AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Hassan A. Al-Shamahy, Faculty of Medicine and Heath Sciences Sana'a University P.O. Box 775 Sana'a, Yemen.
Citation: A Alshamahi EY, Al-Shamahy HA, A Al-Moyed KA, Hizam Al-Arosi SA. (2022). National Comprehensive Trachoma Treatment Campaign: Community Monitoring of Mass Drug Administration (MDA) Coverage and Practices. J. Clinical Research and Reports. 11(2); DOI:10.31579/2690-1919/245
Copyright: © 2022 Hassan A. Al-Shamahy, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 24 March 2022 | Accepted: 15 April 2022 | Published: 25 April 2022
Keywords: coverage; mass drug administration (MDA); mass treatment; monitoring; national campaign; trachoma; Yemen
Background: Trachoma is a communicable infection of the eye by certain strains of the Chlamydia trachomatis. It is the principal cause of loss of sight globally. Mass drug administration (MDA) with azithromycin is a foundation stone of World Health Organization (WHO)’s global struggle to eradicate trachoma by 2020.
Aims: The main objectives of the campaign's third monitoring are to check improvement of interventions and improvement of quality across times and activities implemented in seven selected districts of Ibb and Al-Hodeida governorates, Yemen.
Methods: A community based cross-sectional coverage survey was performed. 68 divisions were selected per selected districts of the two governorates. A disconnect Results Entry Form for each district surveyed was finished, saved and uploaded directly into the online Coverage Survey Analysis Tool to check improvement of interventions and improvement of quality across times and activities implemented.
Results: The national campaign for MDA covers 966 villages in 6 districts of Ibb and Al-Hodeidah governorates by 1932 healthcare workers. A total number of beneficiaries who were monitored from 476 homes reached 3,077, of whom 2,755 (90%, coverage rate) took the dose. The availability rates of trachoma medicines were ranged from 82% to 91%. The improper arrangement of treatment sessions rate was 17%, the incorrect position of the dose pole was 6%, while the correct records of drugs scored the rate of 99%, and the proper storage of drugs rate was 70%. The community collaboration during the treatment campaign the acceptable rate was 92%, while 3% exhibited unacceptable behaviors towards the treatment campaign.
Conclusion: In this survey, the national campaign for MDA in the 966 villages reach the target threshold (i.e. 90%) for effective coverage; with proper rates for the availability of trachoma medicines, good people reactions towards taking treatment, and the community collaboration; while bad rates for the improper arrangement of treatment sessions, the incorrect position of the dose pole and the preparation dosages by MDA team. Hence, programmatic enhancement should be made for the future campaign to achieve the estimated thresholds.
Trachoma is an infectious eye infection produced by certain strains of the chlamydia trachomatis bacteria. Active infection frequently starts at some stage in infancy or childhood and can become chronic. The bacteria are distributed by direct connection with eye and nasal secretions from infected individuals, or by contact with fomites (that is, nonliving objects that carry infectious agents, for example towels or washcloths) or by eye-seeking flies (mainly Musca sorbens). It is the main contagious cause of blindness and is endemic in 53 countries. An estimated 325 million people live in areas where they could be exposed to trachoma, and more than 7 million suffer from trichiasis, the last painful stage of this eye disease [1-3]. In the past three years, researchers in Yemen have become more interested in studying and investigating eye diseases, which include the problem of trachoma, as Yemen is one of the regions endemics with trachoma 4-12. From September 2013 to March 2015 a fieldwork was undertaken cluster-sampled survey in each of 42 evaluation units (EUs) including 166 rural districts of nine Governorates (Al Jawf, Al Hodeihah, Adh Dhale’a, Hadramoot, Lahj, Ma’rib, Taiz, Hajjah, Ibb) by means of the Global Trachoma Mapping Project systems and methodologies. The TF prevalence in children aged 1–9 years was ≥10% in two EUs (7 districts) and 5–9.9% in six EUs (24 districts). In adults aged ≥15 years, trichiasis prevalence was ≥0.2% in five EUs (19 districts). The surveys revealed that more than 2.7 million people in 30 districts need public-health interventions to deal with transmission of the trachoma and its associated morbidity [10]. Also, in a study by Al-Shamahi et al. [9], the prevalence of active trachoma (TF) was 10.93%, which is slightly higher compared to the previous study conducted in Yemen [10].
Researchers have found that it is possible to eliminate blinding trachoma by apply an integrated package of interventions - the so-called "safe strategy" which means: surgery for trachomatous trichiasis, treatment with antibiotics to clear an ocular infection; facial hygiene to decrease the transmission of chlamydia trachomatis in the eyes; and improving the environment, especially improving access to water and sanitation [1-12]. It has been shown that antibiotic treatment for people with trachoma helps prevent the disease from spreading in the community. Presently, azithromycin (Zithromax) is the optional for mass drug administration (MDA). In trachoma endemic areas MDA should be performed annually for a period of three to five years. A coverage survey is necessary to follow movement towards the goals of the program and to find communities with poor coverage in order to allow for appropriate and timely action [2,3]. It is important that MDA is used for the whole population to prevent, control or eliminate neglected tropical diseases (NTDs) such as trachoma, as the drugs (azithromycin for trachoma) are given periodically - using a campaign approach - to all at-risk populations in the area, and the rate is high. Therapy coverage is very crucial for MDAs: the greater the spread of infection, the more imperative it is to achieve high coverage rate. The World Health Organization commends that 80% of the target population should be reached with MDA at last. Country programs normally report treatment coverage by take from the number of azithromycin doses lasting in stock after MDA from the MDA target group, or by gathering reports from drug distributors. Although both methods are better than doing nothing, it is important to routinely verify the accurateness of reported coverage numbers, as they are subject to management and error [2,13,14].
Estimates of drug coverage from population surveys may raise the comprehension of the factors influence MDA efficacy. The survey results should provide valuable information on the existing gaps for projects aimed at preventing and eradicating the disease. Future MDA rounds will be able to take into account the results of this survey and enable trachoma control programs to reach target populations that may have been missed during previous rounds of MDA [13-15]. The main objectives of campaign monitoring are to verify the improvement of interventions and improvement of quality across times and activities implemented in seven selected districts in Ibb and Al-Hodeida governorates, Yemen.
Survey area and period: This survey was conducted from 18 to 20 February 2021 by 1932 health care workers, in intentionally selected intervention areas suffering from trachoma in Al-Hodeida and Ibb governorates. These areas are endemic to trachoma and the first round of a trachoma survey was conducted in 2015. The intended beneficiaries of MDA are over 2 million people in the 7 districts of trachoma intervention.
Survey design: The study was community based cross sectional observational study.
Survey population: All residents/population living in the selected districts was surveyed population.
Eligible population: Everybody living in the survey area (district) based on drug-specific eligibility criteria.
Ethical approval and consent form: Ethical approval was obtained from the Medical Research and Ethics Committee at the Faculty of Medicine and Health Sciences at Sana'a University in Document No. 713 dated January 11, 2021. All data, including participant identification, were kept confidential and informed consent obtained from the people themselves.
Inclusion criteria: Everybody living in the survey area, where current Trachoma MDA was considered to be included in the study.
Exclusion criteria: Those households which were closed during the survey data collection period. In addition, non-resident individuals who came to visit relatives from other location were excluded.
Sample size: The sample size, n, was established by means of the single population proportion formula:
n= (Z α/2)2 x p x q x DEFF/ d2
Where: α is level of confidence; p is the proportion of the population who is expected to have swallowed the drug is 50%. The expected coverage sample size will increase as the reported coverage approaches 50%, which ensures that the sample size is sufficient to meet study objectives; q is (1-p); Z is standard normal distribution curve value for 95% CI which is 1.96 (where α = 0.05); d is tolerable margin of error, i.e. 5% (0.05); and DEFF is the design effect, a measure that reflects the degree to which respondents in the same subunit are likely to be similar in terms of the information provided in response to; we used the suggested default of 4. The sample size was estimated at 1,530; including 35% margin of non-response, the sample size was estimated at 2,615 individuals but for more accuracy we increased the sample size to 3,077 individuals.
Monitoring variables: This component covered total number of beneficiaries who were monitored from 476 houses (surveys) during field visits reached 3,077. Data collected including: the availability of medication and facial cleaning supplies with the MDA team, treatment sessions arrangement in monitor areas, the recipients, preparation, time required for treatment and acceptance of medications, the status of the beneficiaries of accepting used medicines. It also includes: the preparation of beneficiaries for drug dosing according to standard methods by healthcare teams, prior knowledge of the campaign by community members, community collaboration during the treatment campaign and the availability of additional supplies during the comprehensive treatment campaign for trachoma.
Data entry and Analysis: Data were collected by using Android tablets, where all the forms were pre-installed Survey CTO platform. The data were uploaded to our servers using 3G or Wi-Fi internet connections. Then, follows a process of data review, cleaning and verification at the central level. The early handling of data ensured instant quality checks and corrections. Microsoft Excel was used for analysis. Results are presented as frequencies and proportions in tables.
Table 1, shows the names of the directorates and governorates participating in the national campaign for mass treatment of trachoma: Mass drug administration (MDA) in Yemen 2021. The national campaign for mass treatment of trachoma covers 966 villages in 6 districts of Ibb governorates and Al-Hodeidah from 18 to 20 February 2021.
| Governorate | Districts | No of villages |
| Ibb | Al-Sobrah | 94 |
Al-Sayani | 163 | |
Al-Odain | 126 | |
Di- Sifal | 150 | |
subtotal | 533 | |
| Al- Hodeida | Al-Zohra | 189 |
Al-Qanawis | 125 | |
Al-Lohayah | 119 | |
Sub-Total | 433 | |
total | 966 |
Table 1: The number of villages and names of districts and governorates involved in the national campaign for the mass treatment of trachoma: Mass drug administration (MDA) in YEMEN 2021
The total number of beneficiaries who were monitored from 476 homes (surveys) during field visits reached 3,077, of whom 2,755 (90%) took the dose, and the rest 10% did not take medicines for various reasons, including for example (the example is not limited to); being outside the house when visiting or having a chronic disease etc. Table 2 shows the frequency of pre-camping training sessions for the 1932 healthcare workers participating in MDA for the treatment of trachoma in Ibb and Al-Hodeida. 1912 (99%) of healthcare workers attended a one-day training course, while 0.5% attended a two-day training course and 0.5% attended a 3-day training course. Table 3 shows the number of randomly selected house for the monitoring survey in the two governorates, as 186 random homes were selected in Al-Hodiada and 290 homes in Ibb governorate. Table 4 shows the availability of medication and facial cleaning supplies with the MDA team in the selected villages. Azithromycin tablets, azithromycin syrup, tetracycline ointment and facial cleansing kits were available in 90%, 91%, 91% and 82% respectively.
| Course time | number | percentage |
| One day training | 1912 | 99 |
| 2 days training | 10 | 0.5 |
| 3 days training | 10 | 0.5 |
| Total | 1932 | 100 |
Table 2: The frequency of pre-camping training courses for the health care workers (HCW) involved in the mass campaign of trachoma treatment in Ibb and Al-Hodiada.
| Governorates | number | percentage |
| Al-Hodiada | 186 | 39 |
| Ibb | 290 | 61 |
| Total | 476 | 100 |
Table 3: The number of selected houses for the monitoring survey in the 2 governorates
Table 5 shows status of treatment sessions arrangement in monitor areas. The improper arrangement of treatment sessions rate was 17%, the incorrect position of the dose pole was 6%, the return rate of drug application methods was not in 2%, the correct records of drugs scored the rate of 99%, and the proper storage of drugs rate was 70% only. Table 6 shows the recipients, preparation, time required for treatment and acceptance of medications among selected persons on the monitoring survey. In 65% of the cases selected, the task was completed in less than 10 minutes, and in 6% of the cases in 10 minutes, while 6% of the cases the tasks were completed in more than 10 minutes. Table 7 shows the status of the beneficiaries of accepting used medicines. 92% of the selected subjects had a good reaction to taking the medications, 7% had an acceptable reaction while 1% (27 people) had a bad reaction to the drugs used.
| Supplies | Available | Partially available | Unavailable | |||
| No | % | No | % | No | % | |
| Azithromycin Tablets | 428 | 90 | 38 | 8 | 10 | 2 |
| Azithromycin syrup | 433 | 91 | 43 | 9 | 0 | 0 |
| Tetracycline ointment | 433 | 91 | 43 | 9 | 0 | 0 |
| Face cleaning supplies | 390 | 82 | 15 | 3 | 71 | 15 |
| Total 476 | ||||||
Table 4: The availability of medications and face cleaning supplies with the MDA team in the selected villages
| Number | percentage | |
| Proper Arranging of treatment sessions | ||
| Yes | 395 | 83 |
| No | 81 | 17 |
| Proper Position of dose pole | ||
| Yes | 447 | 94 |
| No | 29 | 6 |
| Applying Medicine return methods | ||
| Yes | 466 | 98 |
| No | 10 | 2 |
| Proper records of medicines | ||
| Yes | 471 | 99 |
| No | 5 | 1 |
| Proper storage of medicines | ||
| Yes | 333 | 70 |
| No | 143 | 30 |
Table 5: Status of the arrangement of treatment sessions in the monitoring areas
| Time | Number | percentage |
| Less than 10 min | 1791 | 65 |
| 10 min | 799 | 29 |
| More than 10 min | 165 | 6 |
| Total | 2755 | 100 |
Table 6: Beneficiaries, preparation and time required for treatment and acceptance of medicines
| Number | percentage | |
| Good | 2535 | 92 |
| Acceptable | 193 | 7 |
| Bad | 27 | 1 |
| Total | 2755 | 100 |
Table 7: Beneficiaries status towards acceptance to used the medicines
| Characters | Number | percentage |
| Preparing Beneficiaries for drug doses by MDA teams | ||
| Acceptable | 1570 | 57 |
| Partially Acceptable | 744 | 27 |
| unacceptable | 441 | 16 |
| Advance knowledge of the campaign by the communities members | ||
| Acceptable | 831 | 27 |
| Partially Acceptable | 1077 | 35 |
| unacceptable | 1169 | 38 |
| Community co-operation during the treatment campaign | ||
| Acceptable | 2831 | 92 |
| Partially Acceptable | 154 | 5 |
| unacceptable | 92 | 3 |
Table 8: Preparing Beneficiaries for drug doses by MDA teams, advance knowledge of the campaign by the communities members and the community co-operation during the treatment campaign
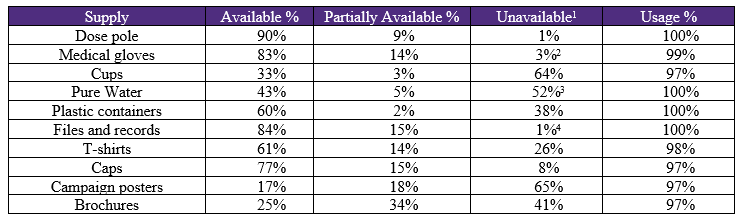
Table 8 shows the preparation of beneficiaries for drug dosing according to standard methods by healthcare teams. In 57% of the cases the preparation dosages were acceptable, 27% partially acceptable and 16% unacceptable (wrong doses). Also, Table 8 shows prior knowledge of the campaign by community members. The acceptable rate was only 27%, the partially acceptable rate was 35%, while in 38% the prior knowledge was unacceptable. In addition, Table 8 shows community collaboration during the treatment campaign. The acceptable rate was 92%, the partial acceptable rate at 5%, while 3% (92 respondents) exhibited unacceptable behaviors towards the treatment campaign. Table 9 shows the availability of additional supplies in selected areas during the comprehensive treatment campaign for trachoma. There was a significant shortage of campaign posters (65%), cups (64%), clean water (52%), brochures (41%) and plastic containers (38%).
This inspection was performed to assess the coverage and achievements of post-MDA trachoma in Ibb and Al-Hodeida governorates. In this survey, the total number of beneficiaries that were monitored from 476 homes (surveys) during field visits was 3,077, of whom 2,755 (90%) took the dose, and the rest 10% did not take medication, i.e. coverage rate of the treatment was 90% which is more than 80% the target threshold for effective coverage recommended by the World Health Organization [16]. The current coverage rate is better than that reported by Bekuma et al. in Ethiopia [17], the coverage rate is 80%. However, the figure cited here is much higher than in Kenya where 65.7% and 64.1% of the respondents had treated for 18. This coverage is also higher than a study in Northern Tanzania which was 76% in 2005 and 76.9% in 2011 19, also higher than study conducted in Nigeria in 2013 reported a coverage of 60.3 . When coverage is greater than 90%, there will be less chance of a recurrence of trachoma until the next round of annual mass drug administration begins; and management may gain higher acceptance over time. Additionally, this may also be due to the great effort made to mobilize and increase access to information as reflected from the qualitative study [21]. The discrepancy between studies may be due to the reason for the current study being conducted in the high rate of drug availability as in the case of azithromycin tablets, azithromycin syrup, tetracycline ointment, and facial cleansing tools available in 90%, 91% and 91% and 82%, respectively (Table 4). Also due to the high rate of pre-camping training sessions for 1932 healthcare workers participating in MDA which was 99% (Table 2). Also in Kenya and Ethiopia surveys, access is more difficult than Yemen due to the presence of damage to roads and bridges caused by the rainy season in these countries during MDA campaigns [16-18,22].
Concerning the status of the treatment sessions arrangement in the control areas in the current study. The improper arrangement of treatment sessions rate was 17%, the incorrect position of the dose pole was 6%, the return rate of drug application methods was not in 2%, the correct records of drugs scored the rate of 99%, and the proper storage of drugs rate was 70% only. These results are almost identical to those reported from Ethiopia and Kenya where improper arrangement of treatment session rate occurred more frequently17,18,22. This indicates that more training is needed for the team of healthcare workers participating in the MDA trachoma campaign.
The incorrect position of the dose pole (dose by height) in the current study was 6%, and as a result, there was a fear that some children might take more than they should. Some participants even stated that it is better to give medication to children based on their age rather than their height. This was also confirmed by the current results in monitoring the preparation of beneficiaries for drug doses according to standard methods by health care teams. In 57% of the cases, the dosages of the preparation were acceptable, 27% partially acceptable and 16% unacceptable (wrong doses) (Table 8). Concerning prior knowledge of the campaign by community members in the current study. The acceptable rate was only 27%, the partially acceptable rate was 35%, while at 38% the prior knowledge was not acceptable. Mass drug administration must be preceded by appropriate mobilization activities for its successful implementation. In this study, among those who received the Zithromax group therapy, 38% had unacceptable prior knowledge and this could lead to obstacles to the MDA campaign.
According to community cooperation during the treatment campaign. The tolerable rate was 92%, the acceptable partial rate 5%, while 3% (92 participants) exhibited unacceptable behaviors toward the treatment campaign (Table 8). This could improve their understanding of the benefits of the drug and contribution in azithromycin (Zithromax) mass drug administration campaign, consequential in improved treatment coverage. Throughout the campaign, health extension workers trained the community on personal hygiene, and to swallow the drug as it prevents from eye disease. Even though the information presented is not inclusive to tackle all the SAFE strategies for trachoma elimination, it could improve the community’s belief for the mass treatment. This is consistent with the health belief model, which says that knowledge, awareness and attitudes about the diseases positively affect the acceptability of the mass treatment [23].
Considering the recipients, preparation, time required for treatment and acceptance of medications among selected persons on the current monitoring survey. In 65% of the cases selected, the task was completed in less than 10 minutes, and in 6% of the cases in 10 minutes, while 6% of the cases the tasks were completed in more than 10 minutes. From a qualitative finding, one of the frequently reported issues was crossing the entire population on the same day. Because large populations may come to intervention sites imposing a disproportionate burden on service providers to administer and register and result in service users complaining about the potentially long waiting hours to get drug administration. Taking a lesson from these results complete the task in less than 10 minutes in a location convenient for most community members. The literature has indicated that detect obstacles that persist across diverse health behaviors for instance lack of time (due to family, household and professional responsibilities), admittance issues (transportation and facilities), established attitudes, limitations in the physical environment and lack of understanding can be useful in designing successful community-based interventions [24].
In this survey, the national campaign for MDA in the 966 villages reach the target threshold (i.e., 90%) for effective coverage; with proper rates for the availability of trachoma medicines, good people reactions towards taking treatment, and the community collaboration; while bad rates for the improper arrangement of treatment sessions, the incorrect position of the dose pole and the preparation dosages by MDA team. Therefore, programmatic improvements should be made for the future campaign to reach the expected thresholds of coverage and managements. Furthermore, health extension workers should be involved in delivering messages that focus on SAFE strategies for eliminating trachoma that can enhance community acceptance of mass treatment.
No conflict of interest associated with this work.
This research work is part of the round 1 monitoring of the national campaign for MDA that done under the supervision of the Ministry of Health and Population, Sana'a, Yemen, and WHO, Sana’a office. All authors were members of this work.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
