AUCTORES
Globalize your Research
Review Article
*Corresponding Author: Maria Goretti Moreira Guimarães Penido, Nephrology Center of Santa Casa de Belo Horizonte R. Piauí, 420 - Santa Efigênia, Belo Horizonte.
Citation: Ana Elisa Souza Jorge, Maria Goretti Moreira Guimarães Penido, Maria Marta Sarquis Soares (2022). Mineral and Bone Disease After Kidney Transplantation: Risk of Fracture, Graft Dysfunction and Mortality - a Review. International Journal of Clinical Nephrology. 4(2); DOI:10.31579/2834-5142/030
Copyright: © 2022 Maria Goretti Moreira Guimarães Penido. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 17 May 2022 | Accepted: 30 May 2022 | Published: 06 June 2022
Keywords: fgf23; calcium; phosphorus; vitamin d; parathyroid hormone; chronic kidney disease; mineral and bone metabolism
The mineral and bone disease of chronic kidney disease (CKD-MBD) is a combination of three components: abnormalities in calcium, phosphorus, PTH, fibroblast growth factor 23 (FGF23) and vitamin D metabolism; abnormalities of bone metabolism, mineralization, volume, growth and strength; and vascular and other soft tissue calcification.
During the natural course of kidney disease, there is an increase in FGF23 levels, inhibition of calcitriol production, secondary hyperparathyroidism, hypocalcemia and hyperphosphatemia. These changes have consequences on the cardiovascular and bone systems. Regarding cardiovascular disease, left ventricular hypertrophy is highlighted, associated with an increase in FGF23 and vascular calcification, directly related to hyperphosphatemia. The main types of bone disease are cystic fibrous osteitis (high turnover) and adynamic bone disease (low turnover), both of which are associated with a high risk of fracture in this population.
Successful kidney transplantation (KT) does not fully correct the mineral, bone and cardiovascular abnormalities generated by CKD-MBD. In the first months after transplantation, PTH and FGF23 levels remain elevated in most patients. There is an increase in the production of calcitriol by the graft. These are the main alterations responsible for a mineral phenotype that resembles primary hyperparathyroidism, with hypercalcemia, hypophosphatemia and elevated PTH levels. Cardiovascular disease does not revert after KT and the transplant patient has a higher cardiovascular risk than the general population. In relation to bone disease, in addition to the pre-existing bone alteration, specific factors of the post-KT period add damage to the bone, especially the use of corticosteroids. The main types of bone disease in renal transplant patients are bone fracture, renal osteodystrophy, osteoporosis and osteonecrosis.
CKD-MBD is a complex disease that affects patients with CKD, increasing their morbidity and mortality. KT does not fully reverse the disease and still adds other specific risk factors that make its approach challenging. This review sought to show the association between the bone mineral disease of chronic kidney disease and the risk of fractures, vascular and other tissue calcifications, graft dysfunction and kidney transplant patient mortality.
Chronic kidney disease (CKD) is a systemic disease and the reduction in kidney function has repercussions on several other organs. CKD generates a clinical phenotype of inflammation, malnutrition, alteration of the autonomic and central nervous system, cardiopulmonary, vascular and mineral and bone disease [1].
The disorder of mineral and bone metabolism associated with CKD is part of a broad clinical spectrum, which encompasses not only bone alterations, but also alterations in the parameters of mineral metabolism and extra skeletal calcification. Mineral and bone disease gained syndrome status; and in 2006, the Kidney Disease: Improving Global Outcomes (KDIGO) group redefined its concept [2]. The term renal osteodystrophy, which referred only to histological bone alterations evaluated by biopsy, was replaced by mineral and bone disorder of chronic kidney disease (CKD-MBD). CKD-MBD is a combination of three components: 1) abnormalities in the metabolism of calcium, phosphorus, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and vitamin D; 2) abnormalities in bone remodeling, mineralization, volume, growth and strength; and 3) vascular and other soft tissue calcification [2]. CKD-MBD starts early in the course of CKD, is progressive and is practically universal in stage 5 of the disease [3,4]. It is associated with increased morbidity and mortality in patients with CKD [2,4].
Kidney transplantation (KT) is the best treatment option for patients with advanced CKD. It reverses several complications of kidney disease, improves quality of life and prolongs survival [5,6]. However, even successful KT does not completely reverse CKD-MBD [5,7]. As in non-transplanted CKD patients, CKD-MBD is also associated with increased morbidity and mortality in kidney transplant patients [7].
2.1 - PTH, calcium, phosphorus, FGF23 and vitamin D metabolism abnormalities
The central role of phosphorus
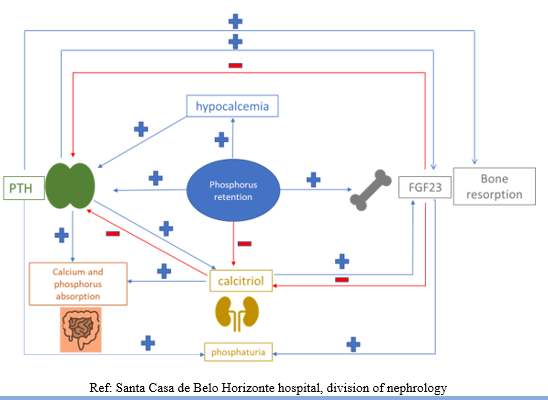
Decreased renal function results in reduced phosphorus excretion and retention of this ion plays a central role in the pathophysiology of CKD-MBD, being the trigger for the development of the disease [8]. Hyperphosphatemia stimulates osteocytes and osteoblasts to synthesize FGF23, a phosphatonin, hormone responsible for regulating phosphorus excretion. Elevated levels of FGF23 is one of the earliest changes in CKD-MBD [3]. FGF23 acts through the FGF23 receptor (FGFR), which is only functional thanks to the presence of the Klotho transmembrane protein, which acts as its co-receptor, and associated with FGFR1 forms the Klotho-FGFR9 complex. The two main effects of FGF23 are: increased renal phosphorus excretion, via inhibition of type II sodium and phosphorus co-transporters (NaPi-2a and Na-Pi2c) in the proximal tubule, and reduced calcitriol production. FGF23 inhibits 1α-hydroxylase, the enzyme that converts 25-hydroxyvitamin D3 into its active form, calcitriol, and increases the activity of 24-hydroxylase, the enzyme responsible for calcitriol catabolism [9]. Decreased production of calcitriol leads to decreased absorption of calcium and phosphorus in the intestine. Hypocalcemia and a drop in calcitriol levels are detected by the parathyroid glands, via calcium sensor receptor (CaSR) and vitamin D receptors (VDR), respectively, leading to gland hyperplasia and increased PTH synthesis and secretion. Beyond the indirect effect of phosphorus on calcium concentration, via FGF23 and reduction of calcitriol synthesis, phosphorus retention itself reduces the concentration of free serum calcium and directly inhibits the synthesis of calcitriol in the kidney [10, 11]. Phosphorus also seems to have a direct action on the parathyroid glands, also contributing to increase PTH secretion [12,13]. Phosphorus therefore exerts direct and indirect actions on PTH secretion, which results in hyperparathyroidism secondary to CKD.
Among all these factors, the reduction in calcium concentration is the main stimulus for PTH secretion [11]. Thanks to the increase in PTH and FGF23, most patients maintain normophosphatemia up to a glomerular filtration rate (GFR) above 20mL/min [9]. Hyperphosphatemia is therefore a late marker of CKD-MBD [11].
The effects of PTH
The classic target organs for PTH are bone and kidney. It acts by binding to the type 1 receptor (PTH1R) on osteoblasts, osteocytes and renal tubular cells. In bone, continuous exposure to higher levels of PTH stimulates the production of activating nuclear factor kappa B receptor ligand (RANKL) and reduces osteoprotegerin (OPG) production by osteoblasts. RANKL binds to its RANK receptor on the surface of osteoclasts, stimulating their activity; and on the surface of their precursors, stimulating their differentiation and survival. OPG binds to RANKL, inhibiting its binding to RANK. The increase in the RANK/OPG ratio results in osteoclastogenesis and increased bone resorption, which culminates in the release of calcium from the bone [14]. In the kidneys, PTH has a similar action to FGF23 in the proximal tubule, and induces phosphaturia. It stimulates 1α-hydroxylase, increasing the production of calcitriol, and promotes renal reabsorption of calcium in the distal convoluted tubule [15]. To complete the complex regulatory loop of calcium and phosphorus metabolism, FGF23 inhibits PTH secretion. Calcitriol and PTH increase the production of FGF23 [4] (Figure 1).
Consequence of advanced kidney disease on mineral and bone metabolism
With the progression of kidney disease and loss of nephrons, high levels of FGF23 and PTH are insufficient to prevent hyperphosphatemia [16]. CKD generates resistance to the actions of PTH and FGF23, which also contributes to the progression of CKD-MBD. There is a reduction in the expression of the Klotho co-receptor in the kidney and parathyroid glands, making the action of FGF23 ineffective. Consequently, PTH rises significantly, even in the presence of high levels of FGF23 [11]. The effect of FGF23 on renal phosphorus excretion is attenuated and PTH becomes the main responsible for phosphaturia [16]. The uremic environment reduces the expression of PTHR1 in the skeleton and kidneys. Modification of the PTH molecule occurs, due to the oxidative stress caused by CKD, which compromises its action. With the reduction of renal function, there is an accumulation of C-terminal PTH fragments, which has the opposite effect to that of the entire molecule [11,15]. These mechanisms that generate resistance to the actions of PTH, associated with hyperphosphatemia, contribute to the hyperplasia of the parathyroid glands and progressive increase in PTH production. Glandular hyperplasia, which is initially diffuse and polyclonal, progresses to a monoclonal nodular form [4]. Glandular proliferation is associated with reduced expression of CaSR and VDR. The glands become autonomous and no longer respond to inhibitory stimuli from calcium and vitamin D [4].
CKD in more advanced stages has a biochemical mineral profile characterized by hyperphosphatemia, hypocalcemia, hyperparathyroidism, Klotho deficiency, increased levels of FGF23 and hypovitaminosis D. In the long term, these changes are harmful to the skeleton and the cardiovascular system.
Consequences of mineral abnormalities of CKD-MBD
Hyperphosphatemia
Elevated phosphorus levels are associated with vascular and valvular calcification and mortality in CKD [12,17]. The osteochondrogenic phenotypic transdifferentiation of vascular smooth muscle cells, promoted by hyperphosphatemia, is an essential pathophysiological component in vascular calcification. The vascular smooth muscle cell loses its contractile property and begins to produce collagen matrix and vesicles rich in calcium and phosphorus, which initiate the process of calcification of the vessel wall [17].
Hypocalcemia
Prolonged hypocalcemia can cause myocardial dysfunction in CKD patients, but it rarely does [18]. It is also associated with higher mortality in observational studies [19]. On the other hand, hypercalcemia is also a concern in these patients. The treatment of hypocalcemia and hyperphosphatemia with calcium-based chelators, in combination with the reduction of renal calcium excretion, lead to a positive balance of this ion in patients with CKD [20]. Calcium has a synergistic effect with phosphorus in the calcification process [21], which is an independent risk factor for cardiovascular morbidity and mortality [22,23].
Hyperparathyroidism
In hemodialysis patients, hyperparathyroidism was associated with abnormalities in left ventricular (LV) function, cardiac hypertrophy, and vascular calcification [12, 18, 24]. Other studies have shown that very low levels of PTH are associated with increased vascular calcification and mortality in dialysis patients [25-27]. Observational studies show a U-shaped association between PTH levels and mortality [19]. In addition to the effect on the cardiovascular system, inappropriate levels of PTH cause both high and low turnover bone disease [11].
Klotho deficiency
Klotho, highly expressed in renal tissue, is cleaved in its transmembrane portion by proteases and secreted into the circulation. The cleaved protein circulates systemically and is called soluble Klotho [28]. It has multiple pleiotropic effects that lead to cytoprotection through anti-oxidant, anti-fibrotic, anti-senescent, stem cell preservation and angiogenesis actions. Both the expression of the transmembrane protein and its soluble form are reduced in CKD [28]. The clinical phenotype of Klotho deficiency is characterized by reduced survival, cardiac remodeling, bone disease, muscle wasting and hyperphosphatemia [29]. The reduction of soluble Klotho is associated with arterial stiffness in patients with CKD, and with mortality and cardiovascular events in dialysis patients [30,31]. The decrease in Klotho co-receptor expression generates resistance to the actions of FGF23, leading to phosphorus retention and increased levels of FGF23. At the same time, there is a reduction in the inhibitory action of FGF23 on the parathyroid glands, accelerating hyperparathyroidism. Hyperphosphatemia, excess FGF23 and hyperparathyroidism have harmful consequences for the cardiovascular and skeletal systems. In short, Klotho is an anti-aging protein and CKD can be seen as a state of premature aging [32].
Excess FGF23
As CKD progresses, FGF23 levels become extremely high.
In addition to hyperphosphatemia, the main stimulus for the production of FGF23, the reduction in Klotho expression has the effect of increasing its synthesis even further. As FGF23 itself inhibits Klotho expression, this becomes a vicious cycle, closing a loop of self-production of this phosphatonin. [32] Elevated levels of FGF23 are associated with LV33 hypertrophy and increased mortality in hemodialysis patients.34 FGF23 appears to have a direct effect on cardiomyocytes through a Klotho-independent mechanism,33 through type FGFR [4,28,35 ]
THIS PARAGRAPH HAS NOT BEEN TRANSLATED...IT IS IN PORTUGUESE YET.
Calcitriol deficiency
1,25-dihydroxycholecalciferol (1,25(OH)2D3, or calcitriol), the active form of vitamin D, is synthesized in the kidneys via hydroxylation of 25-hydroxycholecalciferol (25(OH)D3, or calcidiol) by 1α-hydroxylase [10]. The main actions of calcitriol in mineral metabolism are: increased absorption of calcium and phosphorus in the intestine and calcium in the kidney; and inhibition of PTH synthesis by the parathyroid glands [36]. In situations of negative calcium balance, calcitriol stimulates the production of RANKL by osteoblasts, mobilizing bone calcium and contributing to the preservation of normocalcemia [37]. The biological effects of calcitriol are mediated. via the VDR, expressed in various human tissues in addition to bone, such as the intestine and kidney [36]. The functions of vitamin D are not restricted to the maintenance of calcium and phosphorus homeostasis. Vitamin D exerts pleiotropic effects on various tissues. It acts on cell differentiation and growth, on the immune and cardiovascular system and has anti-proliferative, anti-inflammatory, anti-fibrotic effects, inhibiting the renin-angiotensin-aldosterone system and vascular relaxation [36,37]. Calcitriol deficiency in CKD occurs not only by the loss of renal tissue, but mainly by the action of FGF23 in the kidney, which inhibits 1α-hydroxylase [9]. There is also a resistance to the actions of vitamin D in CKD, by the reduction of VDR expression and affinity binding of calcitriol with its receptor [11]. Calcitriol and calcidiol deficiencies are very common in CKD [38]. An association of vitamin D with progression of kidney disease and death in patients with CKD stages 2-5 has already been demonstrated, with cardiovascular mortality in dialysis patients and with overall mortality in incident hemodialysis patients [35,39,40]. Observational studies have shown that the treatment of patients with CKD with vitamin D was associated with a reduction in overall mortality and diovascular disease, however, this has not been demonstrated in randomized controlled studies [41]. Currently, the indication for the use of calcitriol or vitamin D analogues in patients with CKD with better evidence is the treatment of secondary hyperparathyroidism [42].
2.2 - Abnormalities of bone remodeling, mineralization, volume, growth and strength
The bone disease associated with CKD is known as renal osteodystrophy. Bone biopsy with tetracycline staining is the gold standard for diagnosis [43]. There are three parameters used to assess bone pathology: remodeling (turnover), mineralization and bone volume (TMV system) [2]. According to these parameters, four classic types of bone injury are defined: cystic fibrous osteitis, adynamic bone disease, osteomalacia and mixed uremic osteodystrophy. Cystic fibrous osteitis is characterized by increased bone remodeling and normal mineralization; adynamic bone disease, due to the reduction of bone remodeling; osteomalacia, by reducing bone remodeling and abnormal mineralization; and mixed uremic osteodystrophy, due to increased bone remodeling and abnormal mineralization [2].
The prevalence of adynamic bone disease has increased in recent decades and studies show that this is the most common type of renal osteodystrophy today. This fact can be explained by the increase in the proportion of elderly and diabetics on dialysis; and by treating CKD-MBD with high doses of vitamin D and oral calcium [44]. The main clinical outcome of renal osteodystrophy is bone fractures. Both high and low remodeling diseases increase bone fragility and the risk of fractures [45]. Patients with CKD have a higher risk of fracture when compared to the general population, and hip fractures are associated with higher morbidity and mortality [43,46]. The incidence of fractures is significantly higher in hemodialysis patients compared to the general population, with a 3.7-fold unadjusted relative increase in the risk of death and a 4-fold increase in the combined death/rehospitalization outcome [47]. Fracture prevention should be the main objective in the management of bone disease in CKD.
The diagnosis of renal osteodystrophy has as its main focus the distinction between high and low remodeling disease, which is fundamental in the management of the disease. Despite being the gold standard, bone biopsy has important limitations. It is an expensive, poorly available and invasive procedure [48]. Its performance is considered only if there is doubt regarding the symptoms and abnormalities of biochemical parameters and if the result is to compromise the treatment [43]. Although the assessment of bone mineral density by densitometry predicts the risk of fracture in patients with CKD, it does not distinguish the types of renal osteodystrophy [43]. The Fracture Risk Assessment Tool (FRAX) was shown to be able to predict the risk of major osteoporotic fractures in patients with GFR < 60mL>
PTH is traditionally the most used parameter for the diagnosis of bone disease, based on bone remodeling, since this remodeling depends heavily on the degree of hyperparathyroidism [51]. The study by Sprague et al showed that intact PTH is the best marker to differentiate between high and non-high turnover or low and non-low turnover disease, based on PTH targets recommended by KDIGO (2 to 9 times the upper limit of normal) or the National Kidney Foundation – Kidney Disease Outcomes Quality Initiative (NFK-KDOQI) (150-300 pg/mL) [51]. The optimal value of PTH in distinguishing between low and non-low turnover disease was 104 pg/mL, and the optimal value for differentiating between high and non-high turnover disease was 323 pg/mL. Bone FA added a non-significant additional value to the diagnostic value of PTH. Still in this study, the other biomarker studied, amino-terminal propeptide of type 1 procollagen (P1NP) did not improve the diagnostic accuracy at all. PTH values between 150-300 pg/mL do not guarantee the absence of bone disease, and can occur in both high and low remodeling disease [52].
Bone AF is an enzyme produced by osteoblasts. It is associated with bone formation and is a marker of bone remodeling. It favors tissue mineralization through the inactivation of calcification inhibitors (inorganic pyrophosphate and osteopontin) and generates phosphorus from the hydrolysis of organic phosphates, a substrate for tissue calcification [53]. In the absence of cholestatic liver disease, total AF reflects the increase of bone AF. Both bone and total AF are associated with cardiovascular mortality, all-cause mortality, and fracture risk in the dialysis patient [48]. The aforementioned study by Sprague et al (2016) showed that bone AF was slightly better than PTH for the diagnosis of low-remodeling disease, with a cutoff of 33.1 U/L, but the same was not true for high-remodeling disease [51].
Despite the known benefits of vitamin D on bone health in the population that does not have kidney disease, the values of vitamin D in patients with CKD that protect against fractures are unknown [45, 54]. However, several studies have shown a relationship between vitamin D and bone health in CKD. In the study by Coen et al (2005), patients with 25(OH)D3 levels < 15>
Hyperphosphatemia is associated with reduced osteoblast proliferation, osteoblast apoptosis, and reduced bone formation [45]. However, there is no evidence that treating hyperphosphatemia and reducing phosphorus levels prevent fractures [58].
FGF23 plays an important role in bone mineralization. However, in dialysis patients, bone mineral density does not correlate with FGF levels [23, 45].
Other bone remodeling biomarkers, such as C-terminal propeptide of procollagen 1 (P1CP), C-terminal crosslaps of collagen 1 (CTX) and tartrate-resistant acid phosphate 5b (TRAP5b), were not associated with bone histomorphometry and there is no indication for its use in clinical practice [48].
2.3 - Vascular and other soft tissue calcification
The diagnosis of CKD-MBD includes extraskeletal calcification, which can be arterial, valvular or myocardial. The prevalence of this type of calcification increases with worsening renal function and is higher than that found in the general population. Cardiovascular calcification is associated with cardiovascular events and death [2].
Cardiovascular disease is the leading cause of death in patients with CKD and CKD is an independent risk factor for cardiovascular events. [5,23,59]. Cardiovascular morbidity and mortality is inversely and independently associated with renal function, particularly if GFR < 15>
The calcification process is complex, highly regulated and involves a profound interaction of the bone-vessel axis. The kidney is a key intermediary in this interaction. Disturbances in this axis have devastating consequences for both the skeleton and the cardiovascular system. Among the types of extraskeletal calcification, arterial calcification is the most threatening [67]. There are two patterns of arterial calcification, one that occurs in the intima and that which occurs in the middle layer. Intimal calcification is typically related to atherosclerotic disease, which is associated with Framingham risk factors and pro-inflammatory cytokines. It is a discontinuous process, involving only lipid-rich regions of atheromatous plaques [68-70]. This intimal calcification is associated with atheromatous plaque instability and vascular-occlusive clinical events (acute myocardial infarction and stroke) [69]. Medial layer calcification is restricted to the vascular smooth muscle layer, is continuous and is independent of the presence of atheromatous plaques. It is related to arteriosclerosis, and has the effect of hardening and loss of arterial elasticity. The clinical events associated with this type of calcification are systemic arterial hypertension (SAH) and LV hypertrophy. Atherosclerosis is seen in diabetic, elderly and CKD patients [68-70]. Both types of calcification can be seen in CKD, but middle layer calcification is more specific to CKD [70]. Adult and elderly patients with CKD usually also have the traditional Framingham risk factors and calcification of both the intima and the media is very frequent in these cases [68]. Patients who are previously hypertensive, diabetic, have metabolic syndrome and who develop CKD have, in addition to traditional risk factors, risk factors specific for CKD, such as hyperphosphatemia, hypercalcemia and reduced Klotho expression. Exuberant mineral deposition occurs in the middle layer, superimposed on a previous atheromatous process, which culminates in an extremely high cardiovascular risk [67]. In adolescents with CKD, who usually do not have traditional risk factors, intimal calcification is rarely seen, but they have a vascular phenotype that compares to an 80-year-old patient without CKD, due to arterial media calcification [68]. Patients with CKD who have medial layer calcification are younger, have fewer traditional risk factors for atherosclerosis, have a longer dialysis time and a higher prevalence of disturbances in calcium and phosphorus homeostasis, when compared to CKD patients who have intimal calcification [71]. Medial calcification results in arterial hardening, measured by the wave velocity of arterial pulse, which is associated with a drop in GFR and increased cardiovascular morbidity and mortality in patients with chronic kidney disease [72]. Therefore, CKD is a state of premature arterial aging, whose pathophysiological basis is vascular calcification, more specifically of the middle arterial layer, which leads to its hardening, with an increase in pulse wave velocity and pulse pressure [72]. The consequent clinical outcome is non-vascular-occlusive cardiac events, which are frequent in this population, such as sudden cardiac death, which is responsible for the majority of cardiovascular deaths in these patients [73].
In the past, the process of vascular calcification was understood as a passive deposition of calcium and phosphorus crystals on the vessel wall. It is currently known to be an active and highly regulated process that resembles physiological bone formation [68]. The central point of pathophysiology is the osteochondrogenic phenotypic transdifferentiation of vascular smooth muscle cells, triggered mainly by exposure to high levels of phosphorus [17,68]. Smooth muscle cells are of mesenchymal origin, as are osteoblasts, and are capable of differentiating in response to certain stimuli, such as the entry of calcium and phosphorus (via cotransporter PiT-1) into these cells [68,74]. The phenotypic transdifferentiation process is translated by suppression of the expression of contractile proteins of the smooth muscle cell and expression of proteins that regulate mineralization, normally expressed in bones and cartilage [68]. The differentiated cell under the effect of calcium, phosphorus, inflammatory cytokines and reactive oxygen species secretes matrix vesicles rich in hydroxyapatite and poor in calcification inhibitors (fetuin A and matrix Gla protein). Vesicles serve as a matrix for the calcification process and are rich AF in their membranes [75]. AF provides a source of phosphorus from the degradation of pyrophosphate, which is a potent inhibitor of calcification, favoring crystal growth [68,74,75]. AF is associated with vascular calcification in dialysis patients [76].
Elevated levels of PTH are generally associated with high bone remodeling disease and have the effect of releasing more calcium and phosphorus from the bone into the circulation, which, as described above, are promoters of vascular calcification [74]. Older studies have shown an association between secondary hyperparathyroidism and extra bone calcification, with improvement after parathyroidectomy [77,78]. Low remodeling disease, more specifically adynamic bone disease, is characterized by PTH suppression and low alkaline phosphatase levels. Low bone turnover impairs the bone's ability to act as a buffer in cases of calcium overload. Recent studies have shown that low-remodeling disease is also associated with arterial calcification [79,80]. Asci et al showed a U-shaped association between bone remodeling and coronary artery calcification [81].
The Klotho deficiency seen in CKD is also associated with vascular calcification [82,83]. In addition to its effect on improving phosphaturia, Klotho seems to have a direct action on the vessel by inhibiting the entry of phosphorus into the vascular smooth muscle cell. FGF23 does not appear to be related to vascular calcification [84].
Vitamin D levels were once inversely associated with the risk of developing coronary artery calcification in a population of patients with and without a diagnosis of CKD [85]. In a study with patients on hemodialysis, the levels of nutritional vitamin D and active vitamin D were negatively correlated with aortic pulse wave velocity (a measure of the degree of arterial stiffness) and positively associated with brachial artery distensibility. Arterial calcification scores were not related to nutritional or active vitamin D levels in this study [86]. However, excessive administration of vitamin D in CKD patients is associated with vascular calcification [87]. Vitamin D appears to reduce fetuin A levels, which is an inhibitor of vascular calcification [69]. In addition, excess vitamin D increases calcium and phosphorus levels and can cause adynamic bone disease, factors related to vascular calcification [88].
Kidney transplantation is the best treatment for patients with end-stage CKD. The mortality of the transplanted patient is lower than that of the hemodialysis patient [5,6]. However, even successful kidney transplantation does not fully correct the CKD-MBD that existed in the pre-transplant period [5,7]. After one year of transplantation most patients have a GFR compatible with stage 3 CKD. CKD-MBD after kidney transplantation is a reflection of preexisting BMD associated with de novo BMD in the post-transplant period, influenced by immunosuppression and by graft function [7,89]. CKD-MBD is associated with increased morbidity and mortality in transplant patients, kidney failure and graft dysfunction after transplantation [90-93].
3.1 - Abnormalities of CKD-MBD biochemical parameters after kidney transplantation
Phosphorus overload is a universal abnormality in the pre-transplantation period and is the mainstay of the pathophysiology of CKD-MBD [8]. At an earlier stage after kidney transplantation, there is classically a rapid drop in phosphorus levels, to normal or lower values of normality [89]. Hypophosphatemia can occur in up to 90% of patients in the first three months after kidney transplantation [94]. In the study by Wolf et al, the peak of hypophosphatemia occurred in the second week after kidney transplantation, with improvement at the end of the first year [95]. The persistence of hyperparathyroidism after transplantation is the main cause of hypophosphatemia. PTH acts on the functioning kidney, which is now responsive to its phosphaturic effect [96]. Persistently elevated levels of FGF23 also contribute to the pathophysiology of post-transplant hypophosphatemia [96-99]. FGF23, like PTH, increases renal phosphorus excretion. Relatively high levels of PTH and FGF23 acting on a kidney capable of excreting phosphorus generate hypophosphatemia. In addition, FGF23 reduces the production of calcitriol, which limits the intestinal absorption of phosphorus [98]. Although PTH stimulates the production of FGF23, the negative balance of phosphorus after kidney transplantation is felt by osteocytes, which reduce its production. This is one of the reasons that explains the faster normalization of FGF23 levels compared to PTH, and why hyperparathyroidism is the main cause of hypophosphatemia after kidney transplantation [95,99]. The use of corticosteroids and diuretics is also associated with phosphaturia in these patients [96]. Therefore, hypophosphatemia reflects improved excretory function of the kidney and is usually self-limiting. In the study by Nakai et al (2019), hypophosphatemia in the first three months after kidney transplantation was associated with better renal graft survival [100]. On the other hand, hyperphosphatemia has already been associated with increased mortality after kidney transplantation and graft loss [91, 101]. In a retrospective study, Jeon et al showed that the serum phosphorus level measured one year after kidney transplantation had a U-shaped association with graft loss and patient mortality [102]. The association of hyperphosphatemia with mortality can be explained by vascular calcification. It is known that vascular calcification can progress in renal transplant recipients and its expansion predicts cardiovascular morbidity and mortality in these patients [103, 104]. The reason for the greater graft loss that is associated with hyperphosphatemia is not known, with the main hypotheses being increased phosphate deposition of calcium in the renal interstitium or direct tubular toxicity [105]. The higher mortality related to hypophosphatemia one year after kidney transplantation can be explained by the poorer nutritional status of the patient (malnutrition-inflammation-cachexia syndrome), which is corroborated by the finding of a higher rate of infection in the group with lower phosphorus in the study by Jeon et al [102]. Furthermore, in the same study, hypophosphatemia that persisted until the first year after transplantation was related to persistent hyperparathyroidism, and an association between hyperparathyroidism and higher mortality and loss of kidney graft in transplant recipients [106].
FGF23 levels in patients with CKD are extremely high, exceeding their normal value by a factor of 1000. Despite suffering a rapid drop, greater than 95% in the immediate post-transplantation period, the FGF23 value remains high in the third month after renal transplantation, when compared with patients with CKD with the same GFR. Contrary to PTH, persistence of high levels of FGF23 tends to resolve within one year of transplantation and graft function is the main long-term determinant of FGF23 [7]. That is why hypophosphatemia that persists after one year of transplantation is generally attributed to the persistence of hyperparathyroidism after this period [89]. It is known that high levels of FGF23 are associated with LV hypertrophy in the pre-transplant period [33]. After kidney transplantation, the persistence of elevated FGF23 is associated with higher cardiovascular mortality, for all causes and greater graft loss [90,107].
Parathyroid gland cells, which undergo hyperplasia in the pre-transplant period, have a life span of up to 20 years. Therefore, depending on the degree of gland hyperplasia in the pre-renal transplant period, PTH may remain elevated for a long time, even if graft function is normal [7,89]. When hyperplasia is in early stages (polyclonal and diffuse) and after transplantation there is normalization of mineral parameters, such as calcium, phosphorus and active vitamin D, the possibility of regression is greater. In addition, improvement in uremia tends to restore the expression of CaSR, VDR and FGFR-Klotho receptors in the parathyroid glands. However, if the hyperplasia is monoclonal nodular, the gland remains resistant to the actions of calcium, calcitriol and FGF23 [89,108]. In this case, the possibility of reversing the hyperplasia is lower [109]. This type of hyperplasia related to the persistence of hyperparathyroidism is clinically characterized by high levels of PTH after transplantation, associated with hypercalcemia, considering that the gland has an autonomous secretion pattern, insensitive to calcium levels [110].
The concept and prevalence of persistent hyperparathyroidism after kidney transplantation varies in the literature. In a systematic review, PTH levels after six months of transplantation were consistently lower compared to month zero, with a mean drop of 54%. After two years of transplantation, more than 50% of patients still had elevated levels of PTH. Evenepoel et al showed a prevalence of persistent hyperparathyroidism (defined as a PTH value > 2.5 times the upper limit of normal or need for parathyroidectomy) of 17% in the first four years after kidney transplantation [96]. The greatest decline of PTH was observed in the first three months. The severity of post-transplant hyperparathyroidism correlated with the volume of the parathyroid glands in the uremia period [96]. Wolf et al described a prevalence of persistent hyperparathyroidism (defined as PTH > 65 pg/mL) of 80% during the first year after transplantation, despite good graft function [95]. In more recent studies, Araújo et al and Kirnap et al showed a prevalence of persistent hyperparathyroidism after one year of transplantation, defined as PTH > 100 pg/mL with ionic calcium > 5.3 mg/dL and PTH > 68pg/mL, respectively, of 14 and 62% [112,113]. One study evaluated bone and mineral metabolism after long-term renal transplantation and persistent hyperparathyroidism (defined as a of PTH > 2.5 times the upper limit of normal) was found in 10.7% of patients after 10 years [114]. The main risk factors associated with hyperparathyroidism found in these studies were high levels of PTH, calcium, phosphorus and AF at the time of transplantation, and longer dialysis time [95,96,112-114]. Several studies have linked PTH levels after kidney transplantation with clinical outcomes. Persistent hyperparathyroidism is associated with graft loss and mortality [112,115,116].
The main hypotheses that explain the association of hyperparathyroidism with renal graft loss are: deposition of calcium phosphate crystals in the kidneys, which leads to tubular injury and interstitial fibrosis, caused by hypercalcemia and increased phosphorus excretion secondary to hyperparathyroidism; and vascular calcification of the vessels supplying the graft, known to be associated with hyperparathyroidism, which can interfere with the quality of the anastomosis and vascularization of the renal graft [106,115,117]. It is speculated that the higher mortality is related to the deleterious effects of PTH on the cardiovascular system [118]. Data on the relationship between pre-transplant PTH levels and post-transplant clinical outcomes are scarcer. Roodnat et al showed that high PTH levels in the pre-kidney transplant period were associated with a higher risk of graft loss [117]. In contrast, Isaksson et al showed that lower PTH levels in the pre-transplant period are associated with a higher risk of cardiovascular events in the post-transplant period [119]. The association of low PTH levels with worse cardiovascular outcomes has already been demonstrated in dialysis patients and this is one of the hypotheses that explains the finding of this study [25-27, 119]. Another possible explanation is bone disease associated with reduced PTH, usually with low remodeling. Low remodeling disease reduces the bone capacity to act as a buffer for excess calcium and phosphorus, which favors vascular calcification [79,80]. Despite these associations, in the current literature there is no PTH value in dialysis patients to indicate parathyroidectomy before transplantation, or who contraindicate kidney transplantation.
Calcemia appears to follow a biphasic pattern after successful kidney transplantation. Initially, calcium levels fall, possibly due to cessation of the use of calcium-based binders and vitamin D analogues. After this period, calcium levels increase and hypercalcemia becomes a common finding, especially between three and six months after transplantation [7,108]. Wolf et al showed that hypercalcemia develops in up to 50% of transplant patients, with a peak at eight weeks and subsequent progressive decline until the end of the first year, when it stabilizes [95]. The main factors involved in the pathophysiology of hypercalcemia are persistent hyperparathyroidism associated with reduced bone resistance to the actions of PTH, which results in the release of calcium from the bone into the blood; and increased levels of active vitamin D, whose production is reestablished by the functioning renal graft, in addition to the increase of its synthesis by the drop in FGF23 levels after transplantation [97,120]. The main risk factors for hypercalcemia are persistent hyperparathyroidism, which is associated with more severe hyperparathyroidism before transplantation, and higher calcium levels in the pre-transplant period [95,121]. Several studies have shown the association of hypercalcemia with worse renal outcome [91,101,122,123]. The negative impact of hypercalcemia on the renal graft may be secondary to graft nephrocalcinosis or to the vasoconstrictor, aquaretic and diuretic effect of calcium on the kidney, seen in acute hypercalcemia [124,125]. The first retrospective study that evaluated tubulointerstitial calcifications in protocol biopsies at zero, six and 12 months after kidney transplantation showed that tubulointerstitial calcification was more common in patients with hypercalcemia [126]. Hypercalcemia after kidney transplantation was also associated with higher mortality. It is speculated that vascular calcification is involved in the pathophysiology [91,101].
Calcitriol, the active form of vitamin D, tends to increase progressively after successful kidney transplantation, until it normalizes after one year of transplantation. The recovery of renal function after transplantation, inappropriately high PTH and hypophosphatemia are responsible for the increased conversion of 25(OH)D3 to calcitriol by the kidneys [127]. Although FGF23 inhibits the production of calcitriol, its levels tend to decrease rapidly after kidney transplantation, improving calcitriol production in the first few months after transplantation. Evenepoel et al showed that good renal function after transplantation was the main predictor of increased calcitriol values; high levels of FGF23 and low levels of PTH were independently associated with low levels of calcitriol [128]. Unlike the active form, nutritional vitamin D insufficiency (25(OH)D3 < 30>
3.2 - Bone disease after kidney transplantation
At the time of transplantation, the bone structure of the chronic renal patient is usually not normal. In addition to renal osteodystrophy, which is part of the diagnosis of CKD-MBD, other factors participate in the pathophysiology of bone disease, such as age, sex, etiology and duration of CKD, drug use, gonadal dysfunction, and metabolic disorders. Bone disease in the post-kidney transplant period is a result of preexisting bone disease plus bone damage that begins after transplantation, which is influenced by immunosuppressive drugs, persistent hyperparathyroidism, and kidney graft function [7]. This bone disease increases the risk of fracture, hospitalization and patient mortality. The main types of bone disease in renal transplant patients are bone fracture, renal osteodystrophy, osteoporosis and osteonecrosis [144].
In the first five years after transplantation, up to 22.5% of patients may suffer a bone fracture, an incidence four times higher than in the general population [108]. Ten percent of kidney transplant recipients will have a fracture in their lifetime [145]. Jiménez et al evaluated the prevalence of vertebral fracture in the long term, 10 years after transplantation, in patients with stable renal function (mean creatinine of 1.7 mg/dL), and found a prevalence of 61%, the majority being mild [146]. A BMD at the trochanter, as well as higher levels of PTH at one year after transplantation, were the risk factors for vertebral fracture in this study [146]. However, more recent studies have shown a decrease in the fracture rate, probably related to improved treatment of preoperative renal osteodystrophy and reduced use of corticosteroids after kidney transplantation [145]. A recent prospective study showed an incidence of fractures of 14.1/1000 transplant recipients/year. It also showed that low BMD in the hip and femoral neck at the time of transplantation was a predictor of fracture incidence. PTH and other bone remodeling biomarkers failed to predict fractures in this study [147]. These studies support the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD-MBD) recommendation that indicates a more liberal use of bone densitometry to predict fractures across all spectra of kidney disease, including transplant recipients [42]. In comparison with patients on dialysis, the risk of fracture is 34% higher in the first three years of transplantation. After this period, the risk of fracture becomes lower than in kidney transplant recipients [144]. Patients with fractures have a 60% higher mortality than the general population [108].
Adynamic bone disease is currently the main type of bone lesion found in patients with CKD in the pre-kidney transplant period [44]. Studies with bone biopsy after transplantation are scarce and recruited few patients, and due to this, little is known about the evolution of preexisting renal osteodystrophy in the post-transplant period. In a study with bone biopsy after two years of kidney transplantation, Neves et al showed that 82% of patients had alterations in at least one of the parameters of the TMV system [148]. Regarding bone remodeling, it was normal in 48% of patients, high in 26% and also low in 26%. Forty-eight percent of these patients had delayed mineralization and 37% had reduced bone volume [148]. Evenepoel et al performed paired bone biopsies at the time of kidney transplantation and one year after surgery [149]. Mineralization and bone volume were normal in 83.3% and 91.7%, respectively, and there was little change in these parameters one year after kidney transplantation [149]. At the time of kidney transplantation, bone turnover was normal in 52.8% of patients, high in 2.8% and low at 44.4%. One year after kidney transplantation, 36% of patients had normal bone turnover and 64% had low bone turnover. Accumulated corticosteroid dose was associated with trabecular bone loss. There was no association between bone loss and PTH [149]. Carvalho et al performed kidney biopsy in kidney transplant patients two months, two and five years after transplantation. There was a reduction in bone activity, suggesting an increased risk of adynamic bone disease and loss of bone volume [150]. More recently, Keronen et al performed bone biopsies in dialysis patients and repeated it after two years of transplantation, or after two years of baseline biopsy if the patient was not transplanted [145]. A proportion of patients with high-remodeling disease dropped from 63% to 19% two years after kidney transplantation, while low-remodeling disease increased from 26% to 52% [145]. In patients who remained on dialysis, the proportion of high-remodeling disease remodeling decreased from 69% to 31% and low remodeling increased from 8% to 38% [145]. Abnormal bone mineralization increased in transplant patients. The mineral biochemical parameters analyzed (calcium, phosphorus, 25(OH)D3, PTH, bone AF, and osteocalcin) and bone densitometry were not associated with histomorphometric changes in bone biopsy [145]. These studies show a tendency for bone remodeling to decline after kidney transplant. Low remodeling disease and bone mineralization defects prevail after transplantation. Another notable fact is the lack of correlation between the biochemical parameters, which are much more available, and the findings of bone biopsy, which is rarely performed after kidney transplantation.
Osteoporosis is a bone disease characterized by microstructural changes that result in reduced bone mass, bone fragility and predisposition to fractures [144]. Its prevalence in kidney transplant recipients is close to 30% [108]. Bone loss after kidney transplantation is rapid and the BMD is reduced by 4-10% in the first six months after transplantation [144]. However, studies have shown a decrease in bone loss after transplantation in recent years, which reflects the use of corticosteroid dose minimization protocols [149,151]. Other factors besides preexisting bone disease contribute to the loss of bone mass after kidney transplantation, such as: the use of corticosteroids and hypogonadism [125]. The use of corticosteroids is one of the main factors involved in the development of osteoporosis after kidney transplantation and causes a reduction in BMD through decrease in bone formation and density, especially in the trabecular bone of the axial skeleton [144]. This class of drugs reduces the differentiation and proliferation of osteoblasts, stimulates their apoptosis, as well as that of osteocytes. In addition, they exert indirect effects on bone, by reducing the synthesis of testosterone, estrogen and adrenal androgens [144]. Other factors associated with bone mass loss were shown in the study by Evenepoel et al. Age, female sex, body mass index (BMI), high levels of PTH and bone FA were independently associated with a lower BMD in this study [147]. Bone mass loss is a predictor of bone fracture, associated with high patient morbidity and mortality, as previously described.
Osteonecrosis or avascular bone necrosis is pathologically characterized by the death of bone tissue, and has a strong relationship with the use of corticosteroids [125]. Different studies show a prevalence between 3-40%, but, like osteoporosis, this prevalence has been falling. in recent years due to the use of immunosuppression protocols with lower doses of corticosteroids [125]. Recently, Felten et al showed an incidence of osteonecrosis of 2.2
In view of all this evidence, it is concluded that CKD-MBD has a profound association with relevant clinical outcomes in renal transplant patients. More studies are needed to find optimal levels for mineral biochemical parameters, in an attempt to optimize treatment and minimize the effect of the disease on both the skeletal and cardiovascular systems.
Statements and Declarations:
The authors declare no conflicts of interest between the investigators and the patients, and other institutions.
Funding agency name:
None.
Indication of authors' contribution:
Ana Elisa Souza Jorge, Maria Goretti Moreira Guimarães Penido, Maria Marta Sarquis Soares were responsible for the research idea, study design, data acquisition, supervision, data analysis and article writing.
Acknowledgments
The authors would like to acknowledge all patients for their participation in our study and our colleagues for their valuable clinical assistance.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
