AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Yasser Mohamed Mahmoud El Shahawy, Ass Professor Obstetrics and Gynecology Ain Shams University, Cairo Egypt.
Citation: Mahmoud El Shahawy YM, Mahmoud El Sherbeeny M, (2023), Cabergoline Alone Versus Cabergoline with Gonadotropin Releasing Hormone Agonist in the Prophylaxis of Ovarian Hyperstimulation Syndrome, J. Obstetrics Gynecology and Reproductive Sciences, 4(4) DOI:10.31579/2578-8965/045
Copyright: © 2023, Yasser Mohamed Mahmoud El Shahawy. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 08 December 2020 | Accepted: 16 December 2020 | Published: 23 December 2020
Keywords: ovarian; hyperstimulation; syndrome; vitro surgical insemination;
In this study, cabergoline alone and cabergoline combined with a gonadotropin releasing hormone agonist (GnRH agonist) were used to induce ovulation in patients undergoing in vitro fertilisation (IVF) or in vitro surgical insemination (ICSI) who were at high risk of developing OHSS. Ninety infertile women who were enrolled in the (ART) unit were all included in the study. Patients with one or more risk factors for developing OHSS, such as polycystic ovary syndrome (PCOS), a history of prior hyper-response/OHSS, serum E2 levels higher than 3000 pg/ml on the day of hCG administration, more than 20 follicles with a diameter larger than 12 mm, and/or high egg retrieval rates >20, were enrolled in this study during the stimulation cycle for ICSI.
The long protocol (mid-luteal long GnRH agonist plus a gonadotrophin stimulation protocol) was used to initiate the ovarian stimulation regimen in all patients. The GnRH agonist used was triptoreline acetate, a short-acting GnRH agonist (Decapeptyl), and it was given subcutaneously every day at a dose of 0.1 mg starting in the midluteal phase of the cycle preceding treatment one for two weeks until downregulation took place and E2 dropped below 30–40 pg/ml. Then, using highly purified urine FSH (Fostimon) at the proper doses based on the patient's age, FSH level, past response, and ovarian volume, controlled ovarian stimulation was started. The dose was then modified based on the outcome. three groups were formed from the patients. 30 women from Group I (Control Group) received intravenous albumin only. Thirty women made up Group II (the cabergoline group), who got intravenous albumin first and one 0.5 mg cabergoline tablet every day for seven days at bedtime beginning on the day of oocyte pickup. Group III (Cabergoline and GnRH Group) contained 30 women who received albumin, cabergoline, and a GnRH agonist for one week following hCG injection. When at least two or more follicles with a mean follicular diameter of 17–18 mm were observed, 5000 IU of hCG were given to all patients. 34–36 hours after hCG injection, oocytes were extracted via transvaginal ultrasound-guided follicular aspiration. After 16–18 hours, the injected oocytes were inspected to look for signs of fertilisation. The amount of oocytes that sperm cells successfully fertilise is determined by the fertilisation rate.
The remaining healthy embryos were frozen, and the transcervical transfer was cancelled if symptoms of OHSS (ultrasound evidence of ascites) manifested. One to three embryos were transferred transcervically on day 3 or blastocysts were transferred on day 5.
Results: There was a statistically significant difference in ovarian size (p 0.05) between the tested groups. Regarding the number of patients with ultrasonography ascites, there was a highly statistically significant difference (p 0.001) between the analysed groups, while there was no statistically significant difference (p >0.05) between the studied groups in the number of patients with clinical ascites. Both Group II and Group III saw lower rates of total OHSS than the control group, with a highly statistically significant difference (p 0.001) between the tested groups.
While there was no statistically significant difference (p > 0.05) between the studied groups for patients with severe OHSS, the incidence of both moderate and severe OHSS was lower in Group II and Group III than in the control group in both Group II and Group III. Furthermore, while there was no statistically significant difference (p > 0.05) between the study groups for late OHSS, the incidence of early OHSS was reduced in Groups II and III compared to the control group in both cases. Regarding ovarian size and the number of patients with ascites, however, there was no statistically significant difference (p 0.05) between Groups II and III. As for overall, moderate, severe, early, and late OHSS, there was no statistically significant difference (p 0.05) between Group II and Group III. In conclusion, cabergoline, a dopamine agonist, dramatically reduces both the incidence and the severity of OHSS in high-risk individuals, whether taken alone or in combination with a GnRH agonist. Nevertheless, they do not entirely stop the onset of OHSS
Ovarian hyperstimulation syndrome (OHSS) is an unintended side effect of ovarian stimulation (OS) that can happen in the luteal phase or in the first trimester of pregnancy. Aiming to produce a suitable number of oocytes and embryos while also increasing the risk of OHSS, ovarian stimulation techniques have been developed as part of the advancement of assisted reproductive technology (ART) [1]. Acute fluid shift from the intravascular space to the third space, brought on by an increase in vascular permeability and neoangiogenesis, is a characteristic feature of OHSS, as well as cystic enlargement of the ovaries [2]. The clinical signs and symptoms of OHSS are a reflection of the degree of fluid shift into the third space and the ensuing hemoconcentration from intravascular volume depletion. The symptoms can be as modest as abdominal distention brought on by enlarged ovaries alone or in conjunction with an accompanying fluid shift into the abdomen, to renal failure and death as a result of hemoconcentration and reduced perfusion of organs such as the kidneys, heart and brain [3].
Between 3 and 6% of people are thought to experience mild OHSS, and 0.1 to 3% of people can experience severe OHSS. The incidence is close to 20% among women at high risk [4]. Although OHSS may sometimes arise with ovarian stimulation using clomiphene citrate and even in a spontaneous pregnancy [5], this illness nearly exclusively occurs during assisted reproductive technology (ART) cycles. Young age, low body mass index, greater doses of exogenous gonadotropins, high absolute or rate of increase of serum estradiol (E2) levels, and prior episodes of OHSS are all factors that raise the risk of OHSS [6]
Though the precise origin of OHSS is still unknown, it is most likely that the release of vasoactive chemicals generated by the ovaries in response to human chorionic gonadotrophin (hCG) stimulation is a significant factor in its development (. In the pathogenesis of OHSS, vascular permeability factor (VEGF), also known as vascular endothelial growth factor (VEGF), has emerged as one of the factors most likely to be implicated [7]. Depending on when symptoms first appear, there are two main clinical variants of OHSS: early and late OHSS. Early OHSS is brought on by exogenous hCG given for final oocyte maturation, which typically takes place 3–7 days after hCG. The endogenous hCG produced by an implanting blastocyst, which occurs 12–17 days after hCG, causes late OHSS, which is pregnancy-induced [8].
According to the degree of the symptoms, OHSS is further classified as mild, moderate, and severe. The clinical significance of mild OHSS is negligible, moderate OHSS necessitates careful patient monitoring, and severe OHSS, which is characterised by massive ovarian enlargement, ascites, pleural effusion, oliguria, haemoconcentration, adult respiratory distress syndrome, and thromboembolic phenomena, may necessitate hospitalisation in an intensive care unit, may prove to be critical or even life-threatening [9].Cycle cancellation, coasting, intravenous albumin administration around the time of oocyte retrieval, Gonadotropin-releasing hormone agonist (GnRHa) as an oocyte trigger in GnRH antagonist cycles, natural-cycle in vitro fertilisation (IVF), or in vitro oocyte maturation (IVM) are just a few of the methods that have been tried to prevent OHSS [10]. Unfortunately, none of the methods used today totally eliminates OHSS after hCG treatment.
The goal of this trial was to examine the effectiveness of cabergoline against cabergoline plus a GnRH agonist in preventing ovarian hyperstimulation syndrome in women at risk for OHSS. The use of a dopamine agonist (cabergoline) is a viable novel method to prevent OHSS and lessen its severity [11]. The effects of cabergoline (Cb2) on conception, implantation, and miscarriages are nonexistent. Serum progesterone levels and luteal apoptosis were unaltered, indicating that no luteolytic effects were seen. VEGF/VEGFR-2 ovarian mRNA levels were unaffected by Cb2 treatment as well [3]. According to reports, the GnRH agonist directly causes apoptosis in the granulosa cells of IVF patients. As they previously discovered that GnRH agonist directly inhibited luteal VEGF, one potential mechanism of GnRH agonist action may be a direct effect lowering VEGF expression in the ovary during luteal formation [12].
The purpose of this study was to examine the efficacy of two protocols in the prevention of OHSS during ovulation induction in ICSI patients who were at high risk of developing OHSS. Cabergoline alone, a dopamine agonist, was the initial protocol. The second procedure combines a dopamine agonist (cabergoline) with a gonadotropin-releasing hormone (GnRH) agonist.
This study included a total of 90 infertile women. They were scheduled to undergo ovarian stimulation and intracytoplasmic sperm injection (ICSI). Initially a complete history taking and physical examination were carried out for every participant. Routine investigations were performed for all participants, these investigations included, initial transvaginal ultrasound (TVS), estimation of serum FSH, LH, TSH, prolactin levels, hysteroscopy and semen analysis.
Inclusion criteria:
Age must be less than 40 years old. Satisfactory basal ultrasound examination, normal serum FSH (less than 15 IU/ L) on day three of the menstrual cycle at least not more than three months before the procedures, normal hysteroscopic findings and clear indication for ICSI must be present. Patients were enrolled in this study during the stimulation cycle for ICSI who had one or more risk factors for developing OHSS such as:
Exclusion criteria:
All patients started the ovarian stimulation protocol according to the long protocol (mid-luteal long GnRH agonist plus a gonadotrophin stimulation protocol). if E2 higher than 40 pg/ml GnRH agonist was continued till down regulation occured and E2 became less than 40 pg/ml. T.V.S was performed on the 2nd or 3rd day of the stimulating cycle and revealed thin endometrium (less than 5 mm), no ovarian cysts but if there is cyst, it should be aspirated before starting ovarian stimulation.
Highly purified urine FSH was then administered in the proper dosages to start controlled ovarian stimulation. On day 6 of ovarian stimulation, serum E2 and ultrasonography were performed, and the gonadotrophin dose was then increased or decreased in accordance with the E2 level and follicular activity. Based on their reaction and the necessity to examine the effects of any additional changes to the gonadotropin treatment dose, all patients received serial T.V.S. assessments of follicular growth and measurements of blood E2 levels every one to three days.
Patients at risk of developing OHSS who had serum E2 levels more than 3000 pg/ml on the day of hCG administration and/or more than 20 follicles and fulfilled the inclusion criteria were included. Five thousands IU of hCG were administered to induce final oocyte maturation and ovulation. Ovarian aspiration was performed 34-36 hour afterwards. All patient received 20-gram albumin by intravenous infusion one hour before oocyte retrieval, the duration of infusion was one hour. Once the decision to administer hCG was taken, patients were allocated by the Computer-based randomization method into three groups:
Group I (Control Group):
Group I included 30 women who received intravenous albumin only.
Group II (Cabergoline Group):
Group II included 30 women who received intravenous albumin. Then patients received one 0.5 mg tablet of cabergoline (Dostinex, 0.5 mg; Pharmacia Italia S.P.A, Italy) daily for 7 days at bedtime starting on the day of oocyte pickup.
Group III (Cabergoline and GnRH Group):
Group III included 30 women who GnRH agonist administration was continued for one week after hCG injection and also received intravenous albumin. Then they received one 0.5 mg tablet of cabergoline daily for 7 days at bedtime starting on the day of oocyte pickup.
By using transvaginal ultrasound to assist follicular aspiration, oocytes were extracted. Oocytes were aspirated, inspected, and then incubated before ICSI was carried out. After 18 hours, injected oocytes were checked to see if there was any proof of fertilisation. The number of oocytes that sperm cells successfully fertilise is known as the fertilisation rate. The remaining good quality embryos were frozen, and the transfer was cancelled if symptoms of OHSS (ultrasound evidence of ascites) appeared. One to three good quality embryos were transcervically transferred on day 3 or blastocysts were transferred on day 5. Statistical analysis : Data were checked, entered and analysed using (SPSS version 20).
In terms of age, BMI, duration, and type of infertility, there was no statistically significant difference (p > 0.05) between the analysed groups. PCOS patients, as well as their numbers. Table (1)
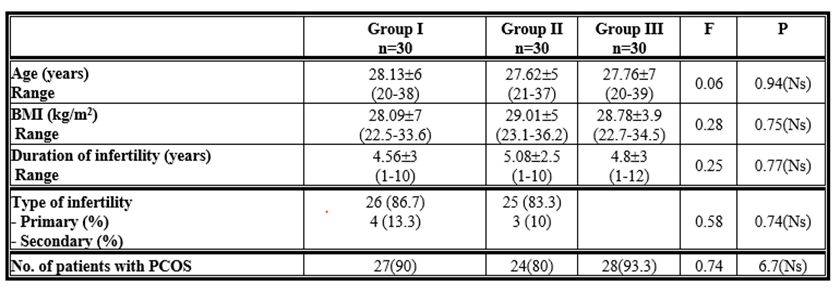
Table 1: Age, body mass index (BMI) and duration of infertility among the studied groups.
Regarding the quantity of follicles, quantity of oocytes recovered, quantity of fertilised oocytes, quantity of fertilised oocytes, fertilisation rate, and ET, there was no statistically significant difference (p > 0.05) between the analysed groups. Table (2)
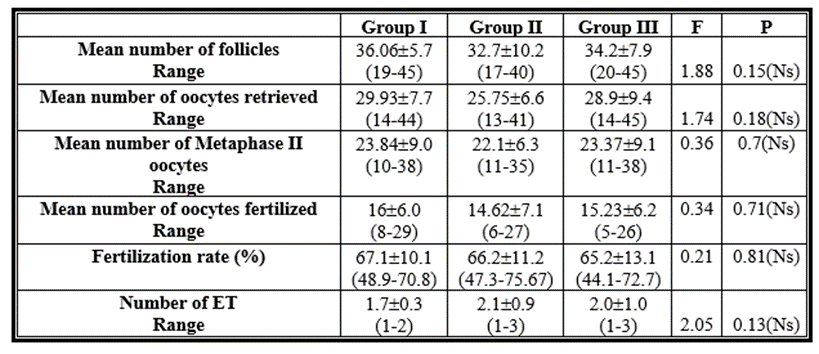
Table 2: Comparison between the studied groups as regards the number of follicles, number of oocytes retrieved, number of oocytes fertilized, fertilization rate and embryo transfer (ET).
The number of patients who underwent a day 3 embryo transfer and the overall number of patients who underwent an embryo transfer were higher in the cabergoline (group II) and cabergoline with GnRH agonist (group III) groups compared to the control group, with a highly statistically significant difference (p 0.001) between the analysed groups. The number of patients with daytime symptoms did not differ statistically significantly (p > 0.05) across the tested groups regarding the number of patients that underwent day-five embryo transfers. With a strong statistically significant difference (p 0.001) between the examined groups, the number of patients with cancelled embryo transfers is lower in the cabergoline (group II) and cabergoline with
GnRH agonist (group III) groups compared to the control group. Regarding the number of patients with day 3 embryo transfers, day 5 transfers, the total number of patients who transferred embryos, and the number of patients whose embryo transfers were cancelled, there was no statistically significant difference (p >0.05) between Group II and Group III. Table (3))
Pregnancy test rates, clinical pregnancy rates, multiple pregnancy rates, first trimester miscarriage rates, and ongoing pregnancy rates did not statistically differ amongst the study groups (p > 0.05). as regards the implantation rate which was lower in cabergoline group than in both cabergoline with GnRH agonist and control groups. Table (4)
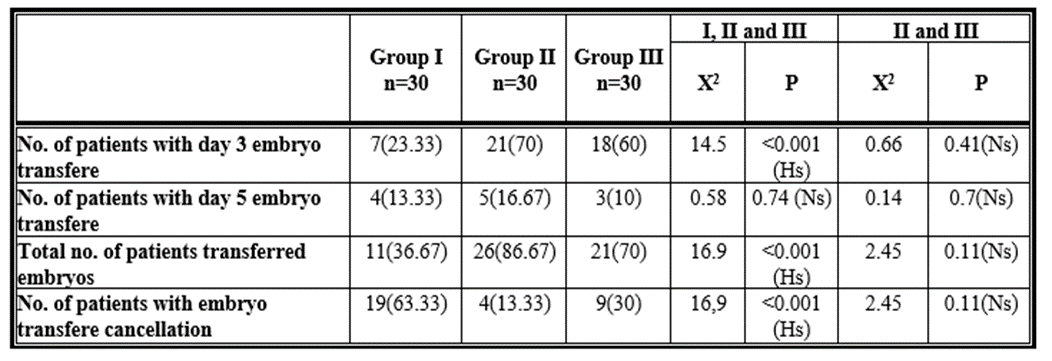
Table 3: Comparison between the three studied groups and comparison between group II and group III as regards the number of patients transferred embryos and the number of patients with embryo transfere cancellation.
Regarding the size of the ovaries, there was a statistically significant difference (p 0.05) between the studied groups. Regarding the number of patients with ultrasonography ascites, there was a highly statistically significant difference (p 0.001) between the analysed groups, while there was no statistically significant difference (p >0.05) between the studied groups in the number of patients with clinical ascites. Both Group II and Group III had
lower rates of total OHSS than the control group, with the difference between the study groups being highly statistically significant (p 0.001). Patients with moderate type of OHSS had reduced incidence of both moderate and severe OHSS in Group II and Group III than in the control group, but there was no
statistically significant difference (p > 0.05) between the analysed groups in patients with severe OHSS.
Additionally, there was no statistically significant difference (p > 0.05) between the analysed groups in late OHSS, although the frequency of early OHSS was lower in Groups II and III than in the control group, with a large statistically significant difference (p 0.001). However, there was no statistically significant difference (p 0.05) in ovarian size or the number of patients with ascites between Groups II and III. Furthermore, there was no statistically significant difference between Groups II and III in terms of overall, moderate, severe, early, and late OHSS (p 0.05). Table (5)

Table 4: Comparison between the three studied groups and comparison between group II and group III as regards positive pregnancy test rate, clinical pregnancy rates, Multiple pregnancy rate, First trimester miscarriage rate, Ongoing pregnancy and implantation rate.
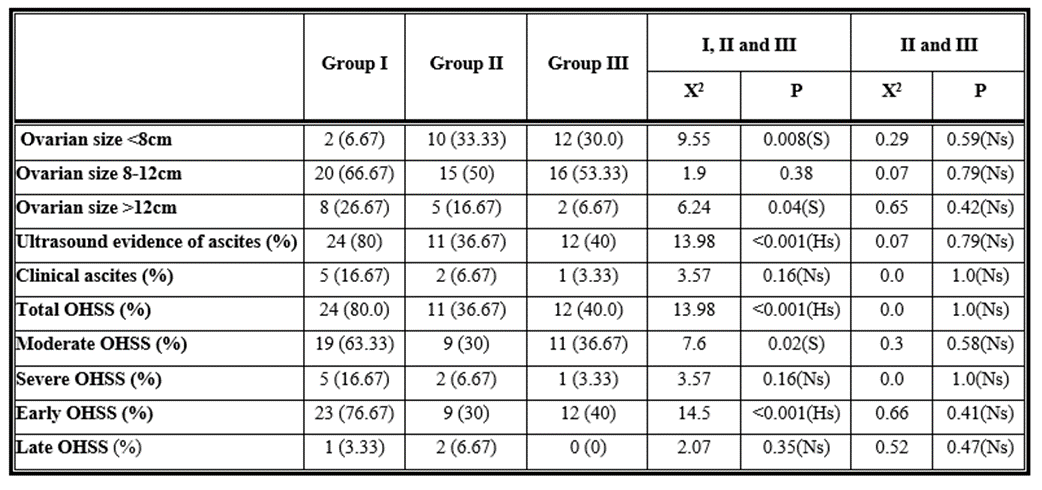
Table 5: Comparison between the three studied groups and comparison between group II and group III as regards ovarian size, ultrasound evidence of ascites, clinical ascites, total, moderate, severe, early and late OHSS.
The purpose of this trial, which was conducted as a randomised prospective study, was to determine how well cabergoline, a dopamine agonist, worked both on its own and in combination with a GnRH agonist to prevent OHSS in individuals who were at high risk for the condition. Our research shown that cabergoline, a dopamine agonist, dramatically reduced both the incidence and the severity of OHSS in high-risk patients. The results of our study show that, whereas cabergoline (group II) considerably decreased the risk of early OHSS, it had no effect on the risk of late-onset OHSS. Additionally, there was a statistically significant decrease in the incidence of mild OHSS; however, this difference was not statistically significant compared to the decline in the incidence of severe OHSS. In this trial, cabergoline administration reduced the frequency of cycle cancellation without having an impact on pregnancy.
Carizza and colleagues (Carizza et al., 2018), The results of a prospective, randomised study that was conducted to determine whether cabergoline had the potential to reduce the incidence of OHSS in high-risk patients receiving ART treatment showed that it did reduce the risk of early OHSS but not the risk of late-onset OHSS. Additionally, there were no differences in any of the parameters between the cabergoline-treated group and the control group including pregnancy, implantation or miscarriage rates. So this study is in agreement with our study except in the implantation rate which decreased significantly in our study in the patients used cabergoline treatment. [13]
Alvarez et al. (2017) to reduces vascular permeability and prevention of OHSS and it showed that cabergoline displyed significant decrease the presence of ascites and decrease the incidence of moderate and severe OHSS. [14]
In our study, patients were given one cabergoline 0.5 mg tablet every day for seven days at bedtime beginning on the day of oocyte pickup. This led to a highly significant decrease in the incidence and severity of OHSS in high-risk patients, indicating the need for additional research to determine the optimal dose and protocol for OHSS prevention using dopamine agonists. By preventing the phosphorylation of VEGFR-2 in response to hCG, Gómez et al. (2020) show that the administration of a dopamine agonist (cabergoline) can lower the incidence of OHSS. Dopamine can lower VEGFR2 phosphorylation, although how it does so is still a mystery. Studies conducted in vitro indicate that the molecular mechanism underlying this action requires the internalisation of VEGFR-2, which is prompted by the activation of the Dopamine receptor 2 (Dp-r2) [3].
Carizza et al. (2018) demonstrated that cabergoline has no effect on pregnancy and miscarriages rates. [13]
One of the strategies studied to prevent OHSS, to continue the administration of the GnRH agonist for one week after hCG administration [15].
Our study showed that when GnRH agonist was added to cabergoline treatment (group III) there was same preventive effect of cabergoline alone aganist OHSS. although there were no cases of late onset OHSS occurred in group III but there was no significant differance between the two groups. The addition of GnRH agonist to cabergoline treatment showed same effect of cabergoline alone in term of cycle cancellation, pregnancy and miscarriage rates but there was improvment in the implantation rate which was decreased in cabergoline treatment alone.
There were many studies done to examine the preventive effects of GnRH agonist on OHSS.
One of those studies was done by Endo and colleagues (Endo et al., 2020) who conducted a three-center open-label, controlled clinical trial research for the treatment of infertility. All of the study participants had a chance of getting OHSS. All pronucleate embryos were cryopreserved in the study group, and GnRH agonist therapy was continued for an additional week following hCG injection. The study found that continuing GnRH agonist for a week after receiving an hCG injection decreased the risk of both severe and moderate OHSS. The continuance of GnRH agonist avoided early OHSS without having a deleterious impact on pregnancy, as evidenced by the study's finding that the pregnancy rate and loss rate were comparable to those of the control group. Therefore, it concurs with our study. [16]
A study by Kitajima et al. 2018 demonstrates that continuing to take the GnRH agonist in the days after receiving an HCG injection lowers the levels of VEGF, VEGFR-1, and VEGFR-2 in hyperstimulated rat ovaries and also lowers vascular permeability. It is hypothesised that GnRH-a therapy could stop early OHSS. [12] For patients who were at risk of developing OHSS, Wada et al. continued GnRH agonist during the luteal phase, but they discovered that this treatment was ineffective [17]. The amount of hCG used in their protocol was significantly different from ours (10 000 versus 5000 IU). The condition of OHSS and the impact of hCG are connected. The 5000 IU hCG dose is crucial for preventing severe OHSS. Our research shown that cabergoline, a dopamine agonist, dramatically reduced both the incidence and the severity of OHSS in high-risk individuals whether used alone or in combination with a GnRH agonist. They do not, however, totally stop the onset of OHSS. Both the cabergoline group (group II) and the cabergoline with GnRH agonist group (group III) considerably reduced the chance of having mild OHSS; however, the risk of developing severe OHSS was only slightly reduced. Although there were no incidences of late onset OHSS in group III and the risk of early OHSS fell significantly in both groups, the trial was typically small and underpowered to detect clinically important outcomes of late onset OHSS.
The rates of pregnancy and miscarriage were unaffected by the administration of cabergoline alone or in combination with a GnRH agonist, which reduced the frequency of cycle cancellation. The implantation rate was much lower in group II, although it was increased when cabergoline was combined with a GnRH agonist. It has been demonstrated that giving short-acting GnRH-a during the luteal phase of IVF cycles greatly raises the rate of implantation and pregnancy. These results imply that elevated levels of hCG during conception cycles are the cause of a direct action of GnRH-a on embryos. [18] Fujii et al. (2019) carried out a comparative prospective and randomised trial to examine the impact of continuous GnRH agonist administration during the luteal phase in an ovarian stimulation programme for IVF. From the midluteal phase of the previous cycle to 14 days following oocyte retrieval, GnRH agonist was intranasally given. According to the study, continuing to administer a GnRH agonist during the luteal phase may help with implantation and prevent a severe gonadotrophin suppression. [19]
In high-risk patients, dopamine agonist (cabergoline) alone or in combination with gonadotropin releasing hormone agonist (GnRH agonist) dramatically reduces the incidence and severity of OHSS. However, they cannot totally stop the onset of OHSS.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
