AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Rehan Haider, Riggs prescription drugs, Department of Pharmacy, University of Karachi-Pakistan
Citation: Rehan Haider, (2023), Dengue Fever: A Narrative Review Article. J. Clinical Research Notes. 4(5); DOI: 10.31579/2690-8816/110
Copyright: © 2023, Rehan Haider. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 21 August 2023 | Accepted: 31 August 2023 | Published: 18 September 2023
Keywords: pharmacoeconomic; healthcare interventions; economic impact; resource allocation; evidence-based decision-making; cost-effectiveness analysis; pharmaceutical companies
Pharmacoeconomic is a multidisciplinary field that evaluates the economic impact of pharmaceutical and healthcare interventions. As healthcare costs continue to rise globally, efficient allocation of resources has become imperative. Pharmacoeconomic provides a structured framework for assessing the value of medical treatments, aiding decision-makers in optimizing healthcare resource allocation.
This abstract introduces key concepts and the importance of pharmacoeconomic. This highlights the growing need for evidence-based decision-making in healthcare, where limited resources must be allocated to treatments that provide the greatest clinical benefit at a reasonable cost. Pharmacoeconomic combines pharmacology, economics, and health outcome research to achieve this goal.
The abstract briefly outlines the main components of pharmacoeconomic analysis, including cost-effectiveness, cost-utility, cost-benefit, and cost-minimization analyses. Each method addresses different dimensions of economic evaluation, such as comparing treatment alternatives based on their relative costs and outcomes, incorporating patient preferences and quality-adjusted life-years (QALYs), and estimating monetary values for health outcomes.
Furthermore, the abstract touches on the relevance of pharmacoeconomic to various stakeholders, including healthcare providers, payers, policymakers, and pharmaceutical companies. Decision-makers can use pharmacoeconomic evaluations to make informed choices about drug formularies, reimbursement policies, and treatment guidelines. Pharmaceutical manufacturers can use pharmacoeconomic data to demonstrate the value of their products and support pricing strategies.
Pharmacoeconomic is a vital tool for promoting efficient and equitable allocation of healthcare resources. Quantifying the economic value of medical interventions assists decision-makers in selecting treatments that offer the best outcomes for patients while considering budget constraints. As healthcare systems strive to provide high-quality care while managing costs, pharmacoeconomic offers a systematic approach to navigating these challenges and making well-informed decisions
Health expenditures in the United States have been increasing as a percentage of the nation’s gross domestic product (GDP) [1]. Although the proportion of GDP spent on total healthcare has increased steadily, but the percentage spent on outpatient prescriptions has remained relatively constant over the past 30 years. Although private health insurance and government programs cover a growing portion of drug expenditures, a sizable portion of drug costs is still paid directly by consumers. Therefore, the cost of pharmaceuticals and pharmacy services has become an essential issue for patients, third-party payers, and governments alike. In the future, it will be necessary to scientifically evaluate the costs and consequences of drug therapy [2].
The primary value of drug therapy for prescribers and patients in the United States is demonstrated by the increased therapeutic use of prescriptions. Community pharmacists dispense Approximately three billion prescriptions annually.[3] The number of prescriptions dispensed per person per year in the United States has increased dramatically over the past 50 years. The nation’s hospitals provide billions of dollars’ worth of drugs and drug products to hospitalized patients [4]. Tablets available over the counter additionally serve an important function in America’s healthcare gadgetry. The income from nonprescription tablets improved from $700 million in the 1950s to billions of dollars.[5] These figures may be indicative of the price and perceived gains that society attributes to medicinal drugs. Most economists may well know that a crude, positive estimate of the charge and advantages of medicine to clients is the amount they spend on this merchandise. Prescription drugs and extraordinary healing interventions have contributed to the important traits recognized in the fitness of our population. Owing to the introduction of recent drug entities over the past several years, the mortality rates for some diseases have declined considerably. Tablets account for a small proportion of expenditures in health center budgets; however, drug remedies play an important role in the green treatment of hospitalized patients. A median hospitalized patient receives 6 to 8 excellent drugs on an ordinary day. An effective drug remedy partially explains why the suggested period of hospital stay has decreased over time. Despite the overall evidence supporting the use of prescription drugs, little data exists regarding the actual charges and blessings attributed to precise drug treatment plans. The primary motive for this is the shortage of methodologies for evaluating medical interventions. Perhaps the present-day popularity of lowering the prices of prescribed drugs and pharmacy services to reduce expenses for the entire healthcare system is inappropriate [6]. "One reason for this study is to present monetary and humanistic size methodologies that can be used now not only to assess the results of drug treatments but additionally to place them on the subject of one-of-a-kind related healthcare expenditure. Outcomes The term “outcomes” is increasingly being used to explain the effects and cost of healthcare interventions. However, depending on the angle, the results of healthcare are multi-dimensional. Clinicians have traditionally been the most involved in the medical outcomes of remedies. Currently, healthcare payers and directors target useful aid use or the monetary final result of healthcare selection. On the other hand, patients have become increasingly knowledgeable and involved in selections concerning their healthcare and are looking for greater records concerning the humanistic outcomes of remedies. Patients need to realize how their lifestyles might be affected or how satisfied other sufferers with their condition have been with numerous remedies. As the healthcare market is hastily converting, there is a chance that the exchange will be generally driven by the choice to comprise price. Fee containment was an important objective of this study. However, a hit to healthcare control, as measured using the objectives of patients, physicians, and other healthcare companies, as well as through societal expectations, calls for additional maintenance. The outcome dimension must not forget financial issues at the same time as recognizing that suitable clinical and humanistic results are also essential goals. The real value of healthcare interventions, applications, and coverage may be best assessed if all three dimensions of the results are measured and considered.
Definition of Pharmacoeconomic Research Economics is a set of trade-offs and picks among desires, needs, and a shortage of sources to meet these needs. While thinking about economics, the general public considers the trade-offs between items, services, and money, but the trade-off might also be expressed in humanistic terms. Therefore, we are cautious about including each useful resource use and humanistic evaluation of drug remedies in the pharmacoeconomic evaluation. Pharmacoeconomic has been described as “the description and evaluation of the charges of drug therapy to health care systems and society” [7]. Within this framework are protected the study methods related to value minimization, fee effectiveness, value advantage, price of infection, cost of software, value consequences, and decision analysis, as well as fine-of-life and different humanistic investigations. In essence, pharmacoeconomic analysis uses tools to examine the effects (proper or undesirable) of alternative drug cures and different medical interventions. The questions that pharmacoeconomic may additionally help to cope with are as follows: What drugs should be blanketed at the hospital for the military? What is a first-class drug for a specific patient? What are the best high-quality drugs for pharmaceutical manufacturers to expand on? Which drug shipping device is best for medical institutions? How do the two medical pharmacy offerings compare? Which drugs must be included in a Medicaid formulary? What is the fee in step with an exceptionally adjusted year of existence prolonged by a drug? Will an affected person's high-quality existence be advanced through specific drug therapy selection? What is the most effective drug for this particular ailment? What are the effects of diverse remedy modalities on the affected people? In essence, pharmacoeconomic evaluation uses critical equipment to inspect the consequences or impact of drug treatments and related healthcare interventions.
Historical Perspective
The emerging discipline of pharmacoeconomic has grown into a health technology area globally through pharmaceutical enterprises, academic pharmaceutical scientists, and pharmacy practitioners. As mentioned previously, it is normally defined as the description and analysis of the costs and outcomes of pharmaceutical offerings and their effect on individuals, healthcare structures, and society. The research techniques utilized by scientists in this subject (e.g., cost-effectiveness, cost-software, and exceptional-of-life reviews) are drawn from many areas: economics, epidemiology, medication, pharmacy, and social sciences. We believe that pharmacoeconomic analysis will have a positive impact on the transport and financing of health care throughout the sector. Moreover, pharmacoeconomic may additionally have an impact on fitness care and pharmacy practice at a value equal to the effect of scientific pharmacy and pharmacokinetics. Throughout the early 1960s, pharmacy began evolving as a clinical subject within the healthcare gadget. It changed all through this time that the pharmaceutical technological know-how disciplines including pharmaceutics, clinical pharmacy, drug statistics, and pharmacokinetics became a crucial part of pharmacy education and technology. In the 1970s, pharmacoeconomic developed its roots. In 1978, McGhan, Rowland, and Bootman, from the University of Minnesota, introduced the concepts of cost-gain and cost-effectiveness analyses [8]". Bootman et al. [9] also published an early pharmacy research article in 1979 wherein value-advantage analysis was used to evaluate the results of individualizing amino glycoside dosages in severely burned patients with gram-negative septicemia using sophisticated pharmacokinetics protocols. The real period of “pharmacoeconomic” did not appear in the literature until 1986, when the primary component presentation via Townsend [10], changed into a post describing the need to broaden research activities in this evolving field. Thus far, most of the efforts in this discipline have been directed toward the refinement of study techniques and their application to evaluating pharmaceutical offerings and precise drug treatments.
Pharmacoeconomic maintains that it conforms to any other exceptionally new pharmaceutical science, pharmacokinetics. Pharmacokinetics surfaced in the 1950s in U.S. colleges of pharmacy and, in the 1970s, became a crucial part of the pharmacy curriculum. Several theoretical models for pharmacy and genetics are based entirely on physicochemical principles advanced by physicists, chemists, and engineers. In parallel, pharmacies and comics have borrowed the most from basic monetary and social sciences for their theoretical models. Mcg Han, Rowland, and Bootman delivered pathology related to pharmacoeconomic in undergraduate and graduate pharmacy curricula, as early as 1976, at the University of Minnesota. However, the educational content emphasized the graduate degree and not the undergraduate professional software ranges. We are starting to see a lot of this fabric incorporated into Pharm D’s schooling stage in the area of pharmacotherapy. Furthermore, upon examining the evolutionary path of Maco kinetics, it is clear that its application within the scientific setting became a pressure that ensured its location in the medical pharmacy curriculum. We agree that pharmacoeconomic will attain an equal degree of popularity, although its software in the clinical setting is more extensive. In other words, while pharmacy practitioners start to apply the outcomes of pharmacy-genomic studies to therapeutic decision-making, positively influencing patient results, the area becomes an increasingly important aspect of the pharmacy curriculum. Like-smart, the successful implementation of “pharmaceutical care” will come about the handiest with enough pharmacoeconomic studies that accurately document the degree to which the advantages of such care outweigh the costs associated with its services. The career of a pharmacy is not likely to achieve its function of providing pharmaceutical care without this crucial body of information. Pharmacists have to turn out to be the most important thing gamers do to ensure that drug remedies and related pharmacy offerings are not only safe and effective but also offer real value in both ecological and humanistic terms.
Overview of Pharmacoeconomic Methodologies
The purpose of this section is to acquaint the reader with simple methodological methods regarding the monetary evaluation of drug therapy. By definition, pharmacoeconomic evaluations consist of any look designed to evaluate the costs (i.e., resources eaten) and results (medical, humanistic) of alternative cures. This consists of methodologies such as fee-gain, value-utility, and fee-effectiveness (see Table 1). Each of these methodologies is discussed with extra intensity in later sections. Re-reading articles that discuss the application of these strategies to healthcare opinions may additionally assist readers in becoming more privy to the function of those tools [11-30] The assessment mechanisms have often been beneficial in demonstrating the cost impact of progressive treatments, consequently granting them extra acceptance by healthcare vendors, directors, and the public.
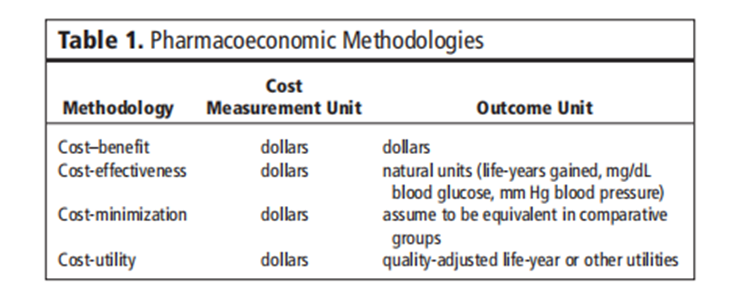
Table 1: Pharmacoeconomic Methodologies
Cost Minimization Analysis
While two or more interventions are evaluated, demonstrated, or assumed to be equivalent in terms of a given outcome or consequence, the prices related to each intervention can be evaluated and compared. This standard cost analysis is known as fee-minimization evaluation. An example of this kind of investigation regarding drug therapy can be the assessment of two generically equivalent capsules, wherein the outcome has been proven to be identical, although the acquisition and administration charges may be drastically different.
Cost-benefit analysis
Price–gain analysis is a primary device that may be used to illustrate the selection process in the allocation of finances to healthcare applications. Even though the general idea of price-benefit evaluation is not always overly complicated, many technical considerations require a degree of explanation and interpretation to understand how it can be or has been carried out.
The cost-advantage analysis consists of determining all the benefits that accrue from the program or intervention and changing them into dollars within the year in which they may occur. This flow of benefit dollars is then discounted to the equivalent gift value at the selected hobby rate. On the other hand, all application charges are recognized and allotted through a particular year, and once more, the charges are discounted to their present fee. Then, if all relevant factors stay constant, this system with the largest gift fee of benefits and much lower expenses is first-rate in terms of its monetary price.
Ideally, all blessings and costs on account of the program have to be included. This presents a huge issue, especially on the blessings aspect of the equation, as many blessings are either hard to quantify, hard to transform into dollars, or both. For example, the benefits of stepped-forward affected persons' high-quality lifestyles, patient delight with the healthcare system, and operating conditions for the medical doctor are not only difficult to measure but are extraordinarily tough to assign a dollar fee to. This problem has been addressed by many researchers in health economics, but it has not been resolved. Commonly, the analyst or researcher will convert as many benefits as possible into monetary ones. Unit. The remaining variables are labeled “intangible benefits” and left to decision-makers to include their very last liberation. Cost-benefit evaluation has frequently been used when comparing the value of varied applications where the effects are in one-of-a-kind units (e.g., value-benefit of getting a neonatal care application vs. cardiac rehabilitation software).
Cost Effectiveness Analysis
Cost-effectiveness evaluation is a way designed to assist a selection-maker in figuring out a desired preference among feasible options. Usually, value-effectiveness is described as a chain of analytical and mathematical methods that are useful resources in the selection of a direction of motion from various opportunity methods. Cost-effectiveness evaluation has been carried out on fitness matters in which the program’s inputs may be effortlessly measured in greenbacks, but the program’s outputs are greater, correctly said, in terms of fitness improvement created (e.g., life-years extended, clinical cures).
A crucial point to be considered in both cost-gain and cost-effectiveness evaluation is that software or remedy providing an excessive gain (effectiveness)-to-cost ratio in terms of a fee to society won't be valued in an equal manner by all contributors to society. As an example, drug therapy that reduced the wide variety of affected person-days in an acute care organization can be positive from a third-party payer’s point of view, but not always from the view of the organization’s administrator, who operated below a fixed level of revenue and relied on a fixed quantity of patient-days to fulfill expenses. What is considered valuable for society as a whole may be considered in another way by plan sponsors, directors, fitness vendors, governmental groups, or even character sufferers. One ought to bear in mind whose pursuits are to be taken into consideration when using those analyses.
Cost-Utility Analysis
In examining Desk 1, one could better recognize the diffused variations between the strategies discussed to this point. Price-application analysis is a monetary device wherein the intervention effect is measured in terms of amount and quality of life. It is a whole lot similar to fee-effectiveness evaluation, with the brought dimension to a selected point of view, most customarily that of the patient. Quite often, the effects of fee-utility analysis are expressed within the intervention cost in line with the fine-adjusted life-year received or adjustments in the satisfactory-of-lifestyles dimension for a given in-intervention value. Although value-application evaluation has been used fairly correctly to resource decisions regarding healthcare programs (e.g., surgical treatment vs. chemotherapy), instruments that are responsible and touchy enough to detect modifications with drug treatments (e.g., one antihypertensive agent vs. some other) are nevertheless needed.
Cost-of-Illness Evaluation
Cost-of-contamination research is critical to pharmacoeconomic critiques of recent treatment plans. By comparing the humanistic incidence of the disorder and the assets utilized in treating a situation before the discovery of a new intervention, the pharmaco-economist can efficiently set up a baseline for contrast. Even though the price and methodologies of price-of-illness research have been debated, people continue to be familiar with the pharmacoeconomic theory [28,29]. As with any pharmacoeconomic assessment, when conducting or comparing the cost of illness, it is far more essential to completely recall the design and purpose of the study. There is a cost to having baseline information; however, absolute conclusions regarding the price of intervention versus an opportunity can be made best after direct evaluation.
Cost Consequence Analysis
A cost-result evaluation has been defined as one “in which expenses and consequences are calculated but no longer aggregated into first-class-adjusted existence years or value-effectiveness ratios.”[31] Without a doubt, this form of analysis contains a list of all applicable charges and outcomes of a drug remedy or healthcare intervention, which include direct clinical charges, direct nonmedical costs, oblique expenses, scientific results, software effects, and pleasant-of-life effects. Fee-concise-sequence analysis offers the most comprehensive presentation of facts describing the value of intervention and has the benefit of being more conveniently understandable and much more likely to be carried out by healthcare selection-makers. [32] In this application, the weighting of various expenses and advantages is left to the choice-maker. although this needs to lead to enhancements in decision-maker welfare From a monetary angle, a possible disadvantage of the dis aggregated presentation of health effects is that decisions made on the person selection-maker degree won't be made inside the affected person’s or society’s nice hobbies. A different potential weakness of fee-outcome analysis is that not every record is consistently of first-class quality. Price-consequence analyses within the literature often encompass a selection of data from scientific trials and different assets since no single source is good enough to offer the breadth of records required [33–37]. Reputation and extrapolation are often important. No matter those issues, cost-result analysis remains a beneficial approach to providing relevant facts for an extensive range of healthcare issues.
Pharmaco economics and Drug Development
The pharmaceutical industry spends billions of dollars annually on the development of new tablets. As a percent of pharmaceutical sales, those studies and development (R & D) fees are virtually higher than the ones found in different industries.2 The massive range of compounds that must be evaluated to bring one drug to market contributes to the high R&D fees of drug expansion. This percentage is also better than that discovered in other industries. It has been estimated that it takes $802 million and 14 years to deliver a new drug to the market.2 The technique by which a drug is evaluated and developed for the market is illustrated in parent 1.
Because pharmacoeconomic facts are becoming more and more critical to practitioners making drug formulary selections, it's far more critical to have these records as quickly as possible after meals and Drug management approval. To try this, discussion and making plans for pharmacoeconomic assessment have to start in the early stages of drug improvement. A primary query arises as to the ideal time to conduct pharmacoeconomic studies and the first-rate system with the aid of which to achieve this. Pharmacoeconomic studies can be planned and carried out at the clinical improvement and Section IV stages of Postmarketing research. Primary research and improvement sports can be partially guided through initial pharmacoeconomic analyses. Therefore, research may also need to be done. performed at several stages of pharmaceutical studies. The following is a summary of the research interests for each section.
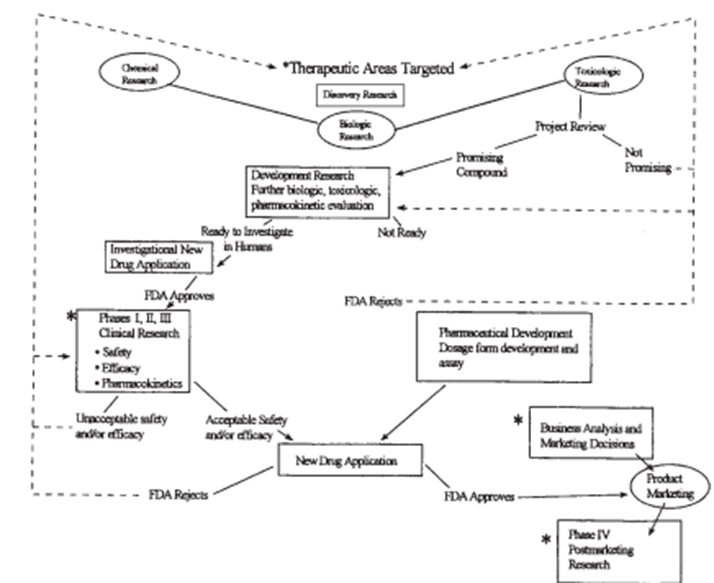
Figure 1: Research and development stages of a new drug. FDA = Food and Drug Administration. * = Pharmacoeconomic evaluations may be designed or conducted at these levels.
Phase I Trials:
The objective of the initial medical trials, or segment I, is to determine the toxicity profile of the drug in humans. The first section I trials normally includes the administration of single, conservative doses to a small number of healthy volunteers. The effects of increasing the size and variety of every day doses are evaluated until poisonous consequences occur or the probable therapeutic dosage is considerably exceeded. It's far in the course of this degree that cost-of-illness research must be finished to aid in determining whether to further broaden the drug and acquire background facts for future pharmacoeconomic opinions. Cost-of-contamination records may additionally be used within the development of preliminary fashions to evaluate the clinical benefits that need to be executed so that it will have a marketable product.
Phase II Trials:
In Section II trials, the drug is administered to a restricted number of patients with the target disorder. Patients without compatible or coexisting medical situations are favored for these trials. This reduces the number of variables that would confound the analysis of the drug’s interest and lets the potential therapeutic benefit of the brand-new drug be better verified.
Even in cautiously decided patients, demonstrating the efficacy of a new drug isn't easy or certain. To provide unequivocal proof of the drug’s therapeutic advantage, it's far more necessary to compare its effectiveness with that of well-known clinically usual treatments or, where ethically suitable, with a placebo. Those comparisons are also used to set up the optimal dosage range for the healing potential of the new drug. During this phase, cost-of-illness research can begin or continue as an initial development of quality-of-existence and aid utilization devices. Fashions may be subtle as more information becomes available about the medical components of the drug.
Phase III Trials:
In phase III trials, larger numbers of sufferers are given the brand-new drug within the hooked-up dosage range and the final dosage form. This large sample length refines the knowledge won at some point in segment II and enables identify sufferers who might have uncommon reactions to the drug. an affected person's choice is still intently supervised in section III, even though a few sufferers with coexisting clinical troubles are intentionally blanketed to permit assessment of the problem inside the drug’s use.
Discussion, making plans, and implementation of pharmacoeconomic research for the duration of this degree of studies are critical. the prospective scientific observation that has incorporated pharmacoeconomic evaluation in the course of the very last degrees of efficacy assessment is near the appropriate state of affairs. Critics of these studies claim that pharmacoeconomic critiques will avoid the new drug application (NDA) procedure. Advocates of pharmacoeconomic evaluation effectively word that, until a new drug remedy has no al- alternatives and is a leap forward, the value of using it ought to be scientifically studied.
Phase IV Trials:
At some point in the Postmarketing segment, or segment IV, retrospective and potential pharmacoeconomic studies may be designed and performed to accumulate records of the use of the drug. publish- marketing Pharmacoeconomic studies are extraordinarily critical in that they permit the assessment of the prices and outcomes of drug remedies without the altered interventions that occur in strictly managed medical trials. Through tightly managed clinical trials, pharmacoeconomic can most effectively place a fee on efficacy; this best approximates the “actual international.” As soon as a product is obtainable, its value-effectiveness may be determined. Observe: Efficacy studies answer, “Can it work?” Effectiveness research evaluates “Does it paint?”
As previously indicated, medical trials are used to assess the efficacy and safety of therapies. The relationships between pharmacoeconomic critiques and medical trials are threefold.
1. The pharmacoeconomic assessment can be a secondary objective of an ordeal designed basically to study safety and efficacy.
2. The pharmacoeconomic assessment can be the most important motive for a medical trial.
3. A pharmacoeconomic evaluation may be done retrospectively using medical records obtained in previous trials.
Once a drug is marketed, either retrospective or extra-prospective pharmacoeconomic research may be designed and performed. Epidemiologic studies are regularly used to assess the efficacy and safety of medications. Epidemiologic information regarding the disorder and treatment under investigation can yield excessively vital data for the financial assessment of a specific drug remedy. Knowledge of the herbal development of disease comorbidities and treatments enables estimation of the variables that could have pharmacoeconomic implications for the cost of illness and quality of life.
Pharmacoeconomic Guidelines:
Researchers and evaluators continue to increase and refine hints for pharmacoeconomic evaluation. The purpose and situation of the proposed recommendations are as follows:
1. Methodologic recommendations might guide researchers to appropriately design, conduct, examine, and report monetary and humanistic critiques.
2. repayment and pricing hints could define the content material, presentation, and evaluation of pharmacoeconomic statistics to decide or justify the rate or repayment of a pharmaceutical product.
3. Approval pointers could set the standards appropriate to a selected government to attain approval to market a new product.
4. Promotional guidelines might set the criteria for using pharmacoeconomic facts in support of pharmaceutical promotion to prescribers and consumers.
Even though the reason for the decision to use pointers is to recognize capability, at present, the science of pharmacoeconomic studies remains growing. It might not be perfect to put in force manual lines that could restrict the development of expertise in this area. Suffice it to say that the substance of any pointers in evolving studies should be well-grounded in appropriate techniques and sound clinical principles.
Challenges of Pharmacoeconomic Research
In the future, we can be mechanically challenged to do pharma-economic studies, even though simply appearing to do the research will not solve all of the issues all the time. To be beneficial, appropriate pharmacoeconomic reviews must be tailored to the precise problem and decision at hand. Our mission, therefore, starts with searching beyond the plain and clear answers. cost-minimization Evaluation is beneficial when comparing interventions with identical scientific and humanistic consequences, but this will be the exception in place of the rule for lots of scientific packages outside of a real, typical substitution. The fee-advantage analysis could, at first glance, be the answer to extra-complex issues in that it might allow for the evaluation of numerous interventions with more than one and varied outcomes. Right here, too, one has to be cautious to be aware of the pitfalls and demanding situations related to changing all of the benefits to economic phrases. How do you locate an economic fee on reduced blood stress and insulin? control, or development in the best of lifestyles?)
allowing effects to remain in herbal and measurable terms The approach of price-effectiveness evaluation can be suitable for plenty of issues and help with many selections when the outcomes of the interventions are measured in the same terms. However, what approximately the affected person and the way the diverse treatments affect everyday living and first-rate existence? Have decisions been made strictly based on supplying a nice medical outcome for the dollars spent? If so, perhaps price-utility evaluation, which takes into consideration patient preference and quality of life, has to be the gold standard of pharmacoeconomic studies. Unfortunately, right here too are the problems of measuring the niceness of life and desire in a converting world.
Gift and future controversies surrounding pharmacoeconomic research also consist of arguments for methodologies of valuation and discounting. What is the most appropriate perspective to take while valuing charges and effects: the affected person, the 1/3-celebration payer, or possibly society? What about ethics? Do we be able to justify our selections completely based on the numbers obtained through scientific studies?
One of the most challenging situations for pharmacoeconomic research lies within the schooling of individuals who are going to be comparing the facts derived from this research. Although the quit users of macroeconomic research records would like to have simple, clear cut answers to their questions concerning the allocation of assets and the healthcare benefits derived from them, in truth, the solutions are quite complicated. Pharmacoeconomic remains an art in addition to a science. Even though the technological know-how may be flawlessly clear, making use of that science ought to be accomplished artfully through the use of professional judgment. Simply as it is impossible to broaden an algorithm for the remedy of a disorder that is suitable for all patients, it might be impossible to broaden one for making macroeconomic choices. In the end, the person responsible for pharmacoeconomic studies statistics must be able to examine the medical appropriateness and robustness of the studies and make a choice concerning their usefulness in a selected scenario. To do this, evaluators will want to recognize the basic principles of pharmacoeconomic studies.
The challenges situations of pharmacoeconomic studies are inexhaustible; many are addressed within the study of this article. The real assignment, however, isn't figuring out the equipment of macroeconomic research, but, rather discovering how and when to apply them.
In undertaking pharmacoeconomic research, diverse strategies are employed to analyze the expenses and results related to pharmaceutical interventions. Not unusual research methods in pharmacoeconomic consist of:
Value-Effectiveness Evaluation (CEA): This technique compares the expenses of various interventions with their results, commonly measured in terms of a commonplace fitness outcome, which includes lifestyle years received or first-rate-adjusted existence years (QALYs).
fee-software analysis (CUA): just like CEA, CUA carries the patient's alternatives for special fitness states, frequently measuring the use of utilities. This allows for an extra nuanced evaluation of interventions in terms of their impact on patient's quality of life.
Cost-benefit analysis (CBA): CBA involves comparing the expenses of an intervention with its advantages, which are normally quantified in financial terms. This approach permits a right-away assessment of costs and benefits to determine if an intervention is economically feasible.
price range impact evaluation: This analysis assesses the financial effect of adopting a new intervention in specific healthcare finances. It makes a specialty of estimating the economic consequences of incorporating a new remedy or drug into the healthcare system.
Modeling techniques: Pharmacoeconomic models, inclusive of choice trees, Markov models, and simulation fashions, are used to assign the lengthy-time period prices and consequences of interventions, especially when long-time period facts are lacking.
The pharmacoeconomic study's consequences offer insights into the financial implications of various healthcare interventions. The outcomes frequently consist of:
Incremental price-Effectiveness Ratio (ICER): The ICER represents the extra fee required to acquire an extra unit of gain (e.g., in step with additional QALY won). This metric allows choice-makers to examine the relative cost of interventions.
Sensitivity Analyses: Sensitivity analyses examine the impact of versions in key parameters on the outcomes. these analyses offer information approximately the robustness of the findings and the degree of uncertainty within the estimates.
Discussion
The dialogue phase of a pharmacoeconomic study includes deciphering the consequences, considering barriers, and contextualizing the findings in the broader healthcare landscape. Researchers often discuss the results of these effects on scientific practice, coverage decisions, and aid allocation. They may additionally compare their findings with those of comparable research and discover reasons for variations.
After a pharmacoeconomic examination, researchers summarize the main findings, restate the consequences, and discuss the examiner's ordinary contribution to the sphere. They may additionally highlight the observation's limitations and endorse regions for future studies.
In short, pharmacoeconomic plays an important role in informing healthcare decisions with the aid of comparing the monetary factors of pharmaceutical interventions. Numerous studies and techniques are employed to evaluate the prices, consequences, and financial prices of interventions. The results and discussions provide treasured insights for healthcare stakeholders to make knowledgeable selections regarding aid allocation and treatment strategies.
The general cost of medical and pharmaceutical care keeps on rising. The delivered value to society, men's or women's healthcare institutions, and sufferers as weighed against value has now not been properly established. The trouble has turned out to be increasingly tough to get dressed due to the lack of information on methodologies for the evaluation of new and present drug therapy.
The completion of this research project would not have been possible without the contributions and support of many individuals and organizations. We are deeply grateful to all those who played a role in the success of this project.
We would also like to thank My Mentor [Naweed Imam Syed Prof. Department of Cell Biology at the University of Calgary and Dr. Sadaf Ahmed Psychophysiology Lab University of Karachi for their invaluable input and support throughout the research. Their insights and expertise were instrumental in shaping the direction of this project.
I at this moment declare that: I have no pecuniary or other personal interest, direct or indirect, in any matter that raises or may raise a conflict with my duties as a manager of my office Management.
The authors declare that they have no conflicts of interest.
No Funding was received to assist with the preparation of this manuscript.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
