AUCTORES
Globalize your Research
Review Article
*Corresponding Author: Sandford Thabit ,Departamento de Neurociências, Hussein University Hospital.
Citation: Sandford Thabit , Current Studies of Immunotherapy for glioblastoma triggers a measurable immune response. J. Psychology and Mental Health Care. 2(3) DOI: 10.31579/2637-8892/029
Copyright: © This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received: 06 March 2018 | Accepted: 26 April 2018 | Published: 25 May 2018
Keywords: immunotherapy; glioblastoma
Glioblastoma is a form of brain tumor with a very high morbidity and mortality. Despite decades of research, the best treatments currently in clinical practice only extend survival by a number of months. A promising alternative to conventional treatment for glioblastomas is immunotherapy. Although proposed over a century ago, the field of cancer immunotherapy has historically struggled to translate it into effective clinical treatments. Better understanding is needed of the various regulatory and co-stimulatory factors in the glioblastoma patient for more efficient immunotherapy treatments. The tumor microenvironment is anatomically shielded from normal immune-surveillance by the blood-brain barrier, irregular lymphatic drainage system, and it’s in a potently immunosuppressive environment. Immunotherapy can potentially manipulate these forces effectively to enhance anti-tumor immune response and clinical benefit. New treatments utilizing the immune system show promise in terms of targeting and efficacy. This review article attempts to discuss current practices in glioblastoma treatment, the theory behind immunotherapy, and current research into various clinical trials.
Glioblastoma, the most frequent and malignant primary brain tumor, stands apart from other neoplasms by its biology and location within the central nervous system (CNS) [1]. In spite of aggressive multimodal treatment including surgical resection, radiation therapy, and cytotoxic chemotherapy, the disease remains incurable with a 2-year survival rate of 26.5% [2]. The failure of conventional oncologic treatment to selectively target glioblastoma cells has prompted investigators to look for new and more targeted therapeutic options as well as prognostic biomarkers that will help us better understand the variation of outcomes. It is clear that new approaches for developing effective and targeted treatment options are needed for patients with glioblastomas. Neurologists and neurosurgeons provide reports that glioma patients who suffer postoperative infections near the tumor bed seem to do better than the average patient similar to the observations made over a century ago [3]. The infection group had a significant advantage in median survival (30 months compared to 15 months) in the non-infected tumor patients. A higher CD4+ counts leads to a significantly longer median survival rate (19.7 months) when compared to a lower CD4+ counts (13.1months) in patients [4]. All of these observations suggest a strong rational to use immunotherapy for glioblastoma patients. Immunotherapy offers a different mechanistic approach from chemotherapy, targeted therapy, radiation, and surgery. Recent success in the treatment of other cancers has fueled a resurgence of interest in this approach [5]. Currently, there are more than 20 FDA approved immunologic products used in treatment of human malignancies [6]. The sevaccine approaches to elicit strong specific immune responses to tumor antigens, approaches involving adoptive transfer of in vitro expanded, naturally arising, or genetically engineered tumor-specific lymphocytes, therapeutic administration of monoclonal antibodies to target and eliminate tumor cells, and approaches that inhibit or destroy the molecular or cellular mediators of cancer induced immunosuppression such as CTLA-4, PD-1, or Treg cells [7]. Unfortunately, these efforts have been unsuccessful in most of other cancers. This may be due to the lack of understanding in immunology of glioblastoma. A major potential pitfall for immunotherapy in glioblastomais due to a number of factors: relative immune-privilege of the brain, this may be due to the blood brain barrier, low numbers of T lymphocytes, and lack of lymphatic systems which makes it challenging for immune cells to enter the CNS [8]. Patients with glioblastoma exhibit a relative systemic immune suppression compared to the general population. The tumor microenvironment is rich with immunosuppressive factors secreted by the tumor like transforming growth factor beta (TGF-β) and vascular endothelial growth factor (VEGF) [9]. It is well known that these factors suppresses T cell proliferation and cytotoxic function by inhibiting dendritic cell (DC)maturation, diminishing absolute counts of CD4+ T cells, and also increased fraction of T-regulatory cells (T-regs) [10]. High proportions of T-regs actively inhibit conventional CD4+ T cells, CD8+ T cells, DCs, and NK cells thus dampening immune responses around tumors [11]. Cells of myeloid lineage have been increasingly associated with immunosuppression in a number of systems. Myeloid-derived cells at different states of maturation have been studied as potent inactivators of both CD4+ and CD8+ T cells and thus may possess immunosuppressive abilities [12]. Another recent study has shown that exposing glioblastoma cells to IFN-γ decreased TGF-β expression but increased expression of PD-1 ligand and Indoleamine-2, 3-Dioxygenase (IDO) [13]. It is reasonable to speculate that other immunosuppressive cytokines exhibit comparably complex interactions. Thus, it is important to understand the role of immunosuppression parameters and factors in tumor progression in patients with glioblastoma. In future immunotherapy, the immune suppression by allowing effective immune targeting of gliomass of those patients with glioma might have less tumor progression and improved outcomes [14]. In a significant number of glioblastoma patients, the blood brain barrier is disorganized by single or combined chemotherapy or radiation which leads to breakdown of the tight junctions between endothelial cells that facilitate migration of leukocytes into the CNS [15]. In that case, activated T cells that encounter their antigen are retained in the CNS. Human leukocyte antigen (HLA) presentation occurs on astrocytes, microglia, and endothelial cells which are essential elements for immune function [16]. The net balance is that CNS immune surveillance still occurs. In spite of these apparently local as well as global aberrations in cellular immunity, most of the glioblastoma patients are generally not systemically immune compromised prior to the growth of their tumor [12,17]. It is therefore likely that tumor -associated immunosuppressive factors will similarly affect clinical attempts to augment antitumor responses.
Therefore, targeting tumor-associated immunosuppression in glioblastoma patients will be critical for the development of meaningful immunotherapeutic strategies. Immunization against glioblastomas can occur in the form of passive or active immunotherapy [3,18,19]. Active immunotherapy provides a boost to the patient’s native immune system (including peptide based therapy utilizing MHC class I molecules and cell based therapy utilizing DCs) by priming it with antigen exposure. By contrast, in passive immunotherapy, a patient is given immune cells or antibodies capable of targeting the tumor cells [19]. Passive immunotherapy does not require activation of the patient’s own immune system, but instead immune cells are active in different ways. Immune cell activity takes place in the following ways: (1) the direct injection of monoclonal antibodies (ex. bevacizumab is a humanized IgG1 monoclonal antibody), (2) stimulation of the immune system with cytokines (Ex. IL-2), and (3) treatment with stimulated immune effector cells by adoptive immunity or cell-based therapy immunotherapy [18,20]. In adoptive immunity, immune cells (lymphocyte-activated killer cells: LAK and cytotoxic T lymphocytes: CTL) activated ex-vivo are administrated to the patient either by systemic injection or directly into the tumor or tumor resection cavity [20,21]. LAK cells are generally obtained by cultivating autologous peripheral lymphocytes in the presence of IL-2, which yields both T and NK cells. The immune reaction provided by LAK cells is non-specifically cytotoxic and is largely not tumor-directed [22]. By contrast, collecting peripheral blood mononuclear cells or tumor infiltrating lymphocytes and then stimulating them ex vivo with antigens prepares CTLs in a tumor-directed fashion [23]. Thus, for glioblastoma immunotherapy, autologous tumor cells are also used for the antigen stimulation thus yielding CTLs that have been activated.
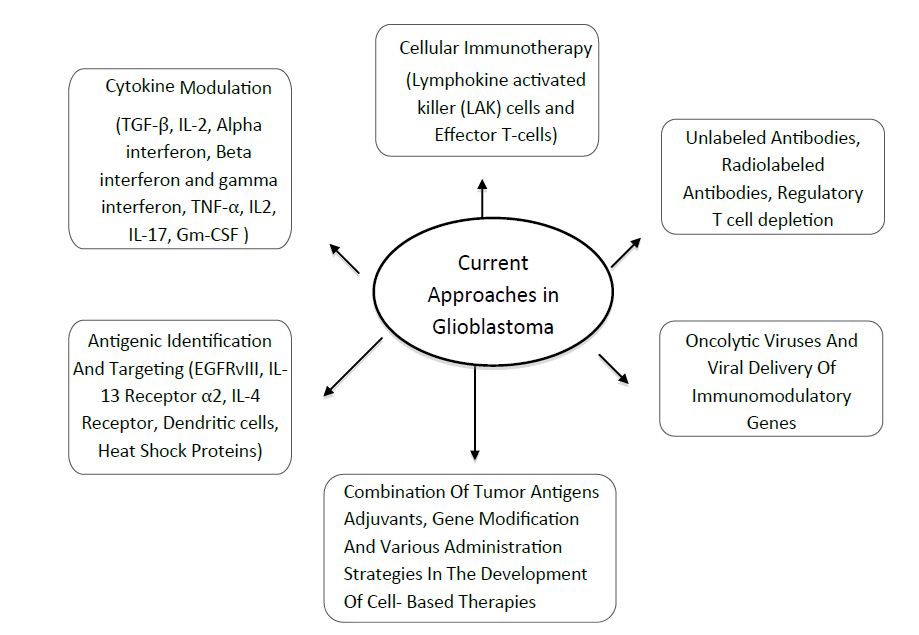
The field of immunotherapy as it is applied to glioblastoma is wide and varied (Figure 1). Although immunotherapeutic approaches have met with mixed success so far, immunotherapy continues to be actively pursued because of its potential to attack infiltrating, high-grade gliomas. Recently, clinical trials demonstrated that using infusion of activated autologous immune cells or active immunotherapy with tumor antigens and dendritic cells successfully induced anti-tumour immunity and some radiological responses [24]. However, large randomised trials are still needed to prove the usefulness of this immunotherapy in brain tumors.
Currently, most drugs under investigation as immunotherapeutic agents depend on designing and confirming immunotherapy in the existing pre-clinical glioblastoma models: both immunocompetent models (normal immune system) and immunodeficient models, which lack specific immune-related molecules. To properly utilize immunocompetent models in an orthotopic context, implantation of genetically compatible tumor cells is required to prevent graft vs. host immunity. This would be analogous to implanting tumor cells derived from a donor C57BL/6 mouse into a host C57BL/6 mouse, which is useful for studying how immune cells infiltrate, respond to, and mediate anti-tumor immunity [25,26]. However, a different approach can be used to study immunotherapy by utilizing an immunodeficient model acting as a litmus test to determine whether a particular therapy requires a specific immune molecule or cell type to mediate an anti-tumor effect. This approach is useful for researchers to understand the mechanism of action for immunotherapy in a brain tumor model that has a T-cell deficiency. This model is more advantageous because it has the ability to isolate which would lead to an unproductive investigation and that T cell functionality is required for translating this therapy into patients with brain tumors.
Our groups and others have shown that all trans retinoic acid (ATRA) can modify the immunogenicity of tumor cells both in vitro and in vivo through differential regulation of MHC class I and intercellular adhesion molecule-1 (ICAM-1) as well as increase the sensitivity of glioblastoma to NK-cells [27,28]. These results suggest that tumor cells can be converted to efficient antigen presenting cells for direct antigen presentation and T-cell stimulation. It has been shown by our group that IFN-γ is an important biomolecule for positive regulation of the MHC presentation machinery [27]. The treatment of glioblastoma cells with IFN-γ induces apoptosis and the extent of cell death is enhanced by pretreatment with ATRA. It was also shown that a combination of ATRA and IFN-γ expressed higher levels of HLA class II and HLA-DM molecules in glioblastoma T98G and U87MG cells than IFN- γ alone suggesting that the combination of ATRA with IFN-γ may overcome the defect in class II-mediated immune recognition of glioblastoma.
Recent studies of human glioblastoma tissue samples have reported tumor-infiltrating lymphocyte populations significantly enriched for T-regulatory cells (Tregs), which are a CD25+, FoxP3+, and subset of CD4+ helper T cells, which suppress immune activation through interactions with T cells, B cells, NK cells, DCs, and macrophages [29]. Tregs have been shown to express CTLA-4, which decrease the secretion of cytokines (IL-2 and IFN-γ), and also skew the immune response away from a cytotoxic Th1-mediated response in favor of a Th2 response [29]. Glioblastoma cells also appear to secrete high levels of CCL22 and CCL2, as compared to low gradeglioma, which facilitates Treg trafficking to the tumor [29]. These observations have led to interest in developing immunotherapies for glioblastoma that target Tregs. Currently, STAT3 inhibitor WP1066, and blocking antibodies against CTLA-4 and CD25, has been shown to decrease Treg proliferation [30]. A new approach being evaluated in clinical trials involves the use of monoclonal antibodies to block immunosuppressive molecules such as CTLA-4 or PD-1 expressed by Tcells. The effectiveness of monoclonal antibodies that block the PD-1 ligand, PD-L1, which can be expressed on tumor cells and normal host cells, is also being explored [31]. A recent phase III clinical trial reported that therapy with CTLA-4–blocking antibodies imparted a significant survival benefit in approximately 30% of patients with other cancer, making this drug a promising treatment for glioblastoma. The success of glioblastoma clinical trials will encouraged interest in blocking other potential effectors of immunosuppression including the soluble (such as IDO and TGF- β) and cellular (such as Treg cells and MDSCs) mediators of the process. Undoubtedly, there is much to be learned about the benefits and risks of inhibiting the different immunosuppressive mechanisms including TGF-β that may be simultaneously operating in the glioblastoma patient [14,32,33]. The involvement of TGF-β in multiple tumorigenic pathways, which promote tumor growth and invasion by sustaining glioblastoma stem cells, promoting angiogenesis, and up regulating MMP-2 expression, makes this cytokine an enticing target for immunotherapy. TGF-β also promotes immunosuppression in glioblastoma by inhibiting T cell activation and proliferation, blocking IL-2 production, suppressing activity of NK cells, and promoting Treg activity. Current approaches to IL-2 and beta interferon (IFN-β) have been extensively studied in cancer immunotherapy either alone or combination with temozolomide [29]. A more recent trial of IFN-α in combination with local BCNU, a chemotherapeutic related to lomustine (CCNU) and semustine, which partially overlaps the activity/toxicity of alkylating agents, delivery in patients with recurrent glioblastoma reported a 6-month progression-free survival in 2/9 patients [29]. These results confirmed by a study, which demonstrated that mice deficient in type 1 interferon and induced to develop gliomas de novo via p53 knockdown exhibited enriched populations of tumor infiltrating myeloid-derived suppressor cells and Tregs, as well as a decrease in the number of tumor-infiltrating CD8+ T cells. Recently, granulocyte-macrophage colony-stimulating factor (GM-CSF) was used as an adjuvant in a phase II vaccination study of patients with newly diagnosed glioblastoma. The proposed mechanism of action was GM-CSF promotion of CD8+ cytotoxic T cell response when combined with antitumor vaccines [29,34,35]. The IL-13Rα2 antigen and IL-4 receptor (IL-4R) are also promising targets for immunotherapy because they are highly expressed on glioma cells but not on host CNS cells [29,36]. Subsequent clinical trials of fused protein (IL-13-PE38QQR and IL-4-PE38KDEL) using the same construct with stereotactic injection as the delivery method, showed similar findings of safety and efficacy.
One well-studied technique is the development of monoclonal antibodies that target specific receptors that are unique to tumor tissue. One such candidate is the vascular endothelial growth factor receptor (VEGF-R). Many of the morbidities associated with glioblastoma are associated with the edema surrounding the primary tumor. This edema is largely produced secondary to disruption of the blood brain barrier and to the production of new vasculature mediated by VEGF released by the tumor [15,29]. Drugs targeting the VEGF pathway have the benefit of reducing edema therefore potentially reducing morbidity and halting further tumor growth. The most successful agents developed thus far have all been monoclonal antibodies. The most well known is bevacizumab, which was approved for use in recurrent glioblastomamultiforme in 2009. In patients with recurrent glioblastoma, patients receiving bevacizumab had6-month progression-free survival rates of 42.6%, and patients receiving combination bevacizumab and irinotecan had survival rates of 50.3%. Both of these rates are significant improvements over prior figures of 9 to 21% [37]. However, relapses still occur despite these advances and other drugs targeting this pathway are being developed. Aflibercept is a drug that binds to VEGF and placental growth factor. In a phase II trial, 19 out of 26 glioblastomapatients with reduced blood levels of VEGF and various cytokines had at least some radiographic improvement in tumor burden after treatment with aflibercept [38]. Antibodies targeting other molecules in the VEGF pathway, such as VEGFR-1,2,3, platelet-derived growth factor, epidermal growth factor, and placental growth factor are also under development in ongoing clinical trials [39].
One problem with targeting growth receptors is that these therapies are not specifically targeted at tumor cells. Damage to healthy brain tissue caused by these agents can result in significant morbidity and, in some of the most serious cases, an allergic encephalomyelitis. Therapies that utilize T cells inoculated against tumor antigens also suffer from this complication due to the fact that many of the anti-tumor antigens produced are shared with normal cells. However, a tumor-specific antigen for glioblastoma has been recently identified. Epithelial growth factor variant III (EGFRvIII) is a common variant that is characterized by an 801 base pair in-frame deletion that causes a split in amino acids 6 and 273. A glycine is inserted between amino acids 5 and 274 [40]. This new arrangement causes the tyrosine kinase domain to be constitutively activated resulting in increased tumorgenicity and resistance to chemotherapy and radiation treatment. This mutation is fairly common -- about 40% of glioblastomas show EGFR gene amplification and 67% of these have been found to carry the EGFRvIII mutation [41]. These characteristics of the EGFRvIII mutation provide a tumor-specific target that is found with high frequency in malignant gliomas.
Preclinical studies on this variant have shown that murine models produce an antibody response to the EGFRvIII-specific peptide PEPvIII. This is a 14-amino acid peptide representing the unique region of EGFRvIII that is conjugated to keyhole limpet hemocyanin (PEPvIII-KLH) [42]. Murine intra-cerebral melanoma models inoculated with the PEPvIII-KLH vaccine along with dendritic cells were found to have developed a humoral response against the variant composed of IgG1 and IgG2a class antibodies. In addition, the presence of antibodies was directly correlated with clinical response and regression of tumor without autoimmunization against the CNS [43]. In humans, phase I and II clinical trials have been concluded. The phase I trial VICTORI showed that patients with glioblastoma could safely be treated with the vaccine composed of dendritic cells inoculated with PEPvIII-KLH [44]. These patients also had a longer survival rate when compared to equivalent patients in other published data. The phase II activate trial endeavored to assess the immunogenicity of the vaccine and progression-free survival in patients with newly diagnosed EGFRvIII-expressing glioblastoma. After the elimination of ineligible patients, 18 patients were included in the trial starting 4 weeks after their last radiation treatment. The first three vaccinations were given every two weeks and were subsequently spaced out to every month until there was radiographic evidence of tumor progression or death. The median progression-free survival for these patients was 14.2 months compared to 6.3 months in the matched cohort. Survival time was also extended in trial patients to 26.0 months compared with 15.0 months in the matched cohort [45]. Currently, phase III trials are underway [46].
Related to the PEPvIII-KLH vaccine is vaccination with dendritic cells by themselves. Normally, dendritic cells exist in most tissue types in an immature state, sampling potential antigens. When dendritic cells present their antigens, they are able to activate both CD4+ and CD8+ cells, a process that is essential for effective cell-based immunity [47]. In addition, dendritic cells have been shown to activate natural killer (NK) cells, providing a powerful method for eliminating glioma cells that do not express MHC-1 molecules on their surfaces [48]. The principle behind a dendritic cell vaccine involves activating dendritic cells removed from a patient with antigens that are tumor-specific, then re-introducing them to the patient. Antigens that have been favored for vaccine production are usually whole tumor-cell antigens, and are isolated in a variety of ways, including acid elution of membrane proteins, various lysates, gamma-irradiation, and isolation of protein from paraffin-embedded samples [49]. Over the last decade there have been a variety of clinical trials demonstrating the efficacy of various dendritic cell vaccines. One recent clinical trial by Chang et al. used a vaccine prepared by using a lysate digested with collagenase. The patients in this trial were treated with the vaccine following surgery and subsequent radiotherapy. They received the vaccine subcutaneously in axillary lymph nodes once a week for 4 weeks, then every 2 weeks twice, then finally monthly 4 times, totaling 10 doses. The 16 patients who completed the trial had a median survival of 525 days and 5-year survival of 18.8%. This was compared to 63 historical control patients with a median survival of 380 days and 5-year survival of 0%. Interestingly, the authors of the study found that patients with relapsed glioblastoma responded better to the treatment than did newly diagnosed patients. Side effects of this treatment were minor and included transient AST/ALT elevations [50]. Many more trials testing various dendritic cell vaccines are currently being done.
One particularly creative avenue of research is the use of oncolytic virus therapy to treat glioblastoma. This technique utilizes oncolytic viruses that are modified so that they selectively infect tumor cells while ignoring normal cells. Glioblastoma is ideal for such a technique due to its relatively isolated tissue of origin and its lack of propensity towards metastasis. Plus, normal brain tissue is static in the post-mitotic phase, making it less of a target for viruses, which require actively dividing cells to propagate [51]. Human viruses used for this technique must be modified so that they do not infect normal tissue. The most successful of these have been herpes simplex virus (HSV) and adenovirus. HSV is a DNA virus with an especially large genome, allowing for the addition or removal of relatively large genes without disruption of viral replication. The first mutant used against gliomas was G207, containing deletions in both gamma (1) 34.5 loci and a disabling insertion of lacZ in the UL39 gene, resulting in a virus that is unable to replicate in normal brain tissue, but proliferates in glioma tissue [52]. A phase I trial using this variant in 21 patients showed that the virus could be administered without the development of encephalitis [53]. A subsequent phase Ib trial also demonstrated safety of administration along with some initial evidence of viral replication in several of the six patients, though the results were not uniform [54]. In light of these and similar results using other modified herpes viruses, oncolytic strains carrying cytokines and other immunostimulatory agents have been developed. So far the G207 variant modified to express murine IL-12 has shown some effect in activating primate lymphocytes in preclinical trials [55]. Techniques utilizing adenovirus include disruption of the E1A and E1B regions of the viral genome, without these regions adenovirus can only infect and replicate in tumor cells that have defective cell cycle regulation through disrupted retinoblastoma protein and protein 53 tumor suppressors [56]. So far only preclinical trials and one phase I trial have been completed in this area, though initial results are promising [57]. In addition to human viruses, vaccine viruses and non-human viruses have been considered as possible oncolytic viruses. The benefit to vaccine viruses is that they have already been inactivated for normal tissue and tend to be less pathogenic, only requiring modification to ensure activation against tumor cells. Promising candidates have been vaccines against measles, polio, and rabies. Non-human viruses are also an interesting avenue of research due to the fact that they do not normally infect healthy cells and must be modified specifically in order to infect tumor cells. Currently, most research on vaccine viruses and non-human viruses remains in the preclinical stage [50]. Recent approaches include: (1) use of autologous tumor transfected with cytokine genes to express cytokine or DC-tumor cell fusions; (2) vaccination with a heat shock protein in complex with autologous tumor derived peptides; (3) delivery of autologous tumor cells via a viral vaccine vector using Newcastle Disease Virus (NDV). NDV offers the advantage of a single stranded RNA virus that poses little health hazard to humans and has the ability to selectively kill human tumor cells.
Future Directions
The future of immune therapies in glioblastoma involves challenges related to enhancing antigen presentation capabilities, effectively breaking tumor-induced immune tolerance, improving a strong and long lasting anti-tumor T cell activation of tumor-specific cytolytic effector cells, and the standardization and upscale production of cell based therapy. Similarly, more clarification is required as to when and how immune therapy should be given with other modalities. The role of steroid and DC based immunotherapy use in this population of patients will require well-designed and appropriately powered clinical trials. There are a few potential targets that could enhance the immune system’s recognition of the tumor. One strategy might be to deplete the regulatory T cells. Once T cells are activated, they up regulate molecules such as CTLA-4 and PD-1 to limit their activity. Use of blocking humanized monoclonal antibodies to these checkpoint molecules appear very promising and have already made it to the clinic in treating patients. To that end, an antibody against CD4 or CD25 could be used to target Tregs, or more general immunotoxins could be used. A phase I clinical trial using a vaccine comprised of autologous tumor cells genetically modified by transforming growth factor–β2 (TGF-β2) antisense vector in 6 patients with recurrent glioblastoma was well tolerated with indications of anti-tumor induced immunity. In the future, we can use this approach to treat glioblastoma patients. Use of additional inflammatory cytokines such as IL-12, IL-7, and IL-15, activating antibodies to co-stimulatory molecules such as CD40, or blocking antibodies to immune inhibitory cytokines such as IL-10 or TGF-β in combination with DC vaccination, can potentially enhance clinical activity which of these strategies in combination with vaccination that will yield the best therapeutic ratio (most effective and less toxic) is still to be determined. The rational to give chemotherapy with immunotherapy may relate to the chemotherapeutic effects on tumor release of relevant antigens, on inhibiting the regulatory compartment, and on the ability to change the tumor vasculature providing better access for effector cells. Another possibility is that vaccination sensitizes the tumor to chemotherapy.
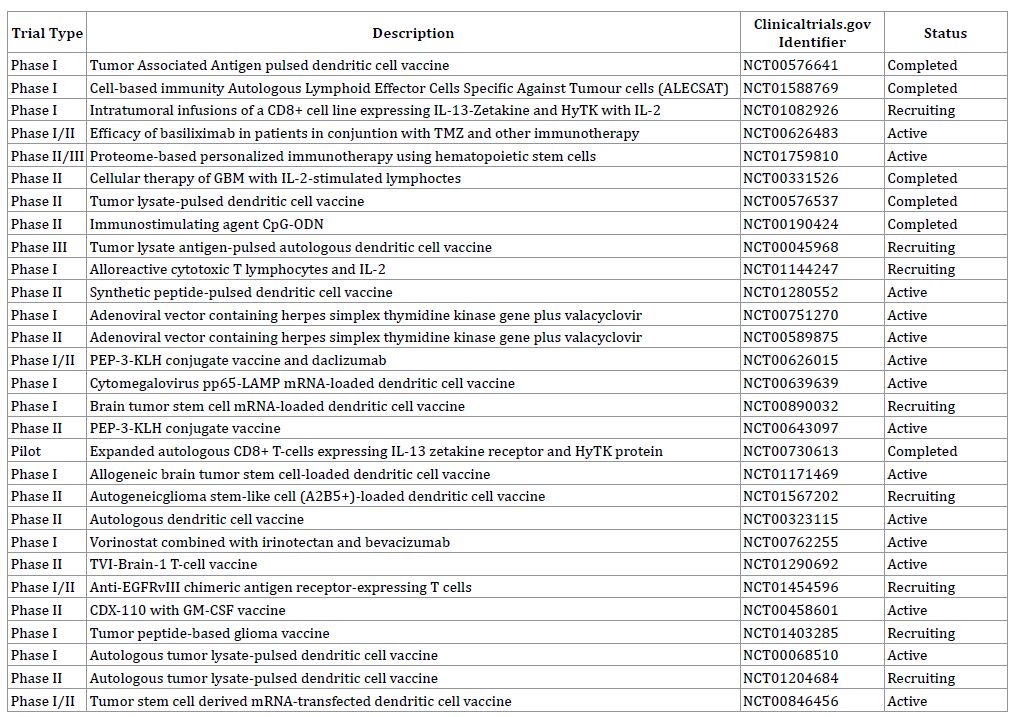
Table 1: Ongoing Clinical Trials
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
