AUCTORES
Globalize your Research
Review Article
*Corresponding Author: Amro A. Amara, Protein Research Department, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab City, P.O. Box 21934 Alexandria, Egypt.
Citation: Amro A. Amara (2023), Old, Running and Future Vaccines: Where are we? J, Biotechnology and Bioprocessing, 4(5); DOI:10.31579/2766-2314/112
Copyright: © 2023, Amro A. Amara. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 01 August 2023 | Accepted: 08 August 2023 | Published: 18 August 2023
Keywords: canine mammary carcinomas; cytological grading; cancer stem cells; cancer model
The vaccine technology is in the core of different disciplines include protein engineering, genetic engineering, drug design, immunology and the like. Linking between the past and the present, the old, new and developed art will let us have a full image about the relationships between the antigens and the antibodies, the pathogens and the immune system, the natural and induced immunity. This review introduces some old tactic, running ones and those still under development. Specific examples represent the old traditional pathogenic infections such as cowpox, smallpox, Rabies, Polio, Rinderpest as well as new one such as SARS-COV-2 are included. Other examples are included as well. Selecting the best tactic to be used with particular pathogen might be need some experience due to the ecological, sociological, economic factors and other ones that might influence the choice. This review is recommended for the vaccine design researcher who might located in the developmental countries and give respective amount of information for those who working in developed countries.
There is some sort of classification for vaccines such as the first, second and third generations based on strategies used to prepare vaccines. Or, based on their types such as viable, inactivated, attenuated, vector, protein, mRNA, DNA, subunits, etc. Nerveless, diverse examples are described to highlight different concepts, strategies and possibilities to prepare different kind of vaccines. One could observe crosslinking between dissimilar strategies. For example, current advances in vaccine strategy use the upper lung as a site for vaccination, a strategy was used hundred years ago to a vaccine against smallpox in China (Figure 1).

Figure 1: Vaccine development timeline include some additional important events
The variolation first known as a process of inoculating people with material taken from a vesicle of a person who has smallpox (Turkey, India, Africa and Middle East). Mild infected or recovered patients were also selected as a source. The method is also used for sheep pox. The dried smallpox scabs were blown up the nose of the patient. Later, procedure took form of scratching arms with a needle. Skilled hands variolation induced a mild infection that stimulated producing of antibodies, creating effective immunity against smallpox. Other strategy was used in China. The first extant and available written record about the Chinese method was in a 1695 medical book by Zhang Lu. He described three methods of variolation: putting a piece of cotton imbued with pox pus into nostrils of healthy children, using squama same way when a fresh pustule was not available, and making healthy child wear clothes that had been worn by a child who had contracted disease. After the child was thus variolated, he would have fever in about 7 days, with a slight and benign case of smallpox (Leung, 2011; Lu., 1695). By the end of the eighteenth century, variolation was even divided into two schools, Huzhou School (Zhejiang) which preferred usage of fresh pus, claiming that it was more effective and other school was Songjiang school (Jiangsu) which preferred to use older, medically treated squama: “cooked pox,” claiming it was safer (Zhu-Yiliang, 1808).
The method was popularized in England in 1721–22 by Lady Mary Wortley Montagu; She learned it in Istanbul and transfer it to England. It has long been known by Turks, Chinese, Africa and other peoples. In America, method was transferred from Africa. Cotton Mather learned of its usage in Africa from his slave, Onesimus, who himself had been inoculated. Its usage spread in America after 1721, and in 1728 it was introduced into South America. It was supplanted by vaccination after 1798. In 1842 an act of Parliament in England made practice of variolation a felony in that country (Variolation., 2013).
Whole virus vaccines
2.1. Viable non-specific virus-based vaccine (cowpox)
In 1796, Edward Jenner demonstrated that cowpox conferred immunity against deadly smallpox virus (Baxby, 1981). He may have heard nursery rhyme: “Where are you going to my pretty maid? I’m going a-milking, sir, she said… What is your fortune, my pretty maid? My face in my fortune, sir, she said” (Bailey, 2011). Whole inactivated and live attenuated virus vaccines cause a diverse immunologic response. Live attenuated vaccines are produced by serial passage of the pathogen virus in cell cultures for selecting a reduced replication potential and reduced virulence (still replicate). These vaccines produce strong and long-lasting humoral and cell-mediated immune responses. Meanwhile, they are a source of infection if used in immunocompromised patients. Inactivated vaccines are inactivated by chemicals such as formaldehyde and beta-propiolactone, they cannot replicate, and safe for the immunocompromised individuals but need multiple doses or adjuvants to achieve immunity (Blumental and Debré, 2021; Carneiro et al.; Frederiksen et al.; Hosseini et al.; Nagy and Alhatlani, 2021; Nakagami, 2021; Patel et al.).
2.2. Chemical inactivation, attenuation and evacuation
2.2.1. Inactivated virus-based vaccine
Traces sign that drying and boiling were used early during practicing violation. Salt used to inactivate snakes and scorpions’ bits and the like. Meanwhile Toussaint introduce first procedure and technique to vaccinate against anthrax using inactivated vaccine, defibrinated blood from a sheep freshly dead of anthrax heated at 55°C for 10 min with or without a filtration through paper or adding carbolic acid. From their name, inactivated viruses are viruses that were killed or inactivated by any means that let them inactive but immunogenic. Inactivation is a time-consuming and costly process, and if not, well applied viruses might become non-immunogenic after inactivation (Bayani et al., 2023; Kyriakidis et al., 2021). Meanwhile, they are safe with immunocompromised patients and need less precaution during their storage. While, they are less immunogenic than live attenuated vaccines with the low capability to induce cellular responses, hence they need adjuvant to enhance the immune response, and the use of large amounts of antigen and booster doses to achieve the desired immunity.
The concept of lethal dosage, dilutions, serial concentrations enable using aggressive chemical compounds in concentrations that could reach purposes.
Strategies were used for microbe deactivation include biological, chemical and physical treatments. Examples about chemical compounds used for virus deactivation include: 2,2’-dithiodipyridine, β-propiolactone, binary ethylene imine, formaldehyde, gamma irradiation and glutaraldehyde. Examples about physical conditions that used to virus deactivation include, pH, temperature, UV (Delrue et al., 2012) and H2O2 (Amara, 2015, 2020; El-Baky, 2014) Other tools are existed in various literatures and patents (Monteil and Mirazimi, 2022).
Some of those compounds are produce naturally during the immune system response against pathogens such as H2O2 and the other compounds that have reactive oxygen (antioxidants) (Amara, 2010). Lung produces H2O2 during infections (e.g., Pseudomonas aeruginosa and viruses). Miscellaneous studies were conducted using H2O2 for virus deactivation. Early study by Mentel et al (1977) conducted studies to prove positive effect of H2O2 on different viruses. They have tested its effect on adenovirus types 3 and 6, adeno-associated virus type 4, rhinoviruses 1A, 1B, and type 7, myxoviruses, influenza A and B, respiratory syncytial virus, strain Long, and coronavirus strain 229E was studied in vitro. Using different H2O2 concentration and time of exposure. H2O2 in a 3 percent concentration inactivated all viruses under study within 1-30 min. Coronavirus and influenza viruses were found to be most sensitive. Reoviruses, adenoviruses and adeno-associated virus were relatively stable (Mentel et al., 1977). H2O2 was used to inactivate a group of viruses including YFV, WNV, LCMV, VV and monkeypox virus (MPV) (Amanna et al., 2012). Using bio-critical concentration of a single compound such as H2O2 enables evacuating Newcastle virus from their RNA constituents using same H2O2 concentration which applied to E. coli JM109 and E. coli BL21 (El-Baky and Amara, 2014). West Nile virus vaccine was deactivated using H2O2 (Poore et al., 2017). This approach needs that virus can be grown to high titer in cell culture or other scalable medium such as hens' eggs; that virus can be successfully and completely inactivated using an agent such as formaldehyde or B-propiolactone without destroying immunogenicity. This approach has had successes in form of vaccines such as inactivated polio vaccine (IPV), hepatitis A (HAV) vaccine, and influenza.
2.2.2. Attenuated virus-based vaccine
Attenuated virus-based vaccines are the closest one to the active virus vaccine or natural infection. Usually confers lifelong immunity with strong induction of cellular and humoral immunity. It needs fewer doses than the inactivated vaccine and adjuvant are usually not needed. The major risk factor is the back-mutation that could turn the attenuated virus to active ones. Require special storage conditions to maintain potency (e.g., temperature), not appropriate to be administered in the immunocompromised patients (Bayani et al., 2023; Yadav et al., 2014)
The first vaccine made using attenuation as a concept came in 1879, for chicken cholera (genus Pasteurella) The cultures of chicken cholera lost their pathogenicity and retained “attenuated” pathogenic characteristics over the course of generations and if left for a long time in culture media (aged culture). Pasteur inoculated chickens with attenuated form and demonstrated that chickens were fully resist virulent strain. Pasteur was coined to be first to introduce an attenuated vaccine against chicken cholera, In July 1880. Chamberland wrote a note dated 18 February 1881, in which he described culture of anthrax bacteria in a chicken broth mixed with a small percentage of potassium bichromate and successful immunization of animals including sheep (H., 2011).
Scientists later understand why attenuation happened after aging microbes. Wild virulent B. anthracis carry two large extrachromosomal plasmids, pXO1 and pXO2, which encode for toxin and PGA capsule. Losing one or two plasmids result in reduce virulence factor (which explained as attenuation at that time) (Green et al., 1985; Sterne, 1959; Uchida et al., 1985; Wang and Roehrl, 2005; Welkos, 1991). Later, scientists learning to eliminate some virulence genes from viruses to obtain attenuated or inactive viruses.
Use of a closely related animal attenuated virus that is not well adapted for efficient and widespread replication in humans and therefore does not cause disease, but nevertheless provokes an immune response that protects against corresponding human viruses. The best-known example of this is used of vaccinia virus to vaccinate against smallpox. Development of an empirically attenuated human virus by multiple passages in tissue culture, typically of nonhuman origin, and/or passage in animals. There is safety perspective in attenuated viruses such as risk of reversion. Attenuated viruses were applied for viruses such as polio – oral polio vaccine (OPV), mumps, measles, rubella, and yellow fever. New art use live-attenuated vaccines prepared by knowledge-based manipulation of the viral genome such as HSV and influenza to develop vaccines (Kusters and Almond, 2008).
2.2.3. Evacuated virus and bacteria-based vaccine (viral antigen expressed on the bacterial surfaces)
Perhaps it is easier to inactivate microbes using chemical compounds or other kinds of treatments. But in special cases evacuations and removing whole genome and cytoplasm is an ideal step such as in case of DNA viruses, highly virulence microbes and microbes with special risk virulence factors. Additionally, progress in molecular biology enable expressing of certain antigens on the surface of bacteria. In such case getting rid of responsible genes is a crucial step. Well purified and prepared BGs do not contain genetic material consider to be safe. There is no risk for horizontal gene transfer. Bacterial and microbial surface antigen can induce immunization (Hoffelner and Haas, 2004). Two major protocols are used to prepare bacterial ghosts (BGs), first one is bacteriophage-based E lysis gene protocol. The second one is sponge-like protocol which uses critical concentration of specific chemical compounds and physical parameters enable calculated controlled evacuation process that maintains 3D structure and surface antigen of evacuated microbes.
Additionally, BGs can easily load nucleic acids, proteins and chemicals; therefore, they are suitable for mass production. BGs can target specific cells, such as APCs, HCDEC, and Caco-2, among others. The immunogenicity and targeting of BGs can be used for tumor immunotherapy and vaccines. In addition, BGs system has huge potential as an oral system for intestinal diseases (e.g., colon cancer, IBD). Oral administration reduces efficacy of some drugs. BG-based delivery systems have potential to override this limitation. However, various challenges are associated with clinical applications of BGs. Through surface modifications and genetic engineering, BGs have potential to become powerful delivery vehicles for drugs or vaccines (Chen et al., 2021).
Examples about the use of evacuated microbes include the use of E. coli bacterial ghosts in transferring and expressing of a chimeric hepatitis C virus gene in macrophages (Miri et al., 2015). Vaccines against Hand-foot-and-mouth disease (HFMD) is primarily caused by enterovirus 71 and Coxsackie virus are prepared using E. coli ghost cells (Gong et al., 2020) Fusion antigen displayed bacterial ghost vaccine candidates against infection of Escherichia coli O157:H7 (Cai et al., 2015). Linear VP1 of enterovirus 71 (EVP1) and Coxsackie virus (CVP1) were displayed on the surface of E. coli O157:H7 BGs based on sandwich vector pSOmpA (Pistor and Hobom, 1988). The outer membrane protein A (OmpA) of E. coli was used in order to construct a novel candidate vaccine named EVP1 bacterial ghosts (EBGs) and CVP1 bacterial ghosts (CBGs). Chimeric BGs vaccine candidates elicit a higher mucosal immune response and provide greater protection for host against HFMD. The vaccine candidates also conferred cross-protection against E. coli O157:H7, indicating that BGs can be used as a relatively efficacious vector for vaccine development against HFMD (Gong et al., 2020). It might be interesting to highlight that one could use the Sponge-Like protocol to optimize the microbes’ evacuations from their genetic materials as a general protocol for cells evacuations.
3.1. Example (1) Cowpox, activating the immune system with similar viruses.
The concept of using similar safe virus to vaccines against another virulence one is well known to the immunologist. Close, safe viruses could satisfy the demand for protecting against dangerous ones by activating the immune system, and producing antibodies that could neutralize the other one. Perhaps the most famous example is smallpox. One will be protected against it if he acquires the cowpox virus. The modern vaccine technology starts with simple observation. This observation tells that knowledge might be here and their but need expert to recognize it. The protection against the smallpox by acquiring the cowpox was well known among farmers but less explained until a physician explained it. The milkmaid who was infected in their hand by cowpox is known that she is immune against smallpox infection. She was happy because her face will be beautiful. She starts to song, and a physician hears this song “I shall never have smallpox for I have had cowpox. I shall never have an ugly pockmarked face.” The cow's name is “Blossom”. The physician started to investigate the case, and then he concluded that the infection with cowpox will protect against smallpox. He made manual infection from arm to arm by the cow lymph node (Amara, 2016). After those different vaccines were developed against the smallpox. For example, the modified vaccinia Ankara (MVA, German: Modifiziertes Vakziniavirus Ankara) was developed in West Germany through 572 serial passages. Vaccinia virus had lost over 14% of its genome (could no longer replicate in human cells) (Malacrida, 1989; Mayr et al., 1975; Volz and Sutter, 2017). Developing freeze-dried vaccines in the 1950s made it possible to preserve vaccinia virus for long periods of time (e.g., Dryvax) (Belongia and Naleway, 2003; WHO, 2017). After that, life vaccinia virus grown in chorioallantoic membrane or cell culture. The Texas Department of Health began producing egg-based vaccine in 1939 and started using it in vaccination movements in 1948 (Fenner et al., 1988). Egg-based vaccine was also widely used in Brazil, New Zealand, and Sweden, and on a smaller scale in other countries. Perceptions about its usage include, temperature stability and avian sarcoma leukosis virus (ASLV) in chickens (Fenner et al., 1988). Cell culture was first used in 1931 by Thomas Milton Rivers. After World War II Enders and colleagues developed prolonged virus culture techniques to attenuate viral strains (Enders et al., 1949). The WHO funded work in the 1960s at Dutch National Institute for Public Health and Environment (RVIM). The Lister/Elstree strain grown in rabbit kidney cells and tested in 1973 (Fenner et al., 1988). Two other cell culture vaccines were developed from Lister strain in the 2000s: Elstree-BN (Bavarian Nordic) and VV Lister CEP (Chicken Embryo Primary, Sanofi Pasteur). Six strains of vaccinia were isolated from 3,000 doses of Dryvax and found to exhibit significant variation in virulence. The strain with most similar virulence to overall Dryvax mixture was selected and grown in MRC-5 cells. The virus was passaged in addition three times in Vero cells to develop ACAM2000 which entered mass production at Baxter.
3.2. Example (2) Rabies (inactivation or attenuation with/without inactivation)
Pasteur conducts his experiments using rabbits and transmitted infectious agent from animal to animal by intracerebral inoculations until he obtained a stable preparation. He desiccated spinal cords of infected animals until preparation became almost nonviolent. In order to attenuate invisible microbes, he desiccated spinal cords of infected animals until preparation became almost nonviolent. On July 6, 1885, Pasteur vaccinated Joseph Meister, a nine-year-old boy who had been bitten by a rabid dog. The vaccine was successful and applied on hundreds of other bites (Britannica, 2013). It might be interesting to describe that in Egypt there is a strange folk practice to handle dog bite. They collect hair from the same dog (that bit) in an iron, then heat it till becomes dark and viscous. The product then applied to injury. Perhaps it is the effect of the carbonic acid or might be the heating process enable virus attenuation/inactivation. Modern rabies vaccines are either human diploid cell vaccine (HDCV) which started at 1967 which are inactivated vaccines made using attenuated Pitman-Moore L503 strain of virus (Mastelic- Gavillet et al., 2019; WHO, 2012), purified chick embryo cell culture (PCEC), and purified Vero cell rabies vaccines adsorbed (PVRV) uses attenuated Wistar virus strain using Vero cell line (Encyclopædia-Britannica, 2013).
3.3. Example (3) Polio (inactivation or attenuation with/without inactivation)
In 1935 Kollmer tried a live attenuated virus consisting of a 4% suspension of PV from infected monkey spinal cord, treated with sodium ricinoleate (Baicus, 2012). The first successful demonstration of a polio vaccine was by Hilary Koprowski in 1950, with a live attenuated virus (oral) (Fox, 2013). In 1935, Brodie tried an inactivated vaccine with 10% formalin suspension of PV taken from infected monkey spinal cord; he tried it first on 20 monkeys, then on Californian children. Live attenuated vaccines (OPV) introduced by Albert Sabin (1956) (Baicus, 2012; Sabin and Boulger, 1973). Developing attenuated PV vaccine starts with passages of PV strains in rats and mice followed by passages in cell culture. The virulence of PV strains was reduced. In 1946, Lansing strain is passaged in rats and mice more than 50 times. Enders, Weller and Robins, who passaged same strain in cell culture. (Baicus, 2012). Polio viruses (PVs) could be grouped into three distinct viral types. PV in vitro led to developing vaccines against poliomyelitis include formalin-inactivated vaccine (IPV) by Jonas Salk (1953) (Baicus, 2012). Salk vaccine, IPV, is based on three wild, virulent reference strains. They are, Mahoney (type 1 poliovirus), MEF-1 (type 2 poliovirus), and Saukett (type 3 poliovirus). They grow in a type of monkey kidney tissue culture (Vero cell line), which are then inactivated with formalin.
3.4. Example (4) Rinderpest (different strategies)
Rinderpest is a fatal disease was known since time immemorial in Europe, and Central Asia with mortality range from 90 to 100%. Rinderpest or the Cattle plague (also steppe murrain) caused by Rinderpest virus (group V ((-) ssRNA. It comprises among the famous historical besets that cause destroyed human farm animals (Amara, 2016; Barrett et al.; Pastoret and Jones, 2004). Rindepest, show selectivity in its attack. Some farm/wild animals are affected by virus while others did not. Early trials to immunize animals against rinderpest are conducted mimicking variolation. Alternatively, evaluable strategies at that time were used to attenuate viruses. For example, using bile duct from dead animals to inoculate others. Also, bile duct was used for attenuating purpose. Survived animals were also used as a source for virus that might be less effective. Robert Koch is the owner of the first publication of the practical method of immunizing cattle against the Rinderpest infections. Robert Koch, doing work in South Africa, recommended that cows could be saved by subcutaneous injection of blood serum, from immunized animals, and bile, from an infected animal. This unsafe formula was shortly substituted by the employ of immune serum, and later by mixing of immune serum, and virulent virus. Subsequently, the method was improved by consecutive passages of the bovine virus through goats, which enabled Edwards to produce a compromised vaccine in India in the 1920s. Runs with inactivated vaccines as well occurred. Subsequently, the successful isolation of the virus in cell culture led to the in vitro developing of a weakened strain, and from this producing safe, and highly efficient vaccine (Amara, 2016; Bento et al., 2015; Mortellaro and Ricciardi‐Castagnoli, 2011).
The dissimilar new types of vaccines reflect the development in the genetic engineering, protein engineering, structure-guide vaccine design, nano-bio-technology and some other de nevo approaches have collectively enabled more opportunities to design diverse kind of vaccines that could compete against different kinds of treatment strategies. Those dissimilar strategies find the chance to react actively and effectively during the emergence demand for fast vaccine preparation to face the new unusual epidemic, the COVID-19. That did not mean that the old strategies (as described above) are less effective, but they might be need more time to be developed. In fact, any single idea or method could play a significant role in our battle against the corona virus and their variants. As well as the other viruses that threaten the human life.
The most attractive and sounded approach was the usage of the mRNA-based vaccine. Some strategies were elevated based on our understanding to the virus and the viruses’ variant behavior and the immune system response of dissimilar individuals. Those strategies include the usage of the circular RNA vaccines (Qu et al., 2021; Zhang et al., 2023), chimeric protein-based vaccines (Liu et al., 2020; Su et al., 2021; Xu et al., 2022; Zhang et al., 2021; Zhang et al., 2023), virus vector-based vaccines (Chen et al., 2022; Zhang et al., 2021; Zhang et al., 2023), and nanoparticle vaccines (Cohen et al., 2020) (Figure 3). Some of those strategies, tools, strategies, and the like, are really and need more time and more chances to give their positive effect and to be optimized. One could not judge their full success right now but on the long-term the time will show their success, side effect or other unexpected perceptions.
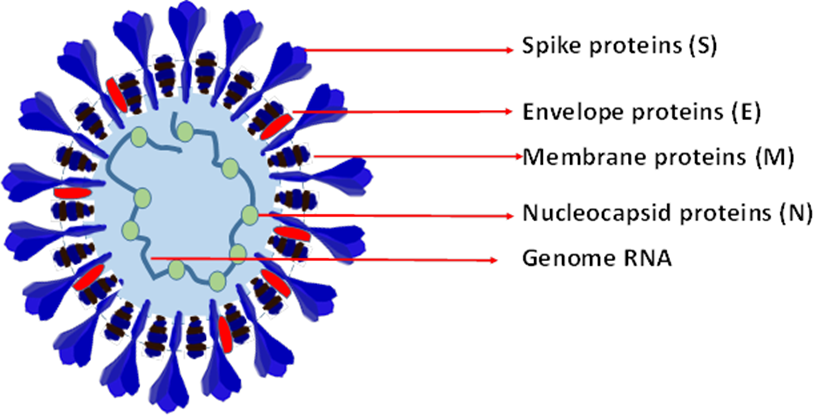
Figure 2: SARA-CoV-2 virus structure and their components

Figure 3: Some important vaccines with different types: 1) the wild type SARS-CoV-2 virus, 2) sttunuated virus, 3) inactivated virus, 4) the virus genome (RNA), 5) one gene (RNA), 6) active mRNA virus vector, 7) inactive mRNA virus vector, 8) mRNA 9) DNA gene, 10) cloning the DNA gene on plasmid (e.g., the gene of the spike protein), 11) expression of the gene in recombinant strains to produce protein used as vaccine
Like rabies, corona virus best choice is to get the vaccine while one still non-infected. COVID-19 vaccine is based on the viruses structural. The most important structure as well function’s part is the spike proteins. Triggering the spike proteins resulting in forming neutralizing antibodies, and T-cell responses (Kantarcioglu et al., 2022).
69.7% of the world population has received at least one dose of a COVID-19 vaccine. 13.32 billion doses were administered globally, and 759,319 are now administered each day. 28% of people in low-income countries have received at least one dose (Updated 8-March 2023) (Mathieu et al., 2021). UK consortium reported that 1 in 5 people who were hospitalized with the disease had a new disability after discharge (Briggs and Vassall, 2021; PHOSP, 2021). Some reports believe that the death rate is higher than the official reports (Adam, 2022). Some believe that time should not be wasting and COVID-19 should be continuously tracked ((Nature), 2022). Developing the vaccines against COVID-19 started as soon as the virus genome was published in early January 2020 (Dai and Gao, 2020; Gorbalenya et al., 2020). Several diverse vaccines were deployed by late December 2020, under emergency use authorization, and mass vaccination campaigns have commenced all around the world. The Pfizer vaccine has full approval as of August 24, 2021, by the US-FDA. In China at 2 Dec 2022 there were eight approved vaccines include, Protein subunit includes, Anhui Zhifei longcom (Zifivax) and Livon Mabharm Inc (V-10). Non-replicating viral vectors include, CanSino (Convidecia) and CanSino (Convidecia Air). Inactivated virus include, Shenzhen kangtai biological product Co (KCONVAC), Sinopharm (Beijng) (Cobilo), Siopharm (Wuhan) Inactivated (Vera Cells), and Sinavac (CoronaVac) and other 35 vaccines are in clinical trial including protein subunit, VLP, DNA, RNA, Non-replicating/replicating viral vectors, inactivated and protein subunits (VIPER, 2023). Sputnik V developed in Russia (combination vector vaccine) was registered on 11 August 2020 by the Russian Ministry of Health (Callaway, 2020).
Generally, the spike proteins generated by diverse approaches result in forming COVID-19 neutralizing antibodies with differential duration of fractions and antiviral spectrum. Whole virion vaccines are derived from chemically or molecularly modified SARS-CoV-2. The viral vector vaccines (VVV) are derived by recombination of genomic sequence encoding trimeric form of spike protein.
RNA vaccines, DNA vaccines, and their hybrid forms. COVID-19 variants cause various pathological responses (Kantarcioglu et al., 2022). Vaccines were tested on many subjects, including young children, immunocompromised patients, pregnant subjects, and other specialized groups (Kantarcioglu et al., 2022).
4.1. Virus-like particle vaccines (VLP).
Virus-like particle’ (VLP) vaccines explore the immunogenicity and safety of empty virus particles presenting several copies of the same antigen on their surface. These are designed to mimic the virus structure, thereby triggering strong immune responses against the antigen(s) presented on their surface. They have good safety profiles because they lack the pathogen’s genetic material. VLPs can be produced in a wide range of production systems, which makes them flexible in terms of production conditions. VLPs vaccines aren’t infectious since they are no viral genomes in them. Oral delivery vaccines could be made from plant-based VLP vaccines. The VLP vaccines are loaded with some proteins at the same time and the degree of immune response induced by it is not clearly known. (Bayani et al., 2023; Deng, 2018; Grgacic and Anderson, 2006; Li et al., 2022). In the mid-1990s, the work of two independent groups led to the self-assembly of L1 human papilloma virus (HPV) protein into VLPs provided the platform for the GlaxoSmithKline and MERCK vaccine design for HPV (Rose et al., 1993). CoVLP is a COVID-19 vaccine candidate developed by Medicago company in Canada and GlaxoSmithKline company in the UK. The VLP are produced by creating a bacterium engineered with genes of the virus, then introducing the bacteria into Nicotiana benthamiana plants. The plants take up the bacteria virus-derived generic material, producing in its leaves. VLP are then harvested and extracted. The method called “molecular farming” or a “plant-based factory”, is rapid, cost effective, large scalability for production, and safety of using plants for pharmaceutical production (Ward et al., 2021).
4.2. Viral Vector Vaccines (VVV)
4.2.1. Vectored or chimeric virus approaches.
This is where an existing virus vaccine can be modified genetically to carry genes encoding antigens from a foreign virus. The chimeric vaccine should retain the attenuation and growth characteristics of the parent vaccine strain but stimulate immunity against the foreign virus. Since viral vectors are common pathogens in nature and that one might acquire it naturally, vector strains that exhibit lower seroprevalence in humans (e.g., chimpanzee Ad, Ad5 and Ad26) are selected to design the vaccine.
4.2.1.1. Nonreplicating VVV
In the nonreplicating vector virus important genes were disabled. Nonreplicating adenovirus (Ad) vectors are the most employed viral vectors. Following the entry of the vector virus into the host cells, the viral vector integrates its genomic code into the host cell nucleus and the S protein antigen is produced by the host cell itself. These expressed antigens generate strong humoral and cellular immune responses without the need for an adjuvant.
4.2.1.2. Replicating VVV
In case of replicating vector virus, some genes that enable it to replicate in infected cells are left. Examples about replicating vector viruses include lentivirus (LV), influenza virus (IFV), MV, MVA, vesicular stomatitis virus (VSV) and Newcastle disease virus (NDV). Because of the replicated potential of these vaccine vector viruses, they are immunogenic and generate robust humoral and cellular-specific immunity. Their use in intranasal formulations may produce better IgA formation and prevent asymptomatic carriage.
ChAdOx1-S, at present named as AZD1222, employs an adenovirus derived from the chimpanzee (to minimize the possibility of interaction with preformed antibodies against adenoviruses). While the E1 deletion blocks the viral replication, the E3 deletion enables incorporating larger genetic cargo into the viral vector. The added sequence encodes for the full-length S protein with a tissue plasminogen activator signal sequence.
Has better safety profiles than live attenuated virus vaccines and is more immunogenic than inactivated virus vaccines. The carrier has a good stimulating response to B cells and T cells and can boost immunity.
In comparison to inactivated vaccines, the manufacturing process for viral vector vaccines is comparatively safe because no live SARS-CoV-2 is involved. Virus vector vaccinations based on adenovirus can trigger side effects, including thrombocytopenia. (Bayani et al., 2023; Holman et al., 2009; Li et al., 2022)
4.3. mRNA based vaccine
Antigens are produced by the host cells just like natural infection by an RNA virus. Degraded within a short time in the body and barely has a chance of changing the genome. The mRNA vaccines can elicit strong Th1 cell responses and GC B-cell responses, and, they produce long-lived plasma cells and memory B cells that can consequently elicit SARS-CoV-2 neutralizing antibodies.
To simplify a timely update because of the elevation of new certain variants, the mRNA vaccines can be directly modified on the original sequence. The mRNA vaccine technology consists of artificial synthesis of the mRNA sequence of the SARS-CoV-2 that encodes the S protein. Incorporating lipid nanoparticles (LNP) into mRNA vaccines protects them from enzymatic degradation and ensures efficient cellular uptake. Following the cellular uptake, mRNA vaccines induce a prompt antigen expression, and the expressed antigens generate both humoral and cellular immune responses. mRNA vaccine needs ultra-cold conditions for long-term storage.
Recently, a broad-spectrum mRNA vaccine, RQ3013, was developed to protect against the VOC. This vaccine consists of mRNAs modified by incorporating pseudouridine and encapsulated in liposomes. These mRNAs code for viral spike proteins that harbor all the mutations detected in VOCs. The vaccine has shown to induce immune responses in various animal models, including primates, hamsters and mice, with high antibody titers that can neutralize the wild type and the α, β, γ, δ and omicron variants of COVID-19. Two doses of the mRNA vaccine showed protection of the respiratory tract from getting infected by the variants. In addition, the vaccine formulation was found to be safe and well tolerated in animal models (Rabaan et al., 2022; Tan et al., 2022). The Low stability of RNA molecules in high temperatures makes global distribution difficult. Complications from mRNA vaccinations, including myocarditis, can be occurred. (Bayani et al., 2023; Heymans and Cooper, 2021; Li et al., 2022; Verbeke et al., 2021; Zhang et al., 2019).
The RNases of animal cells are mainly exonucleases and so circular RNAs are much more stable than the corresponding linear species. Circular RNA-based vaccine induced the production of neutralizing antibodies as well as virus-specific T cells. The circular RNA encodes for the RBD region of the virus spike protein and showed robust protection upon administration in rhesus monkeys and mice. The vaccine sustained antigen production, provided higher and longer-term protection against delta and omicron variants, and could also boost the effects of other vaccines (Qu et al., 2022; Rabaan et al., 2022). circRNAs have possible applications as biomarkers, therapeutic agents, and preventive vaccines in viral infections (Rahmani-Kukia and Abbasi, 2022). Spike protein, namely VFLIP, was engineered by using five proline substitutions in the S2 subunit, a flexible S1/S2 linker, and two cysteine substitutions to introduce an inter-protomer disulfide bond formation (Olmedillas et al., 2021). A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera (Seephetdee et al., 2022).
siRNAs show huge potential in constructing a broad-spectrum vaccine formulation, as they target mRNA and can be artificially modified to target multiple viruses simultaneously. It consists of dsRNA, 20 nucleotides long, which, on entering the host cytosol, modulates the expression of the target gene depending on the sequence complementarity with mRNA (Zamore et al., 2000). The delivery of naked siRNA into pulmonary cells was tried previously through the inhalation route in mice (Bitko et al., 2004; Kandil and Merkel, 2019; Mazzeo et al., 2014). In 2010, it was extended to inhibit syncytial virus replication through the intranasal administration of naked siRNA (ALN-RSV01) through the spray. The treatment showed a significant reduction in RSV prevalence in clinical trials and reduced the risk of developing pulmonary complications post-infection in patients with lung transplants (Gottlieb et al., 2016). These results point out that such a siRNA delivery system could also be applied against all the variants of COVID-19 by constructing siRNA that could target a region conserved in all the variants. Recently, a modified siRNA preparation C6G25S was administered using the aerosol mode to inhibit SARS-CoV-2 variants effectively. This vaccine inhibited all the variants at picomolar concentrations and prevented generating and releasing viral progeny in the lungs. Moreover, it could decrease the viral load by 96% with a concomitant decrease in the virus-induced pulmonary damage, thus providing a practical approach to combat the SARS-CoV-2 variants (Chang et al., 2022; Rabaan et al., 2022).
4.4. CRISPR Based Vaccine Formulations
CRISPR-Cas13d has shown broad-spectrum inhibition activity against various COVID-19 variants. This inhibition depends upon the cRNA co-localization with Cas13d and the target RNA of the COVID-19 variant. Cas13d can also enhance the antiviral activity of small-molecule inhibitors. Using liposome-based RNA delivery, Cas13d can inhibit the COVID-19 variants in human airway epithelium cells. This strategy can work well with both the vaccines and the drugs that fight viruses (Rabaan et al., 2022; Zeng et al., 2022).
4.5. DNA vaccine
DNA based vaccine expressing SARS-CoV-2 Spike-CD40L fusion protein confers protection against challenge in a syrian hamster model (Vohra-Miller and Schwartz, 2022).
This is where a DNA encoding viral antigens plus appropriate expression control sequences is administered directly to the recipient. Expression of the DNA leads to an immune response against the antigens encoded (Kusters and Almond, 2008).
DNA vaccines use plasmids for this purpose. They depend on cloning the SARS-CoV-2 S gene into bacterial plasmids that contain a strong mammalian promoter, such as CMV and/or SV40, followed by large plasmid production in a competent bacterium. The advantages of plasmid DNA vaccines are that they can target and stimulate both humoral and cellular immune responses. They permit for flexible and simple large-scale production and formulation processes over short periods of time, Also, they offer flexibility for multivalence and room temperature storage of the final vaccine. However, because of their low immunogenicity in humans, they need several doses for optimum protection. Long-term risk of carcinogenesis is another perception for DNA vaccines because of integrating plasmid DNA to the host cell. Ease of design and development, relatively inexpensive and scalable, relatively stable at room temperature for storage and shipping. Antigen presentation by both MHC I and II molecules and induce humoral and cellular immune responses. The expression inside the host body may induce the immunological tolerance. Low efficiency probability because of the rapid degradation of naked DNA vaccines by nucleases and diverse biological barriers (Bayani et al., 2023; Khan, 2013).
4.6. Subunits or single protein-Based Vaccines
NVX vaccine against SARS-CoV-2, approved in Canada for adults -CoV2373 (marketed as Nuvaxovid) is a protein-based unable or unwilling to receive an mRNA vaccine (Vohra-Miller and Schwartz, 2022). Subunits or single proteins prepared by recombinant DNA methods and fermentation processes in cell culture (Kusters and Almond, 2008). To produce these recombinant protein vaccines, bacterial expression systems represent the most used technique. These vaccines often need multiple doses and effective adjuvants to obtain a robust immune response. As live-virus handling is not need, the subunit vaccine manufacturing process is safer and simpler, although manufacturing these vaccines can be difficult for mass vaccinations (Blumental and Debré, 2021; Carneiro et al.; Frederiksen et al.; Hosseini et al.; Nagy and Alhatlani, 2021; Nakagami, 2021; Patel et al.). Subunit vaccines available for use include NVX-CoV2373, EpiVacCorona, ZifiVax and FINLAY-FR-2 vaccines. Subunit vaccines have some advantages include their applicability to immunocompromised patients. They are relatively safe with fewer chances of side effects and high-neutralizing antibody titer compared to inactivated virus vaccines Cold chain storage is not required for mass vaccination. Favorable immunogenicity by heterologous or homologous booster dose with some subunit vaccines and efficacy like mRNA vaccines. Their disadvantages include, less immunogenic than live attenuated vaccines, and need adjuvant for stimulating immune responses. The high immunogenic antigen(s) needs to be identified for appropriate efficacy. Multiple doses are required for long-lived immunity (Bayani et al., 2023; Lidder and Sonnino, 2012; Muik et al., 2021; Vartak and Sucheck, 2016).
4.7. Peptide based vaccine
Peptide based vaccine such as CoVac-1 is composed of SARS-CoV-2 T cell epitopes derived from various viral proteins (Heitmann et al., 2021; Nelde et al., 2020), combined with the Toll-like receptor 1/2 agonist XS15 emulsified in Montanide ISA51 VG (Heitmann et al., 2021). Peptides preventing ACE2 binding of the SARS-CoV-2 spike protein, several strategies to prevent the virus from entering the cell are based on interfering with RBD binding to the ACE2 receptor and range from designing peptides derived from the interacting site of the receptors to de novo synthesis of RBD-binding peptides or alternatively, generation of peptides binding the S protein outside the RBD (Cao et al., 2020), Peptides targeting ACE2; ACE2 is a membrane-associated aminopeptidase ubiquitously expressed in the heart, blood vessels, lung, kidney, gut, testis and brain (Hamming et al., 2004); Human defensin 5 (HD5) (Wang et al., 2020); integrin α5β1 (Beddingfield et al., 2021) are also proposed; targeting proteolytic S protein activation. Peptides targeting the fusion mechanism, for more details refer to Schütz et al. (2022) (Schütz et al., 2020).
4.8. Antibody-Based Vaccine Formulations
The neutralizing antibodies isolated from the convalescent sera of COVID-19 patients were recently shown to neutralize many of the existing variants of SARS-CoV-2, thus being termed the Broadly Neutralizing Antibodies (bNAbs). Recently, a combination of 30 antibodies was characterized to offer protection against all the variants of SARS-CoV-2 and the other coronavirus types found in other animals such as bats and pangolins. The antibodies were isolated from 107 COVID-19 patients who had developed hybrid immunity and showed a significant ability to bind to the spike proteins of both SARS-CoV-1 and 2. These antibodies targeted the conserved protein segments common to all the coronaviruses. When mice were given these antibodies and then infected with SARS-CoV-1 and 2, they had less virus in their lungs than mice that had not been given these antibodies (Rabaan et al., 2022; Zhou et al., 2022).
In one of the studies, the convalescent sera collected post-vaccination with the Ad5-nCoV vaccine was used to obtain bNAb against the SARS-CoV-2 variants. A monoclonal antibody termed ZWD12 exhibited efficacy against the Alpha, Beta, Gamma, Kappa, Delta, and Omicron variants through the blockage of the binding of the spike protein with the ACE2 receptor. This mAb provided complete protection against all the variants of SARSCoV-2 in a transgenic mouse model (Chi et al., 2022). In another study, 1737 mAbs were purified from the convalescent sera of a 17-year-old COVID-19 patient (Li et al., 2021). From this pool of mAbs, a mAb termed DH1047 showed broad neutralization activity against not only the SARS-CoV-2 but also the other pre-emerging bat coronaviruses and their variants in mice (Martinez et al., 2021). A mAb known as SP1–77 was obtained from a mouse model in which the B cell repertoire is generated via V(D)J recombination between a human light and heavy chain. This antibody neutralizes all the known SARS-CoV-2 variants through inhibiting membrane fusion (Luo et al., 2022; Rabaan et al., 2022).
Monoclonal antibodies (mAbs) are appealing as possible therapeutics and prophylactics for viral infections. Antibody engineering can be used to strengthen effector function and prolong mAb half-life. Advances in structural biology have enabled selecting and optimizing potent neutralizing mAbs through identifying vulnerable regions in viral proteins, which can also be relevant for vaccine design (Pantaleo et al., 2022). For SARS-CoV-2 patients with immunocompromised or people with mild to moderate COVID-19 who are at high risk of developing severe disease, mAbs can provide an important contribution for vulnerable populations before or after exposure (Kabanova et al., 2014; Zheng et al., 2019).
The spike–ACE2 interaction can be blocked by antibodies targeting the spike receptor-binding domain (RBD) (Taylor et al., 2021). S protein was used to develop neutralizing antibodies. It is expected that generating anti-infective mAbs that are urgently needed will benefit from the recent clinical successes and case studies (Crowe, 2022). The potential for mutations in the viral targets of antibodies to permit viruses to escape neutralization has recently been highlighted with SARS- CoV-2 Omicron VOCs (Choi et al., 2020; Rockett et al., 2022; Vajda et al., 2021; Vellas et al., 2022).
Novavax vaccine, a recombinant nanoparticle vaccine made of a stabilized form of the coronavirus spike (S) protein, to be safe with an efficacy of 89.7% (Bangaru et al., 2020; Heath et al., 2021; Sung et al., 2021). Nanotechnologies induce multivalent display of antigen enhances B-cell responses and can provide longer-lasting immunity than monovalent antigens. Examples include, Mosaic nanoparticles (Cohen et al., 2020; Kang et al., 2022; Royal et al., 2021), Ferritin-based nanoparticles (Ma et al., 2020; Zhang et al., 2023), etc. The principle behind this technique was to build a nanocage consisting of engineered proteins that provide a tagging site for the viral proteins in the form of surface appendages. These nanocages may be modified to display proteins from only one or multiple viruses and may be termed homotypic or mosaic nanoparticles. Upon administration into the host, these engineered nanocages show viral antigenic fragments to the immune system and elicit producing specific humoral and cell-mediated adaptive immune responses (Cohen et al., 2022; Rabaan et al., 2022). The spike protein of COVID-19 was encapsulated within a ferritin nanoscaffold and liposomes. Ferritin monomers undergo self-assembly to generate scaffold systems that were used as adjuvant or drug delivery systems (Bradley et al., 2016; Rabaan et al., 2022). When introduced in primates, this new vaccine, termed Spike Protein Feritin Nanoparticles Vaccine (SpFN), induced both virus-specific B and T cells. The serum obtained from vaccinated animals showed high titers of neutralizing antibodies effective against various COVID-19 variants. Two doses of SpFN (50 ug) within a 28-day interval between them induced a TH1 response and generating neutralizing antibodies against the wild-type viral strain and its variants. Inducing humoral and cell-mediated immune responses inhibited virus replication in the upper and lower respiratory tracts in non-human primates (Joyce et al., 2022; Rabaan et al., 2022).’
In t-cell-based vaccines design is used to cover the shortage happened because of the antibodies number decrease through time or the inability to confer correct immune response because of illness or an emergence temporal health condition. Circulating antibodies may be short-lived, or of low magnitude and/or potency, T cells have an important role for COVID-19 outcome and maintenance of SARS-CoV-2 immunity. Peptide vaccine (Heitmann et al., 2021), MVA-S (García-Arriaza et al., 2021; Routhu et al., 2021), etc.
Dendritic cells are tailoring innate and adaptive immune responses against viral infections. SARS-CoV-2 infection leads to a rapid reduction of host DCs along with T-cell function abnormalities. These alterations can harm the induction and persistence of immunological memory and the preparation and efficacy of vaccines. (Galati et al., 2022; Sabado et al., 2017). Studies were established the clinical safety and potency of DC- based vaccines to activate NK cells, CD8 and CD4+ T lymphocytes (Sabado et al., 2017). DCs can move between lymphoid and non-lymphoid tissues and can modulate cytokine and chemokine secretion. Regulating lymphocyte homing and inflammation, represents a fundamental feature for immunotherapy (Mastelic- Gavillet et al., 2019). Personalized vaccines employing ex vivo generated DCs, principally obtained from peripheral blood mononuclear cells (PBMCs), mo- DCs, or CD34+ hematopoietic stem cell progenitors loaded with dissimilar Ags, were extensively investigated in numerous preclinical and clinical studies (Galati et al., 2022; Sabado et al., 2017).
Since 2009 Corona virus show three epidemics, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS)” and SARS-CoV-2 “COVID-19”. In six months (End of August 2022 to 25 February 2023) more than 300000 death were reported. Some comment that SARS-CoV-2 might become endemic. Large economical loses and social problems are reported. All the world collaborates to face this sever attack. Governments, ministries, organization (WHO/FAO/etc.,), groups CDC, ECDC, public organizations, etc., collectively collaborated to install a successful strategy that could be accepted and applied everywhere. The sever sudden attack elaborate the new progress in the science, technology and informatics particularly in the field of the molecular biology, genetic engineering, nanotechnology, informatic, bioinformatic and the like. The brains of thousands of researchers everywhere were sparked to find solutions. All those positive points and other did not capacitate the virus. There is a real need for installing advanced remote sensing points, advanced strategies and policies to warn us from any new attack happened by a new variant. Some basic activity must be considered like avoiding the contact with wild animals. Vaccines prove to be the best solution particularly with our modern lifestyle which did not give us a trained immune system. New strategies and approaches were applied particularly the immunopeptidome, gene surveillance, vaccine mixing and matching approach, and the like. The new vaccines strategies include, DNA, mRNA, VVV, VLP, protein subunit and peptides. However, some other strategies were used as in the text. During the human live many other strategies were used some of them could be used nowadays as well like using the upper lung as a site of vaccination. Other strategies still need more chances like viral evacuations and using BGs expressed viral epitopes on their surfaces. Beside the vaccines, non-pharmaceutical activities are proved to be useful in reducing the virus transmissions. Perhaps, the most important point is the avoidance of any contact with the wild animals either directly or indirectly through our domesticated and farm animals. Other idea still waits for a chance include strategies for avoiding transfer the viruses from the wild animals. Or even vaccinating might wild animals in our backyards. More studies are needed to understand the role of excessive sanitization and losses due to our modern life style. Linking between the old and the new protection strategies and vaccination arts could lead to developing new vaccines and developing simpler strategies to protect us and let us to win the games against the viruses.
The authors declare no conflict of interest.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
