AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Yolanda Carrascal, Cardiac Surgery Department University Hospital Avda. Ramón y Cajal, spain.
Citation: Yolanda Carrascal, Echocardiography Parameters Predicting Postoperative Atrial Fibrillation: Their Influence On Early Left Atrial Remodelling And Right Ventricular Function After Heart Valve SURGERY, Clinical Cardiology and Cardiovascular Interventions.2(1);Doi:10.31579/2641-0419/012
Copyright: © 2019, Yolanda Carrascal, This is an open-access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in anymedium, provided the original author and source are credited.
Received: 03 February 2019 | Accepted: 28 February 2019 | Published: 08 March 2019
Keywords: atrial fibrillation; echocardiography; cardiac surgery
Objective: To identify relationship between echocardiographic parameters variations and risk of postoperative atrial fibrillation (POAF) after cardiac valve surgery.
Design: Prospective. Case-control study.
Setting: A university hospital.
Participants: We analysed the incidence of POAF in 90 patients undergoing elective heart valve surgery. POAF was considered when episodes equal or longer than 5 minutes, and those under 5 minutes with hemodynamic disturbances.
Interventions: None.Measurements and main results: POAF incidence was 36.7%. Preoperative echocardiographic study showed higher systolic pulmonary pressure (p: 0.047) and longer atrial electromechanical interval (AEI) (0.049) in POAF group. Postoperative echocardiographic evaluation revealed higher TAPSE decreasing related with preoperative values (8.18± 4.33 mm in No-POAF vs. 10.35 ± 3.83 mm in POAF group) (p: 0.026). In multivariate logistic regression POAF correlated with age> 65 years (p: 0.007) OR: 4.80; IC 95% (1.52-15.14), longer preoperative AEI (p: 0.042) OR: 1.029 IC 95% (1.001-1.059), higher TAPSE reduction (p: 0.040) OR: 1.15 IC 95% (1.006-1.316) and postoperative left atrial volume index> 36 ml / m2 (p = 0.0203) OR: 3.63; 95% CI (1.23-11.92).Conclusions: After heart valve surgery, POAF favoured right ventricular dysfunction (evidenced by higher postoperative TAPSE decreasing) and impaired early left atrial remodelling. In older patients and those with preoperative longer AEI, biatrial pacing and pharmacological prophylaxis might prevent these undesirable POAF effects.
Up to 50% of patients undergoing heart valve surgery presents postoperative atrial fibrillation (POAF).1-3 Atrial structural remodeling is considered a risk factor for their appearance. Variations in some echocardiographic parameters have been related to increase risk for atrial fibrillation (AF) after coronary artery bypass graft (GABG) surgery. Longer A-wave and atrial electromechanical interval (AEI), increased E/A ratio, and higher diameter and indexed left atrial volume were more prevalent in patients suffering from POAF.4-6 Patients with preoperative abnormal right ventricular performance index were more likely to present POAF after GABG.7 Anyway, their predictive value has not been adequately validated after heart valve procedures.
Cardiac surgery leads to atrial remodeling due to the cumulative effect of fibrosis, scarring, ischemia and myocite necrosis.8-10 In patients with heart valve disease, these factors were associated with increased atrial volume and diameter.8-10
We aimed to evaluate characteristics and predictive value of echocardiographic parameters associated with POAF in patients undergoing valve surgery. Their preoperative identification would permit to apply prophylactic measures in order to prevent POAF and its related complications in this group of patients.
MATERIALS AND METHODS
Clinical and echocardiographic records were prospectively analyzed in 90 patients with primary diagnosis of heart valve disease (isolated or associated with coronary artery disease) satisfying requirements for heart surgery under cardiopulmonary bypass (CPB). Informed consent was obtained from each patient and the study protocol, conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
Previous episodes of AF, preoperative beta-blocker therapy, severe left ventricular dysfunction (left ventricular ejection fraction< 30> 2 mg/dl) or urgent surgery were considered exclusion criteria.
Correlation between preoperative and postoperative echocardiographic data and prediction of POAF was included as primary end point. We considered POAF episodes equal or longer than 5 minutes and those less than 5 minutes, with hemodynamic disturbances. Clinical and echocardiographic variables were preoperatively collected. After surgical procedure, all patients had continuous electrocardiographic monitoring until seventh POD (inclusive). Events related to cardiac rhythm disturbances were recorded daily and analyzed by a cardiologist independent of cardiac patient clinical management. Before hospital discharge (sixth POD) all patients underwent postoperative transthoracic echocardiography with Doppler imaging analysis [Vivid 7; GE Medical Systems, Vingmed, Norway].
Echocardiographic methodology (definitions):
Atrial Electromechanical Interval (AEI): time (in milliseconds) from the onset of electrocardiographic P wave to the beginning of atrial systole (movement backward in the atrioventricular plane) measured in the lateral face of left atrium. Four chambers were obtained in apical view, from the mitral lateral ring in transthoracic echocardiography and measured as the average of 3 cardiac cycles.A-wave: late peak ventricular filling velocity during atrial systole (m/s). It was measured using pulse-wave Doppler across the mitral valve plane in 4-chamber view and calculated from the average speed of 5 consecutive beats in M-Mode.
E-wave:doppler peak wave velocity during early diastolic ventricular filling.
E/A ratio: ratio of the peak early (E-wave) and late (A-wave) ventricular filling velocities.
TAPSE (tricuspid annular plane systolic excursion) assessed with M-mode in an apical 4-chamber view (0º angle related to right ventricular free margin) describes longitudinal right
ventricular myocardial shortening. It measure the amount of longitudinal displacement of tricuspid annulus at peak-systole. (Normal value > 16 mm).
Left atrial measures: Left atrial linear dimension was measured in 2-D mode and parasternal long-axis view and left atrial volume, in apical 4-chamber view at the maximal atrial dimension, using modified Simpson’s rule.
Statistical analysis was conduced with SPSS 22.0 software. Quantitative variables were expressed as mean ± standard deviation or as median, for asymmetric distributions. Qualitative variables were expressed as absolute value and percentage. Association between variables were identified using χ 2 or Fisher exact test when qualitatives and t of Student or U of Mann Whitney in quantitatives. Association between risk factors and analyzed events in univariate analysis (p < 0>
90 patients were prospectively recruited between 2011 and 2013. Incidence of POAF was 36.7 %. In all patients, POAF developed during 2º POD, with a median of 45 hours after surgery. Clinical, demographic and surgical variables are detailed in Tables 1 and 2.
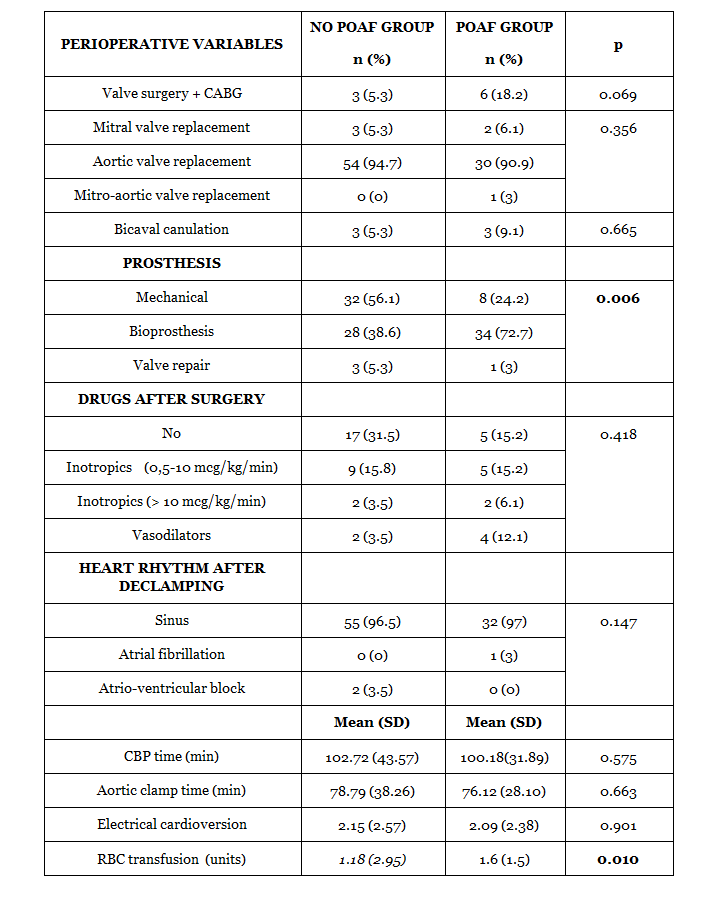
Patients who developed POAF were significantly older, with a median of 74 years vs. 66 years in patients who did not present arrhythmia. No clinical or surgical analyzed risk factors were related with atrial arrhythmia except an increased trasfusional demands. In multivariate logistic regression, POAF correlated with age> 65 years (p: 0.007) OR: 4.80; IC 95% (1.52-15.14), longer preoperative AEI (p: 0.042) OR: 1.029 IC 95% (1.001-1.059), higher TAPSE postoperative reduction (p: 0.040) OR: 1.15 IC 95% (1.006-1.316) and postoperative left atrial volume index> 36 ml / m2 (p = 0.0203) OR: 3.63; 95% CI (1.23-11.92).
In all patients, left atrial diameter (LAD) and left atrial volume index (LAVI) were increased and pulmonary hypertension (at least moderate) was identified. Preoperative echocardiography parameters showed a certain grade of diastolic dysfunction, which seems more related to advanced age than to hypertension. Although E/A ratio values were normal, A-wave was greater than E-wave. Age ≥ 65 years correlated with an increased A-wave (p: 0.035) RR 5.56 (95% CI 1.12-28.82) and increased systolic pulmonary artery pressure (p: 0.016) RR 4.47 (95% CI 1.25-15.93).
When comparing both groups, a significant increasing in AEI delay, systolic pulmonary artery pressure and gradient between right atrium and ventricle were observed in POAF group (Table 3).
However, there was no difference in A-wave velocity or in E/A ratio, traditionally associated with atrial remodeling and proposed as incremental risks factor for POAF.
In postoperative echocardiogram, decrease in LAD and LAVI were observed, nevertheless, early atrial remodeling was only statistically significant in No-POAF patients. When calculating (for each patient), the difference between pre and postoperative LAVI, this value decreased in No-POAF group and increased when POAF was present (3.62 ± 8.15 ml / m2 vs. -1.09 ± 11.88 ml / m2, respectively) (p = 0.043). Average rate of echocardiographic left atrial reverse remodeling was 5.82 ± 20% in No-POAF vs. -7.67 ± 30.2% in POAF group (p: 0.002). In conclusion, there was no significant reverse atrial remodeling when POAF appeared. Early atrial remodelling was significantly reduced over 65 years (3.22±8.4 ml/m2 vs. -2.4±10.04 ml/m2) (p: 0.036) and was not influenced by age under 65. No influence of pulmonary hypertension was observed on atrial remodeling. Right ventricle functionality was indirectly assessed by TAPSE measurement. Concerning preoperative values, TAPSE decreased in both groups with no significant differences(Table4).
However, when analyzing the difference between pre and postoperative TAPSE values in each patient, showed statistically significant reduction when POAF occurred (10.35 ± 3.83 mm vs. 8.18 ± 4.33 mm, respectively) (p = 0.026). Although TAPSE decrease is independent of patient age, POAF impact over right ventricular function was higher in patients younger than 65 years (11.42±4.27 vs. 7.36±5) (p: 0.083). No other factors influencing TAPSE reduction were found and no changes related to age were observed in the rest of echocardiographic variables considered.
Follow-up was conducted in 100% of survival patients. Median follow-up was 49 months (interval: 32-64 months). Only 1 patient died during follow-up due to a late prosthetic valve endocarditis. No mortality or neurological events related with new onset AF were identified. In 14 patients (15.9%) at least one episode of AF was observed during follow-up. AF was more frequently detected in patients with previous POAF episodes (29.1% vs. 8.9%, respectively) (p: 0.031). In multivariate analysis, age (p: 0.0218) OR: 1.11; IC 95% (1.01-1.22) and longer postoperative AEI (p: 0.0273) OR: 1.07 IC 95% (1.0078-1.14), were identified as incremental risk factors for AF during follow-up.
The interpretation of the influence of preoperative echocardiographic variables on the appearance of POAF in patients with valve disease is complex, since we analyze values unusual among the normal population. In heart valve disease, preoperative diastolic dysfunction is considered frequent and manifested by an increased A-wave value, although the E/A ratio maintains within the normal range. Relationship between diastolic dysfunction and impaired atrial electrical activity is uncertain, but it is known that risk of AF is higher when E-wave is greater than 0.84 m/s, as baseline data in our sample.11 Furthermore, increased baseline atrial volumes and mean values of pulmonary systolic pressure were common. These changes were especially significant over 65 years, although in the logistic regression analysis, only the longer duration of AEI was an independent preoperative risk factor for POAF appearance.
In the same way than previous works, age over 65 was main risk factor for POAF after heart valve surgery in our population.9,13-15 Ageing is associated with atrial fibrosis and structural changes9,15,16 which impair and/or slow stimulus conduction between sinus and atrioventricular node and prolong AEI.6,8 Increasing in E and A-waves velocity, E/A ratio and LAD, positively correlated with higher incidence of AF, both among general population and after cardiac surgery.4-6,17,18 However, our results suggest that valve disease and age-related changes in atrial function could modify these preoperative values8-10,19 invalidating their effectiveness as predictors of POAF. It seems that, after heart valve surgery, POAF genesis is mainly related to atrial conduction impairment, instead of to a contractility disorder. Prolonged preoperative AEI is the only independent predictor of POAF. Atrial electromechanical delay reflects structural atrial changes that retard transmission of intra- and interatrial electrical impulse and might be due to diastolic dysfunction. AEI duration among 115-147 ms is related to adequate prediction of POAF after coronary artery bypass graft surgery.4,6,17,20 In the analyzed population, AEI was significantly lower, probably due to the exclusion of patients with preoperative beta-blocker therapy (with a recognized prophylactic effect on POAF) in our sample.4,6,17,20 Postoperative echocardiographic controls showed an early reduction of LAVI and LAD after valve surgery.21,22 Early atrial reverse remodeling was also appreciated in our sample, but only among No-POAF patients. This phenomenon was significantly higher among elderly patients. Consequently, in patients older than 65 with preoperative increased A-wave and AEI, it seems appropriate to apply pharmacological POAF prophylaxis and/or consider biatrial pacing during the first 48 hours after surgery. Right ventricular dysfunction is a strong predictor of developing AF in patients with acute decompensate heart failure.23 Shymony7 has confirmed the association between impaired right diastolic ventricular contractility and increased risk of POAF after CABG. No influence of preoperative right ventricular function on POAF was demonstrated in our analyzed population; nevertheless, using each patient as a case and control, TAPSE decreasing after surgery was significantly related to POAF. It is difficult to establish the real cause-effect relationship between these two variables. The etiology of postoperative right ventricular dysfunction is considered multifactorial: basal substrate altered by preoperative pulmonary hypertension, ischemia during CPB, trauma associated to surgical procedure, postoperative changes related to volume overload, effects of inotropic drugs…etc.24,25 Probably, postoperative TAPSE decreasing is a secondary effect of previously mentioned factors. However, considering that its effect was higher in younger patients, in whom POAF was less frequent, we might conclude that POAF contributed to postoperative right ventricular function impairment and was associated to increased TAPSE decline after heart valve surgery.
According to aforementioned data, POAF after valve surgery in patients with preoperative sinus rhythm, impaired early atrial reverse remodeling and contributed to postoperative right ventricular dysfunction, not only in aged patients.
Age is main risk factor for POAF related to heart valve surgery. The etiology of this disorder is supposed to be multifactorial. Echocardiographic parameters previously identified as risk factors for AF were ineffective predictors after heart valve surgery in our population. Increased AEI reflected an atrial conduction disorder and was the only preoperative risk factor identified in this group of patients. Diastolic dysfunction probably played an important role in the genesis of POAF after valve surgical procedures. POAF favoured right ventricular dysfunction evidenced by higher postoperative TAPSE decreasing. Adequate prevention in higher risk groups (elderly and patients with increased preoperative AEI) would promote early reverse atrial remodeling after surgery and might prevent or reduce impaired right ventricular function after CPB as well as AF recurrence during follow up.
This is a single-center clinical trial, conducted in a small group of patients. The findings may be affected by reduced sample size.