AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Monica A. Schmidt, BIO5 Institute, University of Arizona, 1657 E. Helen St, Tucson AZ 85718.
Citation: Monica A. Schmidt, Joseph Opoku, Hillary L. Mehl, (2023), Enzymatic Degradation of Aflatoxin Accumulation in Dry Maize Kernels as a Promising Post-Harvest Mitigation Strategy. J, Biotechnology and Bioprocessing 4(3); DOI: 10.31579/2766-2314/090
Copyright: © 2023, Monica A. Schmidt. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 26 April 2023 | Accepted: 11 May 2023 | Published: 16 May 2023
Keywords: maize; aflatoxin; enzyme; degradation; food security; post-harvest
Background: Aflatoxin is one of the most problematic fungal-produced toxins as it is responsible for massive global agricultural losses and is deleterious to both human and animal health. Contamination of crops by certain strains of Aspergillus fungi accumulate aflatoxin in both post- and pre-harvest conditions.
Methods: In this report, we tested the aflatoxin-degradation efficiency of an endogenously expressed enzyme in harvested maize kernels. In post-harvest conditions, equivalent loads of A. flavus were used to infect harvested maize kernels previously engineered to express an aflatoxin-degrading enzyme from the Honey mushroom fungus.
Results: No measurable, or significantly reduced, levels of aflatoxin were detected in all the enzyme-expressing harvested kernels initially and then 3 days post –harvest the transgenic kernels amassed aflatoxin.
Conclusions: This is the first report of an enzyme degradation of aflatoxin in a crop in harvested kernels. This demonstrates the potential of this strategy to aid in the mitigation of aflatoxin in post-harvest conditions.
Mycotoxins are toxic fungal-produced compounds that cause global economic and health issues. Estimates currently cite as much as 80percentage of world crops are contaminated with detectable levels of one or more mycotoxins [1] with at least 25percentage of crops exceeding regulated Codex Alimentarius limits [2]. Although there is difficulty in accurately measuring the prevalence of mycotoxins in global crops, the consumption of these compounds in diets has been correlated to deleterious health effects in humans and animals. These fungal-produced secondary compounds are responsible for massive economic losses and they adversely affect global trade. Aflatoxins are considered the most toxic group of mycotoxins as they are known carcinogenic compounds, being linked to liver cancer [3-4], implicated in the stunting of children’s growth [5] and sometimes cause acute death [6]. Aflatoxin-producing Aspergillus species can infect many crops but have an affinity for cereals and nuts. Maize is often the most impacted crop, with annual global losses due to aflatoxin contamination of $160 million in the US [7] and $450 million in sub-Saharan Africa [8].
Infection of maize with aflatoxin-producing Aspergillus species, including A. flavus, can occur both pre-harvest while the crop is actively growing and in post-harvest during storage. Pre-harvest aflatoxin mitigation strategies include breeding [9] or genetic engineering [10-13] for fungal resistant cultivars, use of RNAi suppression technology to inhibit aflatoxin production [14-16], competitive exclusion of aflatoxin-producing fungi using non-toxigenic strains [17-18], and agricultural practices such as insect control and fungicide applications. Post-harvest strategies largely focus on proper storage with moisture levels of particular concern as Aspergillus species grow in moist, humid environments, insect control as bite wounds serve as a gateway for the opportunistic fungus, low oxygen or high ozone environments and detection and sorting of contaminated kernels/cobs ([19] for review of post-harvest mechanisms).
A promising post-harvest decontamination strategy involves the enzymatic conversation of aflatoxin to innocuous compounds. Prior research [20] demonstrated the successful pre-harvest degradation of aflatoxin from contaminating A. flavus conditions in developing maize kernels that were expressing an endoplasmic reticulum (ER)-targeted embryo-expressed aflatoxin-degrading enzyme isolated from the edible Honey mushroom (Armillariella tabescens) [21]. In this research, we investigate if reducing /eliminating aflatoxin by enzymatic degradation in maize kernels would also be an effective post-harvest strategy in dry stored kernels.
Plant Material
Dry kernels were harvested from a control nontransgenic maize plant (Null) and three independent transgenic maize lines (Enz7, Enz8, Enz10) (Zea mays Hi II hybrid A 188 and B73 background) previously shown to be expressing an inserted aflatoxin-degrading enzyme (GenbankAccession AY941095) [20]. The inserted cassette in these transgenic maize plants consisted of a codon-optimized 2.166 kb open reading frame of an ER-targeted aflatoxin-degrading enzyme expressed by an embryo-specific maize globulin-1 promoter (Genbank Accession AH001354.2) [20]. Cobs from three transgenic lines (Enz 7, Enz 8, and Enz 10) and the nontransgenic maize line (Null) were removed from ears grown in the greenhouse. Kernels from the 3 transgenic enzyme-degrading aflatoxin and null control maize plants were harvested, dried, and stored at room temperature until they were used for infection experiments.
Aspergillus flavus inoculum preparation
Aspergillus flavus isolate AF13 [22] was obtained from the USDA-ARS Aflatoxin Biocontrol Lab culture collection. A granule from a silica gel stock vial of the isolate was transferred to 5/2 agar (5percentage V-8 vegetable juice, 2percentage agar, pH 5.2) and the plate was incubated in the dark at 31°C for 5 days. Approximately 10 agar plugs colonized with mycelia and spores of the isolate were transferred to a vial containing 3.5 ml of sterile water, and this was used as the working stock for growing up inoculum. Conidial suspensions (15 µl) from the water vial stock were transferred to wells in the center of 5/2 agar plates. After incubation at 31°C for 6 days, conidia were picked up from plates using sterile cotton swabs and suspended in 10 ml of sterile 0.02percentage Tween-80. Conidial suspensions were vortexed and transferred into nephelometric turbidity unit (NTU) vials, and turbidity was measured using a calibrated turbidimeter (model 965-10, Orbeco-Hellige, Farmingdale, NY) as described previously [23]. The final spore concentration was calculated using a standard curve for NTU versus spores/ml using the formula: spores/ml = NTU × 49,937. The spore suspension was diluted to a final concentration of 50,000 spores/ml in sterile water.
Kernel inoculation
Five-gram samples of kernels from each maize line were disinfested by submerging kernels in 10percentage commercial bleach (sodium hypochlorite) for 3 min. followed by 70percentage ethanol for 5 min. and a final rinse in sterile water. Kernels were air dried on sterile paper towels in a biosafety cabinet then transferred to 250 ml Erlenmeyer flasks (5 g per flask). The moisture content of a subsample of each maize line (4 g) was measured using a Halogen Moisture analyzer (Model HC103/03, Mettler Toledo, Columbus, OH). The moisture content of kernels in each flask was then adjusted by adding 10 µl (5 × 104 spores) of A. flavus isolate AF13 in the volume of water needed to bring kernel moisture to 25percentage. Flasks were then gently shaken to evenly coat kernels with the inoculum. For the non-inoculated control of each maize line, only sterile water was added to flasks. Flasks were sealed with gas permeable Bug Stopper plugs (Whatman, Piscataway, Nj) and transferred to an incubator set at 31°C. Three replicate flasks of each maize line were taken out of the incubator one, two, and three days after inoculation. Spores were washed off the kernels by adding 20 ml of 0.01percentage Tween-80 to each flask followed by shaking at 150 rpm for 10 min. on a Model HS 501 Digital Shaker (IKA Works Inc., Wilmington, NC). Spore suspensions were filtered through Miracloth and collected in 50 mL Falcon tubes. Kernels were washed again with 20 ml of sterile water, and the suspension was again filtered through Miracloth into the 50 mL tube. The ~40 mL spore suspensions were vortexed, and spore concentrations were measured using turbidity as described above. Washed kernels were dried at 60 °C for 36 h then ground for 35 s using an IKA A11 basic S1 grinder (IKA Works Inc., Wilmington, NC).
Aflatoxin quantification
For each sample, total aflatoxins (aflatoxin B1 + aflatoxin B2) were extracted from 1.5 g ground kernels with 15 ml of 70percentage methanol. Extracts were separated using thin layer chromatography (TLC) and aflatoxin was quantified using scanning densitometry as described previously [20, 24]. Briefly, 12 µl of extract was spotted on 20 × 20–cm TLC glass plates (Silica Gel 60 F254, Millipore) along with an aflatoxin standard (Aflatoxin Mix Kit-M, Supelco, Bellefonte, PA), and plates were developed with diethyl ether: methanol: water (96:3:1). The presence or absence of aflatoxins B1 and B2 were confirmed visually under ultraviolet light (365 nm) and quantified on plates using scanning fluorescence densitometry with a CAMAG TLC Scanner 3 (Camag Scientific Inc., Wilmington, NC). Quantities of aflatoxin relative to the standard were used to calculate total nanogram (ng) aflatoxin per gram (g) kernels (equivalent to parts per billion; ppb).
Means and standard error for all metrics measured from the maize kernel transgenic lines were determined. All quantitative metrics were performed in triplicate. Significant difference from the nontransgenic control to any experimental line was determined by student T-test analysis, plessthan0.05 levels.
An aflatoxin-degrading enzyme from the Honey mushroom Armillariella tabescens was subcellularly targeted to the ER to enhance its stability in maize kernels. The localization of inserted enzymes into the endomembrane system is a technique that has been demonstrated to be effective at both enhancing an enzyme’s stability and allowing an introduced enzyme’s accumulation. The ER-targeted enzyme cassette was under the regulation of an embryo-specific promoter as previous research had shown embryo metabolic activity was positively correlated with aflatoxin resistance in maize cultivars [26]. The inserted aflatoxin-degrading enzyme was detected via mass spectroscopy analysis of ground transgenic kernel tissue in all three transgenic Enz lines in both developing kernels and dry stored kernels [20]. In pre-harvest conditions, previous research on ER-targeted aflatoxin-degrading enzyme expressing maize kernels found undetectable aflatoxin accumulation in all 3 transgenic Enz maize lines after 14-days of infection and significantly reduced (90percentage reduction) aflatoxin loads after 30-days, compared to nontransgenic control levels [20]. To determine the effectiveness of this successful pre-harvest strategy in a post-harvest situation, non-inoculated aflatoxin-degrading Enz transgenic maize kernels were harvested, dried, and stored at room temperature prior to being infected with A. flavus strain AF13.
Inoculated kernels were incubated for 1 day, 2 days or 3 days at which time the aflatoxin load was quantified by TLC analysis. Kernels were incubated under non-optimum conditions for storage (e.g., warm temperature and high moisture/humidity), but conditions simulated those that could occur in poorly maintained storage environments. After 1-day, infected dry null kernels had an average 2.5 +/- 0.3 ppb log aflatoxin load compared to undetectable aflatoxin amounts in all three Enz transgenic maize kernels (Figure 1).
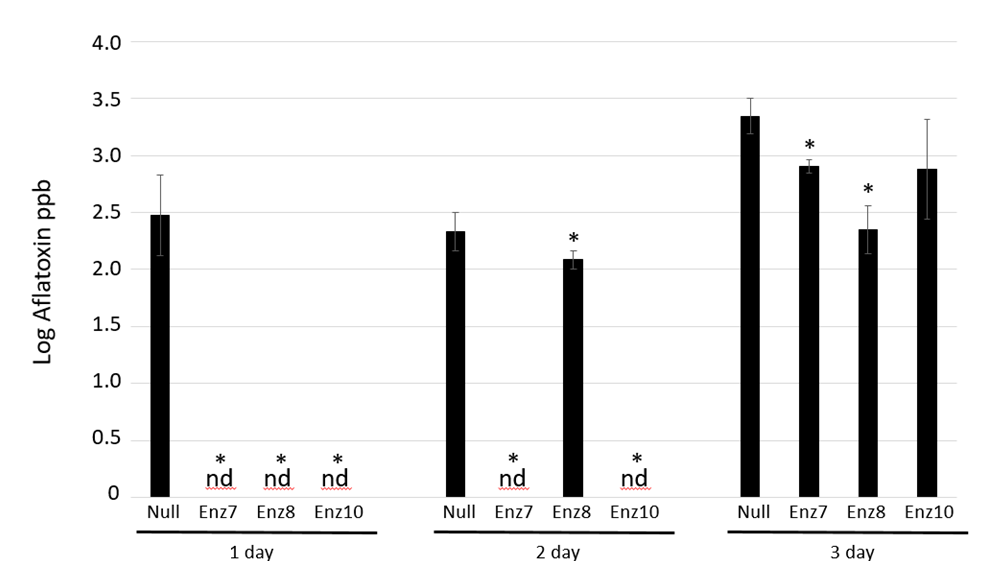
Harvested kernels were inoculated with Aspergillus flavus strain AF13 and infection was allowed to occur for 1 day, 2 days or 3 days. Aflatoxin loads were quantitated via thin layer chromatography (TLC) analysis at the three time points. Log aflatoxin means ± SE are shown. * Denotes significant difference from the nontransgenic control at plessthan0.05 levels, based on student T-test. Replicates were performed in triplicate. nd denotes not detectable at a limit of 20
Figure 1: Harvested Kernel Infection Assay of Aflatoxin-degrading Transgenic Maize
After 2 days of infection, the null kernels had 2.3 +/- 0.1 ppb log aflatoxin compared to undetectable in two Enz transgenic lines (Enz7 and Enz10) and 2.1 +/- 0.1 ppb log in Enz 8 line. The TLC methodology used to quantitate aflatoxin has a detection limit of 20 pbb (log value 1.3 ppb), hence the dry kernels with non-detectable aflatoxin loads had less than 20 pbb. Three days post-Aspergillus inoculation, null kernels contained 3.3 +/- 0.2 ppb log compared to Enz7, Enz8 and Enz10 having 2.9 +/- 0.1 ppb log, 2.3 +/- 0.2 ppb log and 2.9 +/- 0.4 ppb log aflatoxin, respectively. After a single day after inoculation, kernels from all 3 transgenic aflatoxin-degrading maize lines had no detectable aflatoxin accumulation compared to considerable levels already accumulated in the null controls. At two days post Aspergillus-infection, two (Enz7 and Enz10) of the three transgenic maize lines still displayed undetectable amounts of aflatoxin while one line (Enz8) had some aflatoxin accumulation but was significantly lower than the amount accumulated in the null controls as determined by student t-test plessthan0.05. Three days after inoculation, all dry kernels, both transgenic and null, accumulated aflatoxin with two of the lines (Enz7 and Enz8) accumulating significantly lower amounts compared to null controls (student t-test plessthan0.05). Considering most countries have regulatory aflatoxin limits around 20 pbb of aflatoxin as a consumption threshold, this methodology would be able to keep maize kernels within an acceptable range in short-term post-harvest conditions.
To investigate if the Enz transgenic maize dry kernels had reduced aflatoxin accumulation because they had a reduced initial Aspergillus infection, we quantified the amount of fungal growth on each sample by spore counts. Figure 2 shows the results of fungal spores detected in all samples across the 3 days of the infection experiments. After 1 day infection, spores on the null kernels were 7.07 +/- 0.2 log spore count compared to Enz7, Enz8 and Enz10 having 6.65 +/- 0.2 spores, 6.78 +/- 0.3 log and 6.50 +/- 0.1 log, respectively. Day two post infection, no significant difference in spore count was noted as null kernels had 7.84 +/- 0.1 log, compared to the three transgenic maize lines Enz7, Enz8 and Enz10 had 7.43 +/- 0.1 log, 7.40 +/- 0.3 log, 7.60 +/- 0.3 log, respectively. Finally, day 3 post infection, spore count on null kernels was 7.61 +/- 0.1 log compared to Enz7, Enz8 and Enz10 being 7.66 +/- 0.02 log, 7.70 +/- 0.01 log and 7.61 +/- 0.04 log, respectively. Consistently, no Enz transgenic dry kernels samples were determined to be significantly different from the null controls regarding the number of spores detected. This indicates that the Aspergillus infections were done consistently, and the amount of fungal inoculum and subsequent growth were comparative across all samples, yet when correlated with aflatoxin accumulation (Figure 1) it demonstrated the inserted aflatoxin-degrading enzyme was, at least initially, able to degrade the produced aflatoxin from the contaminating A. flavus.
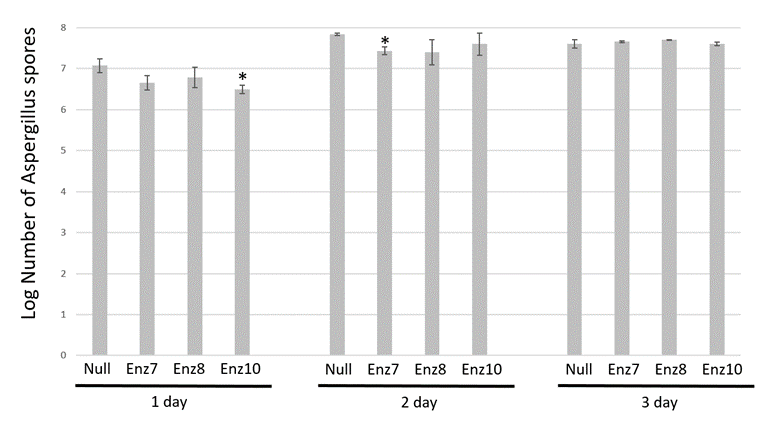
Spore counts are shown as log mean ± SE of three replicates. * Denotes means are significantly different from null nontransgenic as determined by student T-test analysis, plessthan0.05.
Figure 2: Spore count comparison of Aspergillus-infected transgenic maize harvested kernels.
Enzymatic degradation has been an effective post-harvest aflatoxin mitigation strategy typically employed by the exogenous mixing of contaminated food items with a microorganism, or its isolated degradation enzyme, capable of converting aflatoxins to innocuous compounds [25, 27].
This research is the first report of engineered crop plants capable of degrading contaminating aflatoxin in post-harvest conditions. Future reiterations of this research could focus on the enhancement of the aflatoxin-degrading enzyme accumulation in seeds, perhaps by expressing the enzyme under a strong kernel-specific promoter.
Aflatoxin has been a major global economic and health concern for decades and it is predicted that this food contaminant will increase in both frequency and severity if global climate temperatures continue to increase [28]. The degradation of accumulated aflatoxin by contaminating Aspergillus species on food items by engineered crops might play a role in eliminating, or at least reducing, this carcinogenic compound thereby enhancing global food security and safety.
This work was financially supported by United States Department of Agriculture National Institute of Food and Agriculture from the Improving Food Safety program, grant # 2019-67017-29644, title “Characterization and Enhancement of Higs in Aflatoxin-Free Transgenic Maize”. We thank Cyrus Mao for maintaining and harvesting the transgenic Enz maize kernels. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
The authors declare they have no known competing financial interests that might influence the work reported in this paper.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
