecular hydrogen bond of the hydroxyl group. Furthermore, compound NHM01 exhibits absorption bands at 1675-1619cm-1 corresponding to the C=C and C=O, respectively. Additionally, the band at 1446−1450-1577 cm-1 corresponds to C=C of compounds NHM06, NHM04 and NMH11, respectively.
DNA binding studies
UV Absorbance Spectra
UV-spectroscopy being an important qualitative and quantitative analysis technique has been exploited to investigate the interaction of ligands with DNA. The electronic absorption spectrum of DNA exhibits a broad band in the range of 200-350nm and shows maximum absorption at ~260nm. This maximum is a consequence of chromophoric groups in purine and pyrimidine moieties responsible for the electronic transitions (González-ruiz et al., 2011). Therefore, the changes in the absorption spectra of the compound alone and compound–DNA mixture is an indication of DNA–compound interaction, and the mode and strength of binding can also be determined from the shift. The absorbance spectra of DNA could show hypochromism (deceased absorbance intensity) and hyperchromism (increased absorbance intensity) upon titration with the ligand. In general, the intercalative mode of binding usually results in hypochromism and a red-shift because of the strong stacking interaction between an aromatic chromophore and the nitrogen bases of DNA. This results in structural changes of the DNA that entailed the local unwinding of the double helix. Whereas for weak interaction such as hydrogen bonding, groove binding and electrostatic interaction no significant shift of the absorption maxima occurs (Banerjee et al., 2016). The absorption spectra resulting from titrations are depicted in Fig. 2. As observed from spectral curves, the complexes showed pronounced hypochromism with a slight red shift of 2nm. The results suggest that these compounds bind to DNA through an intercalation mode.
The resultant hypochromic effect was found to be 0.244nm for NMH01, 0.201nm for NMH04, 1.53nm for NMH06, and 1.42nm for NMH11 followed by a red shift of magnitude 2.01nm, 1.89nm, 1.73nm and 1.88nm for NMH01, NMH04, NMH06, and NMH11, respectively.
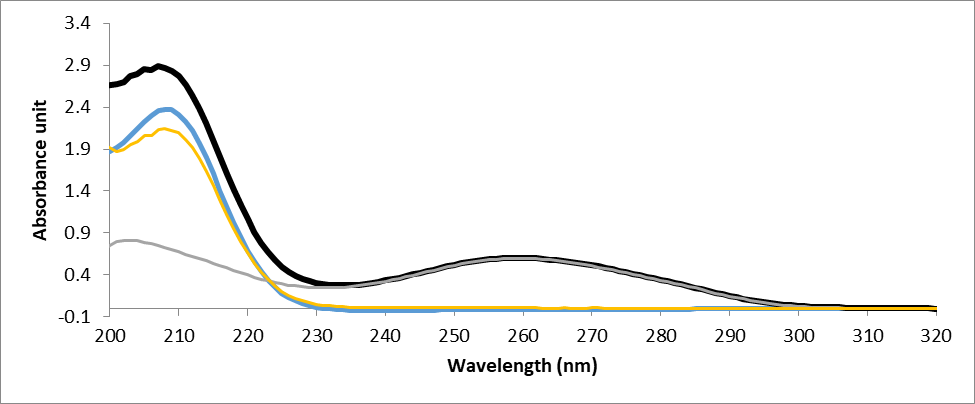
Figure 2: UV–Vis absorption spectra of DNA in the absence and presence of NMH01 (A) the G-DNA-NMH01 complex, (B) the 30 µm NMH01, (C) G-DNA-NMH01 complex subtracted from the G-DNA absorbance to show the hypochromic shift (D) GDNA alone. The experiment was conducted in Tris buffer solution (0.01M Tris, 0.1M NaCl, at pH 7.4). Genomic DNA was used in a concentration of 75 μg/ml and NMH01 30 µM.
Ethidium bromide (EB) Competition Assay
A competitive displacement assay was conducted to obtain further evidence of the binding pattern of the tested compounds and DNA. Ethidium bromide (EB) is a strong fluorescent dye, known to bind to DNA through intercalation with maximum emission at 661 nm upon binding to double stranded DNA. Free Ethidium bromide exhibits very low fluorescence. However, with the addition of DNA, due to its intercalation, the fluorescence intensity magnifies remarkably (Zhao et al., 2013).The gradual addition of compounds 1, 4, 6, and 11 to the EB–DNA complex cause a decrease of the emission (quenching effect) (Figure 3). The hypochromic shift appears as a result of formation of a complex between compound and DNA and this reduction of the fluorescence intensity is a characteristic sign of intercalation. The fluorescence spectra of the EB-DNA complex in the absence and presence of NMH01 is shown in Fig. 3. The figure shows that the fluorescence intensity of EB-DNA can be quenched significantly by gradual addition of NMH01. This result indicates that NMH01 was able to replace EB in the DNA helix indicating an intercalative NMH01 DNA binding mode.
The fluorescence quenching data were analyzed by the Stern–Volmer equation and the quenching constants (Ksv) were calculated in which the synthesized compounds were the quenchers:
I0/I = 1 + KSV [Q] (1)
I0 and I represent the fluorescence intensities in the absence and presence of quencher, respectively; KSV is the linear Stern-Volmer quenching constant; Q is the concentration of quencher. The KSV values were given by the ratio of the slope to intercept. The KSV value suggests a strong affinity of the compounds to EB-bound G-DNA and that it can competitively displace EB from DNA via an intercalative mode of binding. As shown is Table 8, compound NMH01 had the highest KSV value suggesting that compound NMH01 bound most strongly to G-DNA even more than daunurobicin. The KSV values for the tested compounds are listed in Table 8.

Figure 3. Fluorescence changes of the NMH01 system that contains (A) the G-DNA-ethidium bromide complex, (B) the G-DNA-ethidium bromide complex with 30 µm NMH01 and (C) ethidium bromide alone. The experiment was conducted in Tris buffer solution (0.01M Tris, 0.1M NaCl, at pH 7.4), λex = 480 nm. Inset: plot of F0/F versus different concentrations of NMH01 (µM) for the titration of NMH01 to G-DNA-EB complex. Genomic DNA was used in a concentration of 75 μg/ml and ethidium bromide 72 µM.
Based upon the variation in absorbance and the fluorescence intensities the binding constant for the compounds to determine the strength of the interaction of compounds with DNA at 298 K, was determined from fluorescence titration data using the Benesi–Hildebrand (BH) equation:
1/ΔA =1/ (KA.ΔA0) [DNA] +1/(ΔA0) ······ (2)
Here, the difference in fluorescence was denoted by ΔA, ΔA0 is maximum florescence intensity change and [DNA] is the concentration of DNA. By plotting the reciprocal of the difference in fluorescence intensity against the reciprocal of DNA concentration, the Benesi−Hildebrand plot was constructed. To determine the extent of DNA‐binding affinities of compound towards G‐DNA, the intrinsic binding constant (Kb) values were obtained from the linear regression plots of [1/(ΔA0)] vs. {1/[DNA]} M−1, and the ratio of the slope to intercept calculated. The Kb binding constants of compounds are given in Table 8(Benesi & Hildebrand, 1949).
The Schiff’s base derivatives of o-phenylenediamine compounds showed similar binding constants and these areclose to the binding affinity of the intercalating agent daunorubicin (7.2x104 ±0.004M-1 ), which agrees well with those reported in the literature (7.8 X104 M-1 ) (Hajian et al., 2009). The overall measured Kb findings for all compounds were in the subsequent sequence: 1>4 > 6>11.They were within the range of the intercalation mode (K = 1 × 105 × 1011M-1), where intercalative mode of interactions had been assigned (Wolfe et al., 1987;Zhou et al., 2016).
The free energy (ΔG) was calculated by using equation ΔG RT = − ln K (3), where ΔG is the Gibbs free energy, R is the universal gas constant (taken as 2cal/mol K), T is the absolute temperature (298 K), and K is the binding constant. The observed values of ΔG were in the range of-6.12 to -6.44kcalmol-1(Table 8), which also indicates that the compounds spontaneously intercalate to DNA. Compound (NHM01) exhibited the most excellent binding potency compared to others.
| Name | KSV (M-1) | Ka (M-1) | ΔG kcal mol−1 | R2(5 points) |
| Taxol |
5.90X103 | 5.3 X104 ±0.002 | -6.44 | 0.99 |
| Daunorubicin | 7.91x103 | 7.2 X104±0.004 | -6.62 | 0.97 |
| NMH01 | 9.00X103 | 5.9 X104±0.006 | -6.50 | 0.99 |
| NMH04 | 6.02X103 | 4.3 X104±0.001 | -6.31 | 0.98 |
| NMH06 | 3.02X103 | 3.9 X104±0.004 | -6.25 | 0.978 |
| NMH11 | 2.86X103 | 3.1 X104±0.002 | -6.12 | 0.968 |
Table 8. The key selection vector (KSV) values, the binding constant (Kb)and the free energy (ΔG)of synthesized Schiff’s base derivatives of o-phenylenediamine.
A new series of fifteen compounds (1-15) possessing ortho-phenylenediamine Schiff’s base scaffold were designed. Next, ADMET studies were conducted by using SwissADME software and all produced compounds satisfied the Lipinski criterion. Furthermore, the results of docking studies revealed that the docked compounds displayed strong binding affinity with DNA and have a similar binding mode to that of daunorubicin (DNA intercalating agent used as reference) with binding energies ranging from -6.3 to -10.9kcal/mol. Among all these compounds, four compounds (NMH01, 04, 06, and 11) have been synthesized and characterized by FT-IR, 1H and 13C NMR spectroscopy. The interaction of these compounds with genomic-DNA (G-DNA) has been explored by using absorption spectral and fluorescence displacement studies using ethidium bromide. The results indicate that the compounds bind strongly to G-DNA via an intercalation mechanismNHM01 showed the highest key selection vector (KSV) value, suggesting that compound NHM01binds most tightly to G-DNA. Therefore, NHM01, NHM04, NHM06 and NMH11are proposed as effective cytotoxic agents with potential DNA intercalation and may serve as lead compounds for the discovery of new anticancer agents. Further biological evaluations are necessary to confirm our findings.
We gratefully acknowledge Garabolle Research Center (GRC) for performing FTIR study.
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest and is compliant with ethical standards.
This article does not contain any studies with human participants performed by any of the authors.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.
