AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Fatiha Tasmin Jeenia, Department of pharmacology, Chattagram International Medical College, Shamsherpara, Chandgao, Chattogram
Citation: Fatiha T Jeenia, Ferdaush A Sojib, S Rahman, Tasneem Ara, Rafiquzzaman Khan, Jamal U Tanin6 (2022). Neuroprotective Effect of Vitamin B6 and Vitamin B12 against Vincristine- Induced Peripheral Neuropathy: A Randomized, Double- Blind, Placebo Controlled, Multicenter Trial. J. Pharmaceutics and Pharmacology Research. 5(5) DOI: 10.31579/2693-7247/080
Copyright: © 2022 Fatiha Tasmin Jeenia, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 19 March 2022 | Accepted: 29 March 2022 | Published: 20 April 2022
Keywords: vincristine induced peripheral neuropathy; chemotherapy induced peripheral neuropathy: acute lymphoblastic leukemia; neuropathy; neuroprotection; vincristine; vitamin b6; vitamin b12
Background: Vincristine leads to development of debilitating neuropathy in 40-45% patients with resultant compromised efficacy of chemotherapy, suboptimal treatment and worse prognostic outcome. Vitamin B6 and vitamin B12 improves non- oncological neuropathies. Therefore, this study investigated vitamin B6 and vitamin B12 to prevent vincristine- induced peripheral neuropathy (VIPN) by reducing incidence, absolute risk, relative risk, severity as well as delaying the onset.
Methods: Patients with ALL undergoing induction phase were randomly assigned into intervention or placebo arm in a double- blind manner. Vitamin B6 (25 mg Pyridoxine) two tablets were given three times daily for 5 weeks. Vitamin B12 (500 μg/ml Methyl cobalamin) was administered intravenously on day 1, 3 and 5 of every week for 5 weeks during induction period. Placebo arm received oral and intravenous placebo for same duration. Patients were evaluated on the outset of every week by FACT/GOG-NTX questionnaire. Severity was assessed per NCI-CTCAE grading scale.
Results: 102 patients were enrolled. Among them 81 completed the study, where 42 received vitamin B6 and B12 and 39 received placebo. There was significant difference in incidence of neuropathy between arms (26.19% intervention arm, 56.41% placebo; P-0.01). Relative risk of neuropathy was significantly (RR-0.46) lower in intervention arm. Besides, absolute risk reduction (ARR) was 30% and relative risk reduction (RRR) was 54%. NNT was 3.33. Significant trend was observed in difference of severity of VIPN between groups (P-0.03). No significant difference observed in between arms for time to onset of neuropathy.
Conclusion: Vitamin B6 and vitamin B12 significantly reduced the incidence, relative risk and severity of VIPN. NNT was encouraging too. Henceforth, the status of vitamin B6 and vitamin B12 as neuroprotective agent against VIPN can be recommended as a promising one.
This trial was registered in Clinicaltrials.gov on 4th October 2016 with ID: NCT02923388.
Chemotherapy Induced Peripheral Neuropathy (CIPN) is one of the most challenging and complex complications of chemotherapy [1]. CIPN is observed in up to 80% of patients [2]. Chemotherapeutic regimen completion with proper dose and adequate duration is crucial in determining the survival of patients with cancer. Unfortunately, CIPN significantly limits the dose and numbers of chemotherapy and may lead to premature termination of treatment [3]. However, these dosage adjustments affect the prognostic outcome and increase chance of relapse. Moreover, neurotoxicity has negative impact on patient’s quality of life, particularly on daily living activities [4].
Acute lymphoblastic leukemia (ALL) is most common and accounts for three fourths of leukemia cases [5]. Vincristine is an integral constituent of the regime recommended for treatment of acute lymphoblastic leukemia. It is one of the most neurotoxic antineoplastic medicines [6]. Vincristine Induced Peripheral Neuropathy (VIPN) accounts for around 45% of neuropathy incidence [7].
Two clinical trials attempted to explore the efficacy of ACTH (4- 9) analogue in preventing VIPN. The pilot study showed promising result, but a large double blind trial annulled the possibility with its negative result [8, 9]. One isolated study evaluated glutamic acid but no recommendations can be drawn [10]. Another trial by Kautio et al., (2009) demonstrated no efficacy of amitriptyline in amelioration of VIPN [11].
Vitamin B12 plays a fundamental role in neurological function [12]. It maintains the integrity of neuronal membrane [13, 14]. Methylcobalamin is one of the two coenzyme forms of vitamin B12. Intravenous methylcobalamin has no interference with the action of other drugs and is relatively well tolerated [15]. However, chemotherapeutic agents may render vitamin B12 inert that leads to deficiency [16]. The neurologic sequel is peripheral neuropathy [12] which may lead to decline in physical functions and disability in patients [17]. On the other hand, vitamin B6 is an essential cofactor synthesis of myelin sheath [18]. Besides, vitamin B6 has potential antioxidant property [19].
Broadly, VIPN develops as a result of two proposed mechanisms, which include neurodegeneration and oxidative stress [2]. Eventually, both vitamin B6 and vitamin B12 have significant role in synthetic pathway of myelin sheath and maintenance of its integrity [13, 18]. Moreover, these vitamins also got antioxidant activity to some extent [19, 20]. Five distinct case reports has been published between 2004 to 2016 figured out the potential efficacy of pyridoxine and pyridostigmine in treating VIPN with cranial involvement [21-25].
Clinical experience shows that CIPN is a difficult problem to deal with. Different strategies to prevent CIPN have been assessed in cell and animal models, though none has reached clinical application. To date no effective strategy to prevent or treat CIPN is available [26]. In the premise of scarce number of RCTs to alleviate VIPN and encouraging results of few case reports generated enthusiasm to conduct the present study. Considering the potentials of vitamin B6 and vitamin B12, this randomized, double-blind, placebo-controlled multicenter trial was undertaken to investigate the effect of these vitamins in reducing VIPN in ALL patients.
Study sites and participants
This study was conducted in Bangabandhu Sheikh Mujib Medical University (BSMMU) and Dhaka Medical College Hospital (DMCH). Prior to the study conduct, the research protocol sought approval from the Institutional Review Board (IRB) of Bangabandhu Sheikh Mujib Medical University (BSMMU). The study protocol, questionnaire and informed consent form as well as the methodology of the study was reviewed thoroughly by the institutional review board of BSMMU. Eligibility of each patient was assessed and they were informed about the intervention and the study objectives. They were informed that there is least possible chance of any harm to the patient by inclusion in this study and if any complication arises due to the intervention, they will avail treatment for that complication free of cost. Patient was also informed that they can participate in this study by their free will and also they are free to refuse to participate or to withdraw at any time without compromising their medical care. Patients who took part in the study willingly with written consent interviewed and patients’ confidentiality was strictly maintained. Patient’s personal data regarding name, age, sex and other information was not disclosed anywhere and used for research purpose only.
The study protocol was approved by Institutional Review Board of Bangabandhu Sheikh Mujib Medical University (BSMMU) in compliance with the provision of Declaration of Helsinki and Good Clinical Practice guidelines. This trial was registered in clinicaltrials.gov and trial ID is NCT02923388. Patients were informed about the investigational nature of the study and signed informed written consent.
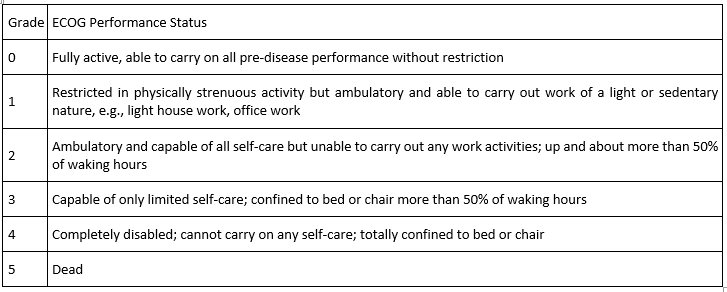
Newly diagnosed acute lymphoblastic leukemia patients of 18 years or older going to receive induction phase of chemotherapy were eligible for this study. Patients were required to have an ECOG performance status of 0- 3. Patients were not allowed on trial if they had a pre- existing peripheral neuropathy of any grade; were receiving anticonvulsants, antidepressants, opioids, vitamin E or any other agents specifically being given to prevent or treat neuropathy. Pregnant women, nursing mothers and patients with head neck tumor were excluded.
Study Design
This study was a randomized, double- blind, placebo- controlled, multicenter trial comparing vitamin B6 (pyridoxine) and vitamin B12 (methylcobalamin) versus matching placebo for 5 weeks. Randomization, blinding, sequence generation was done prior to patient enrollment.
Randomization and Sequence Generation
After determining the sample size, patients were randomly allocated into two arms prior to the patient enrollment for the study. Randomization was done by online graph pad software by using computer. Necessary inputs were given to the software regarding sample size, number of sets required and the software automatically generated two distinct sets of random numbers.
Online graph pad calculator also generated the sequence of patient numbers while procreating random numbers and thus equally distributed the patients into two comparable groups. The randomization and sequence generation process was conducted by a competent third person, a distinguished Professor of University who has no relationship with this research. The probability of an individual to receive intervention was made independent of the plausibility of any other individual receiving the same.
Blinding
Immediately after randomization and sequence generation, random numbers of the two sets were assigned as patient code number. One set was designated as intervention group and another set was placebo group. Then the set of code numbers that belongs to the intervention group were written as patient ID numbers on the packages contained methylcobalamin and pyridoxine. On the other hand, the set belongs to the placebo group were designated as patient ID numbers on the packages contained placebo. This total procedure was conducted by the persons unrelated to this research. Thus the participants, caregiver, outcome assessor and the analyst, who require being blind for such study, were effectively blinded from the knowledge about intervention allocation.
Allocation Concealment
In order to prevent selection bias, concealment of allocation was done. Third person allocated two distinct sets of random numbers into intervention group and placebo group. After entering into this trial, according to that the participants were assigned into their respective group. This allocated code was recorded in two paper documents and in two separate pen drives, which were sealed within two different envelops. The sealed envelopes containing paper documents and pen drives were preserved to another two distinguished Professors not involved in the randomization and blinding procedure. Thereby, intervention allocation was not known to any person involved in the research in advance or during enrollment.
Patient Enrollment and Intervention
After taking informed written consent patient enrollment started. Patients on intervention arm were provided with pyridoxin (Vitamin B6) 2 tablets 3 times daily and one ampoule methylcobalamin injection (Vitamin B12) on day1, 3 and 5 of every week for 5 weeks starting from the 1st day of chemotherapy. Each tablet contained 25 mg of pyridoxine and each ampoule contained 500 μg of 1 ml methylcobalamin. On the control arm patients got similar amount of placebo tablet and injection at same duration. Each placebo tablet contained placebo and injection placebo was in similar amber colored ampoule contained 1 ml of normal saline.
Vincristine Chemotherapy
Patients received vincristine according to standard Berlin-Frankfurt-Münster (BFM) protocol and MRC UKALL X protocol at a dose of 1.4 mg/ m2 of body weight. Maximum 2 mg of vincristine was administered to a patient according to body weight.
Patient assessment
Patients were assessed for neuropathy at baseline and at the outset of every week during the induction period for 5 weeks. Development of neuropathy was assessed by 11- item Functional Assessment of Cancer Therapy Neurotoxicity (FACT- NTX) questionnaire. Severity of neuropathy was graded according to National Cancer Institute- Common Toxicity Criteria for Adverse Events (NCI- CTCAE) Neurotoxicity grading scale version 4.
Outcome measures
The primary outcome measure for this study was to compare incidence of neuropathy between pyridoxine, methylcobalamin treated group and placebo group. Secondary outcome measures were determination of absolute risk, relative risk, risk reduction of neuropathy in pyridoxine, methylcobalamin treated group in comparison to placebo group and comparison of severity and time of onset of neuropathy in between groups.
Statistical analysis
This study, planned to accrue 102 patients, was designed with 80% power (β= 0.20) to demonstrate a statistical reduction of 25% of the neuropathy events in vitamin B6 and vitaminB12 treated groups. All the raw data were recorded, processed and analyzed by using computer software SPSS version 23.0. The statistics used to analyze the data were chi- square (x2) test, independent t- test, Fisher’s exact test, Kaplan- Meier analysis and log rank test. The chi- square (x2) test was employed to compare some patient baseline characteristics and incidence of neuropathy. Data implies independent t- test for analysis were patient age, ECOG performance status, FACT- GOG- NTX neurotoxicity scoring. Furthermore, Fisher’s exact test was performed to compare severity of neuropathy. Kaplan- Meier analysis and log rank test was done to detect the delay in onset of neuropathy. P value <0>
Ecog Performance Status
Accrual and Evaluability
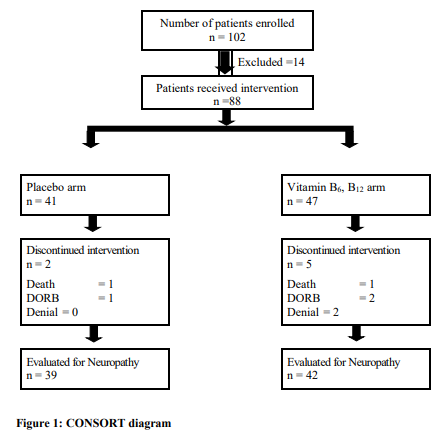
102 patients were accrued. 14 patients were excluded based on the eligibility criteria for the study. Among remaining 88 patients, 47 received intervention and 41 received placebo. 7 patients were dropped out during the research due to the reasons like, DORB (n= 3), denial to take medication (n= 2), death from the disease complication (n=2). For these patients, determination of the study end point was not possible and therefore they were excluded from the per-protocol-analysis. At the end of the research the evaluable patients for neuropathy were a total of 81 [Figure 1].
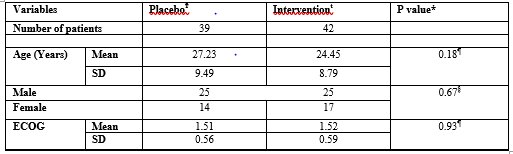
Patients Characteristics at Baseline
Table I shows the demographic and clinical characteristics of patients at baseline. There was no significant difference between arms in age, gender and ECOG performance status.
ϮPlacebo tablet and injection given
ŧVitamin B6 and B12 given
¶Independent t- test was done
§Chi- square (x2) test was done
*P ≤0.05= statistically significant
Incidence of Neuropathy
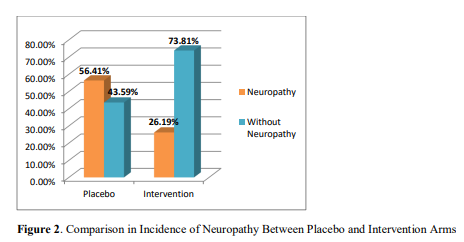
Figure 2 demonstrates that in placebo arm 22 patients out of 39 developed neuropathy, whereas in intervention arm, 11 patients out of 42 developed neuropathy. The incidence of vincristine induced neuropathy in placebo arm was 56.41%, which was 26.19% in intervention arm. Difference in the incidence of neuropathy between placebo and intervention arm was statistically significant (p=0.01).
Absolute Risk Reduction (Arr), Relative Risk Reduction (Rrr) and Number Needed to Treat (Nnt) Of Placebo and Intervention Arms
Table II demonstrates the Absolute Risk Reduction (ARR), Relative Risk Reduction (RRR) and Number Needed to Treat (NNT) of the given intervention. Absolute Risk Reduction (ARR) for this study is 30%. Relative risk (RR) for this study is found to be less than 1, which indicates that risk of occurrence of neuropathy is significantly less in intervention arm than in placebo arm. Relative risk reduction (RRR) is 54%.The calculated Number Needed to Treat (NNT) is 3.33. Therefore, approximately three patients need to be treated with vitamin B6 and vitamin B12 in order to prevent neuropathy in one patient.
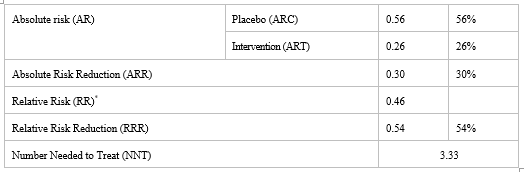
*RR < 1>
Neurotoxicity Scoring By Fact/Gog-Ntx Subscale Score
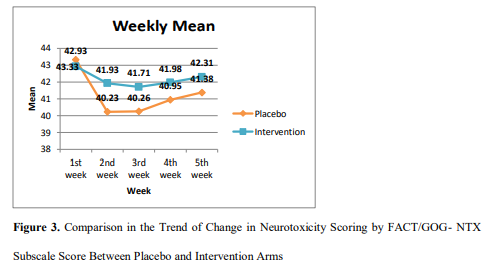
The observed values of neuropathy assessment per FACT/GOG- NTX questionnaire and trend of change are shown in Figure 3. On 1st week, the baseline neurotoxicity score difference was not significant between arms (p=0.16). However, on assessment of 2nd week (after 1st dose of vincristine), the score of placebo arm was significantly (p=0.01) lower than intervention arm. Consequently, on assessment of 3rd (p= .01), 4th (p= .03) and 5th week (p= .04), the difference between score of placebo and intervention arm were consistently more significant.
Severity of Neuropathy According to NCI-CTCAE Grading Scale
Severity of neuropathy according to NCI-CTCAE scale has been shown in Figure 4. There was statistically significant (p =0.03) difference in the severity of neuropathy between intervention (tablet vitamin B6 50 mg and injection vitamin B12 500 µg) group in comparison to placebo according to NCI-CTCAE grading scale.
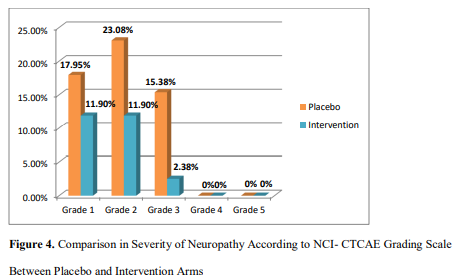 |
| Figure 4. Comparison in Severity of Neuropathy According to NCI- CTCAE Grading Scale between Placebo and Intervention Arms |
NCI- CTCAE Version 4.0
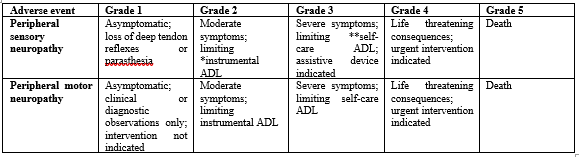
Activities of daily living (ADL):
*Instrumental ADL refer to preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc.
**Self-care ADL refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden.
Time of Onset of Neuropathy
The overall comparison in time of onset of Grade 1, 2 and 3 neuropathies between placebo and intervention arms showed no significant difference in the Log Rank
Neuroprotection is a tempting alternative to prevent neuropathy induced by cancer chemotherapeutic agents. Review of the existing literatures regarding this alluring option reveals inconsistent results left with almost no recommendation. This trial evaluated the neuroprotective potential of vitamin B6 and vitamin B12 against vincristine- induced peripheral neuropathy.
One of the primary endpoints of this study was to determine the incidence of neuropathy in intervention and placebo arm. However, the incidence of neuropathy was significantly lower in the patients treated with vitamin B6 and vitamin B12 (26.19%) in comparison to the patients treated with placebo (56.41%). The incidence of VIPN in placebo arm corresponds with the findings of previous studies like, 43%, 45% and 48% [27, 7, 28]. The positive findings of previous two studies conducted with vitamin E and omega- 3 fatty acid appeared to reduce the incidence of neuropathy in cisplatin and paclitaxel treated patients actually support the finding of the present study [29,30]. Though, the negative finding of two studies conducted with amifostine against incidence of paclitaxel- induced peripheral neuropathy not in inclination with the present study [31,32]. In addition, vitamin E while attempted in patients received taxanes and platinum compounds, there was no significant reduction of neuropathy incidence [33].
Besides the significant result of the present study regarding incidence of neuropathy, the risk reduction analysis which is a powerful tool for interpreting randomized controlled trial also demonstrated significant positive findings. Vitamin B6 and vitamin B12 resulted into “Absolute Risk Reduction (ARR)” and “Relative risk reduction (RRR)” of vincristine- induced neuropathy by 30% and 54% respectively. The Relative Risk (RR) was 0.46, which indicates significantly lower possibility of developing neuropathy in intervention than placebo. Similar result regarding significant risk reduction by vitamin E and oxcarbazepine was observed in prevention of cisplatin and oxaliplatin- induced peripheral neuropathy [34, 35].
Regarding FACT/GOG-NTX questionnaire scoring, the graphical presentation from first week towards second week showed a drastic fall in placebo arm, which is much smaller fall in intervention arm indicating more worsening in placebo arm than intervention. Consequently, significant difference between vitamin B6 and vitamin B12 treated patients and placebo arm was observed in second, third, fourth and fifth week, which might be explained by the fact that more patients appeared to develop neuropathy with worsening of neuropathic symptoms (Grade 2 and Grade 3) in placebo arm. Guo et al., (2013), who studied with oral alpha lipoic acid to alleviate neuropathy by platinum based compounds, could not reveal any statistically significant difference between placebo and intervention [36]. However, Hershman et al. (2013) reported worsening of CIPN in acetyl- L carnitine treated group indicating contraindication of acetyl -L carnitine in neuropathic symptoms induced by taxanes [37]. The current study revealed safety of vitamin B6 and vitamin B12 as these vitamins have not worsened vincristine- induced neuropathy.
Another objective of this trial was to compare severity of neuropathy between two arms. Significant difference has been observed in severity of neuropathy between placebo and intervention arms. This particular result is consistent with Milla et al. (2009) who reported significant reduction in severity with glutathione in oxaliplatin treated patients [38]. Aside than that, Ishibashi et al., (2010) where difference of severity of neuropathy between the groups asserted as insignificant when calcium and magnesium was investigated against oxaliplatin- induced neuropathy and findings of Leal et al. (2014) which showed an insignificant reduction of severity by glutathione in paclitaxel and carboliplatin treated patients not in concordance with this present study [39,40].
However, the Kaplan- Meier Curve for the onset of neuropathy corresponds to the log- rank test which showed insignificant difference in the time of onset of neuropathy between two arms. Study finding of Chay et al., (2010) demonstrated an insignificant delay in the time of onset of neuropathy which is compatible with the present study [41]. Grothey et al., (2011) concluded with a significant delay in the onset of neuropathy in intervention arm [42]. These studies were investigating the role of calcium and magnesium in prevention of oxaliplatin- induced neuropathy and were aborted prematurely as calcium and magnesium reported to interfere with the anti-tumor efficacy of oxaliplatin. The conceptual strength of this regime is that theoretically as well as in the present study; vitamin B6 and vitamin B12 did not interfere with the antitumor activity of vincristine.
This study has not evaluated the autonomic neuropathy, a common side effect of vincristine chemotherapy that is more pronounced in adult patients [43]. Risk factors of developing neuropathy like, diabetes mellitus, alcohol consumption, preexisting neuropathy also needs to be taken into consideration [44, 45].According to Quasthoff and Hurtung, (2002), vincristine more than 4mg is considered as the neurotoxic cumulative dose [26]. In this study, all the patients received vincristine at a dose of 1.4 mg/m2 with an upper limit of 2 mg and therefore below the recognized neurotoxic dose. Besides, patients with history of diabetes mellitus, neuropathy and alcohol abuse were excluded from the study.
Genetic variation is evident in development of VIPN [46, 47]. Thereby, analysis for genetic variant of VIPN might be beneficial to determine the susceptibility of neurotoxicity in this population.
Moreover, this study has not utilized the maximum tolerable dose of vitamin B6 and vitamin B12. Thereupon, dose escalation might be another preference as both vitamin B6 and vitamin B12 are water soluble, non- toxic vitamins.
This trial was endeavored with two of the commonly used, safe and inexpensive medicines of peripheral neuropathy with an endurance to generate a potential candidate for preventing VIPN. In this study, vitamin B6 and vitamin B12 have explicitly and unquestionably reduced the incidence, severity and the risk of vincristine- induced neuropathy in terms of both absolute and relative risk.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
