AUCTORES
Globalize your Research
Research Article
*Corresponding Author: Delia Teresa Sponza, Dokuz Eylül University, Engineering Faculty, Department of Environmental Engineering, Tınaztepe Campus, 35160 Buca/Izmir, Turkey.
Citation: Oztekin R., Delia T. Sponza, (2023), Bio-Electrochemical Cell Assisted Production of Biogenic Palladium Nanoparticles with Shewanella oneidensis MR-1 Bacteria for the Catalytic Removal of Ofloxacin (OFX) and Doxycycline (DOX) Micropollutants in Pharmaceutical Industry Wastewaters, International Journal of Clinical Case Reports and Reviews, 13(4); DOI: 10.31579/2690-4861/300
Copyright: © 2023, Delia Teresa Sponza. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 01 March 2023 | Accepted: 22 March 2023 | Published: 04 April 2023
Keywords: anova statistical analysis; antibiotics; bio-electrochemical cell; biogenic palladium nanoparticles (bio-pd nps); catalytic removal; coronavirus disease-2019 (covid-19); cost analysis
In this study, biogenic-palladium nanoparticles (bio-Pd NPs) with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalyts was examined during catalytic degradation process in the efficient removal of Ofloxacin (OFX) and Doxycycline (DOX) micropollutants from pharmaceutical industry wastewater plant, İzmir, Turkey. Different pH values (3.0, 4.0, 6.0, 7.0, 9.0 and 11.0), increasing micropollutants (OFX and DOX) concentrations (5 mg/l, 15 mg/l, 30 mg/l and 45 mg/l), increasing Bio-Pd NPs concentrations (5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l, 40 mg/l and 60 mg/l), different Bio-Pd NPs/cell dry weight (CDW) mass ratios (5/5, 6/4, 7/3, 8/2, 9/1, 1/9, 2/8, 3/7 and 4/6), increasing recycle times (1., 2., 3., 4., 5., 6. and 7.) was operated during catalytic degradation process in the efficient removals of OFX and DOX micropollutants in pharmaceutical industry wastewater. The characteristics of the synthesized NPs were assessed using Diffuse reflectance UV-Vis spectra (DRS), Energy-dispersive X-ray (EDX), Field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FTIR), Inductively coupled plasma mass spectrometry (ICP-MS), Transmission Electron Microscopy (TEM), X-Ray Diffraction (XRD) and X-Ray Photoelectron Spectroscopy (XPS) analyses, respectively. The catalytic activity was first assessed by the degradation of methyl orange. The Bio-NPs showing the highest catalytic activity were selected for the removal of micropollutants (OFX and DOX) from secondary treated municipal wastewater. The catalytic degradation mechanisms of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalysts and the reaction kinetics of OFX and DOX micropollutants were evaluated in pharmaceutical industry wastewater during catalytic degradation process. ANOVA statistical analysis was used for all experimental samples. The maximum 99% OFX removal efficiency was obtained catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l OFX concentration, 40 mg/l Bio-Pd NPs concentration, Bio-Pd NPs/CDW mass ratio=6/4, after 24 h catalytic degradation time, at pH=6.0, at 25oC, respectively. The maximum 99% DOX removal efficiency was observed catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l DOX concentration, 40 mg/l Bio-Pd NPs concentration, Bio-Pd NPs/CDW mass ratio=6/4, after 24 h catalytic degradation time, at pH=9.0, at 25oC, respectively. Finally, the combination of a simple, easy operation preparation process, excellent performance and cost effective, makes this Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts a promising option during catalytic degradation process in pharmaceutical industry wastewater treatment.
Emerging contaminants (ECs), sometimes known as contaminants of emerging concern (CECs) can refer to a wide variety of artificial or naturally occurring chemicals or materials that are harmful to human health after long-term disclosure. ECs can be classified into several classes, including agricultural contaminants (pesticides and fertilizers), medicines and antidote drugs, industrial and consumer waste products, and personal care and household cleaning products (Idham et al., 2017; Idham et al., 2021). Antibiotics are one of the ECs that have raised concerns in the previous two decades because they have been routinely and widely used in human and animal health care, resulting in widespread antibiotic residues discharged in surface, groundwater, and wastewater.
Antibiotics, which are widely utilized in medicine, poultry farming and food processing (Arenas and Melo, 2018; Pellerito et al., 2018), have attracted considerable attention due to their abuse and their harmful effects on human health and the ecological environment (Fridkin et al., 2014; Tamma et al., 2017). The misuse of antibiotics induces Deoxyribonucleic Acid (DNA) contamination and accelerates the generation of drug-resistant bacteria and super-bacteria (Huo, 2010; Ferri et al., 2017; Tan et al., 2018); thus, some diseases are more difficult to cure (Tong et al., 2018). A number of studies have revealed that the level of antibiotics in the soil, air and surface water, and even in potable water, is excessive in many areas (Alygizakis et al., 2016; Casanova and Sobsey, 2016; Zhang et al., 2016), which will ultimately accumulate in the human body via drinking water and then damage the body’s nervous system, kidneys and blood system. Therefore, it is necessary to develop an efficient method to remove antibiotics present in pharmaceutical industry wastewater.
The uncontrolled, ever-growing accumulation of antibiotics and their residues in the environment is an acute modern problem. Their presence in water and soil is a potential hazard to the environment, humans, and other living beings. Many therapeutic agents are not completely metabolized, which leads to the penetration of active drug molecules into the biological environment, the emergence of new contamination sources, the wide spread of bacteria and microorganisms with multidrug resistance (Jiménez-Tototzintle et al., 2018; Kerrigan, et al., 2018; McConnell et al., 2018). Modern pharmaceutical wastewater facilities do not allow efficient removal of antibiotic residues from the environment (Karthikeyan and Meyer, 2006; Dinh et al., 2017), which leads to their accumulation in ecological systems (Dong et al., 2016; Siedlewicz et al., 2018). Global studies of river pollution with antibiotics have shown that 65% of surveyed rivers in 72 countries on 6 continents are contaminated with antibiotics (Barry, 2019). According to the World Health Organization (WHO), surface and groundwater, as well as partially treated water, containing antibiotics residue and other pharmaceuticals, typically at < 100>
The removal of antibiotics and their residues from water and wastewater prior to their final release into the environment is of particular concern (Yang et al., 2021). Modern purification methods can be roughly divided into the following three categories depending on the purification mechanism: biological treatment (Akyon et al., 2019; Zhang et al., 2019), chemical degradation (de Souza Santos et al., 2015; Yang et al., 2021), and physical removal. Each of these methods has its own advantages and disadvantages. For example, biological purification can remove most antibiotic residues, but the introduction of active organisms into the aquatic environment can upset the ecological balance. Various chemical approaches (ozonation, chlorination, and Fenton oxidation) cannot provide complete purification and, in some cases, lead to the death of beneficial microorganisms due to low selectivity. Photocatalysis is widely used in new environmental control strategies (Zhong et al., 2018; Alagha et al., 2021; Yang et al., 2022). However, this method has a number of key disadvantages, such as insufficient use of visible light, rapid annihilation of photogenerated carriers, and incomplete mineralization, which greatly limits its application (Yang et al., 2021).
Common techniques for the removal of antibiotics are adsorption, membrane filtration, and advanced oxidation processes (AOP) (Abtahi et al., 2018; de Andrade et al., 2018; Pandey et al., 2020). Adsorption and membrane filtration are only temporary solutions in which, the micropollutants are transferred away from the wastewater rather than being treated (degradation in metabolites), while the wastestream also contains a high ionic load (cations and anions) (Bobu et al., 2013; Yagub et al., 2014). The disadvantages of AOP are the high energy consumption and costs, as well as the production of secondary toxic wastestreams (Abtahi et al., 2018; Anjali and Shanthakumar, 2019). An environmentally friendly and highly efficient method that does not produce toxic and high concentrated wastestreams alongside the removal of antibiotics.
Ofloxacin (OFX) is a quinolone antibiotic useful for the treatment of a number of bacterial infections (The American Society of Health-System Pharmacists, 2016). A quinolone antibiotic is a member of a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone (Andriole, 1989). They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production (Johnson et al., 2003). OFX is well-known for their antimicrobial and anti-inflammatory capabilities (Kritas et al., 2020). OFX is used to treat pneumonia, skin and urinary tract infections (Amini et al., 2020). Severe acute respiratory syndrome (SARS)-CoV-2 (COVID-19) pandemic, which has killed and infected people in 216 countries/territories, has become the most significant pandemic of the century (Sen Gupta et al., 2020). OFX combined with other drugs, has been widely used to minimise COVID-19-induced inflammation in 2020 (Sen Gupta et al., 2020). OFX is a typical fluoroquinolone antibiotic administered to both humans and animals, and after administration, approximately 78% of OFX is excreted (Tong et al., 2011). OFX pharmaceutical compounds enter water resources in various ways, such as human and animal excretions and inefficient industrial wastewater treatment (Amini et al., 2020). In the class of antibiotics, OFX is also recognised as highly refractory and persistent in aquatic water systems. As the biodegradation of OFX is difficult, sewage treatment plants (STPs) have a low removal rate, and the OFX concentrations in the STP effluents of Beijing, Hangzhou, and Vancouver have been determined to be between 6x10-7 and 1.405x10-3 mg/l (Xiao et al., 2008; Tong et al., 2011).
Doxycycline (DOX), a tetracycline antibiotic, combined with other drugs, has been widely used to minimise Coronavirus Disease-2019 (COVID-19) induced inflammation in 2020 (Sen Gupta et al., 2020). So, of a sudden, the demand and production of DOX have increased many folds. Due to the high consumptions, there are many chances that DOX will come in effluent treatment through the urinal of COVID-19 patients. DOX will also come in pharmaceutical industries wastewater, which are producing DOX. These compounds can enter water resources through various channels, including human waste and inefficient industrial wastewater treatment (Amini et al., 2020). DOX is also used to treat chest, skin, and dental infections. DOX pharmaceutical compounds enter water resources in various ways, such as human and animal excretions and inefficient industrial wastewater treatment (Amini et al., 2020). They also enter the environment due to the improper disposal of expired pharmaceuticals in the garbage or sewage system. The traditional methods for treating such wastewater like coagulation, flocculation, or precipitation lacks efficiency and cannot remove them completely (Adams et al., 2002; Stackelberg et al., 2007; Garg et al., 2016). On the other hand, biological treatments are time-consuming and produce a large quantity of sludge that cannot be used further (Bansal et al., 2016). However, most of these technologies have been reported to have drawbacks such as low efficiency, secondary pollution, and high capital costs. As a result, it is necessary to develop practical technologies based on modern world engineering science to find a better and more cost-effective solution to treat water and wastewater for human consumption. In recent years, advanced oxidation processes (AOPs) have piqued the interest of many researchers due to their potential application in the efficient mineralisation of refractory substances (Rezaei et al., 2019), more effective and sustainable in the long term (Wei et al., 2020; Babaei et al., 2021).
Palladium (Pd) is a precious metal and is one of the most important elements in chemical catalysis, (Xu et al., 2019) electronic circuits, jewelry, semiconductors, ornaments, corrosion-resistant equipment and thermocouples (Folens et al., 2016). With the increasing use of palladium in the past 30 years, a substantial amount of waste Pd is discharged into the environment, which results in the presence of higher levels of Pd in road dust, airborne particulates, groundwater tables and soil. Moreover, the chemical catalysis and electroplating industries generate a large amount of wastewater containing palladium. In epidemiological studies, exposure to Pd has been veried to cause not only acute toxicity, hypersensitivity with respiratory symptoms, and urticaria, but also some immune system diseases, indicating it is a signicant hazard to human health because Pd ions are one of the most signicant metal sensitizers. Thus, the recovery and reuse of this metal, based on the notion of recycling waste, (Sattayarut et al., 2019) are necessary to recover this valuable material and to treat wastewater 4 to promote green chemistry, economic efficiency and sustainability (Noah et al., 2016).
Under suitable conditions, Pd adsorbs more than 900 times its volume of hydrogen (H2) (Dekura et al., 2019). This metal is an essential catalyst for energy conversion, chemical reaction, and abiotic electrochemical processes (Guo et al., 2013; Wu et al., 2018). The Pd is frequently applied in different methods due to its catalytic activity; therefore, it is often used as a catalyst in its NPs size (Coy et al., 2020). Smaller sizes allow a monodisperse distribution of the particles and provide a high surface area that increases the catalytic activity (Saldan et al., 2015). The NPs can be chemically/electrochemically or biologically produced (De Windt et al., 2006; Saldan et al., 2015; Wu et al., 2018). Chemical synthesis of Pd NPs requires the use of a series of toxic and expensive agents, whereas strong reductants and stabilisers are required to reduce the metals to zero-valent and as carrier materials (De Corte et al., 2012). Electrodeposition of Pd is also used and is obtained by using an underpotential deposition condition, e.g. potentials higher than the Nerst one (Previdello et al., 2017; Espino-López et al., 2018). Biological synthesis is less harmful to the environment than chemical production as no secondary toxic wastestream is produced (Hennebel et al., 2009a; Hennebel et al., 2009b).
Production of biogenic palladium nanoparticles (bio-Pd NPs) occurs through microorganisms or plants, where they are responsible for the conversion of Pd+2 to Pd0 (Yong et al., 2002; De Windt et al., 2005; Sathishkumar et al., 2009; Sahin and Gubbuk, 2022). Different microorganisms and plants have been used through extensive research to obtain a higher functionality (Courtney et sl., 2016; Tuo et al., 2017; Xiong et al., 2018). When biological carriers are used for the production of bio-Pd NPs, all Pd+2 that is taken up inside or on the biological carrier will be converted to Pd0 (Xu et al., 2018; Sahin and Gubbuk, 2022). No difference in terminology is made for bio-Pd NPs inside or outside the cells when production occurs through microorganisms. While the production pathway of most of the microorganisms remains unknown, it was recently unravelled for Shewanella oneidensis MR-1. Shewanella oneidensis is a facultative bacterium (Venkateswaran et al., 1999) and the special interest in Shewanella oneidensis MR-1 revolves around its behavior in an anaerobic environment contaminated by heavy metals such as iron (Fe), lead (Pb) and uranium (U). Experiments suggest it may reduce ionic mercury (Hg) to elemental Hg, (Wiatrowski et al., 2006), and ionic silver (Ag) to elemental Ag (Ng et al., 2013a; Ng et al., 2013b). Cellular respiration for Shewanella oneidensis MR-1 is not restricted to heavy metals though; can also target sulfates (SO4-2), nitrates (NO3-) and chromates (CrO4-2) when grown anaerobically. Shewanella oneidensis MR-1 was shown that nicotinamide adenine dinucleotide (NAD) + hydrogen (H) (NADH) dehydrogenase and hydrogenase enzymes played an important role in the production of bio-Pd NPs, when converting all Pd+2 present in the cell to Pd0 (Fredrickson et al., 2008; Deplanche et al., 2010; Hou et al., 2017; Yang et al., 2020). It was found that Escherichia coli was still viable after exposure to Pd, and hence metabolic active but not culturable (Joudeh et al., 2021). For plants, the biomolecules are responsible for the production, e.g., polyols, proteins, flavones, flavonoids, and tannins (Qazi et al., 2016; Sarmah et al., 2019). Besides these carriers, the potential use of anaerobic granular sludge for production was recently discovered (Suja et al., 2014; Quan et al., 2020). Other than using different biological carriers, the performance of bio-Pd NPs can be tuned through modification in production methods. One of the commonly used techniques is the formation of bimetallic NPs; Pd has been combined with other metals of interest, e.g., gold (Au), silver (Ag), iron (Fe), and ruthenium (Ru) (De Corte et al., 2011; Omajali et al., 2019; Sivamaruthi et al., 2019).
The use of bio-Pd NPs for the removal of pharmaceutical compounds has been tested with promising results (Forrez et al., 2011; Wang et al., 2018). However, the application of bio-Pd NPs for the removal of fluorinated pharmaceutical compounds (e.g., ciprofloxacin, citalopram, and atorvastatin), which are highly used and for which removal efficiencies tend to be low, have not been well studied yet (Forrez et al., 2011; Miao et al., 2018; Osawa et al., 2019; Antonelli et al., 2020). Martins et al. (2017) assessed the removal of several antibiotics by bio-Pd NPs, but no removal of fluorinated antibiotics as a result of the catalytic activity of the NPs was detected. The study was carried out in a synthetic medium, where the matrix composition is fairly simple in contrast to real environmental matrices. Nevertheless, a removal of 87.7% of ciprofloxacin was found after 25 hr and at pH = 3.2 with 30 mg of Pd in the form of bio-Pd NPs (He et al., 2020). The disadvantage of this method is the low pH and high concentrations of Pd needed. Moreover, the degradation of persistent fluorinated antibiotics, other than ciprofloxacin, with bio-Pd NPs required further study, especially in environmentally relevant matrices. Ideally, effective removal is accomplished with the use of a lower concentration of Pd, shorter reaction time, higher pH, and in environmentally relevant concentrations are required.
The produced bio-Pd NPs are widely utilised to remediate micropollutants in the environment (e.g., pharmaceuticals and antimicrobial agents) (De Corte et al., 2012; Hoffmann, 2012; Hazarika et al., 2017). Several “hard to degrade” micropollutants such as diatrizoate, trichloroethylene, diclofenac, chromate, chlorophenols, and lindane have been efficiently transformed by bio-Pd NPs (De Gusseme et al., 2011; De Corte et al., 2012; Quan et al., 2019). Microbial degradation often stalls or slows down at micropollutant relevant concentration range due to mass transfer limitation across the cell membrane (Verlicchi et al., 2010; Ehrl et al., 2019; Kundu et al., 2019). Some of the micropollutants are even resistant to microbial enzymes; nevertheless, enzymatic removal of micropollutants can also produce toxic by-products which is not desired (Feng et al., 2021). Therefore, oxidative techniques are often used; however, dangerous explosive compounds are needed (Silva et al., 2017). Hence, developing alternative sustainable approaches is of utmost importance. The bio-Pd NPs hold promise to overcome the hurdle of micropollutants removal in a sustainable way (1) reusable, (2) bio origin, environment-friendly production, and (3) high catalytic activity.
A more recent application of bio-Pd NPs can be found in electrochemical systems. The bio-Pd NPs are coated on anodes and used to increase electricity generation in a microbial electrochemical fuel cell (MFC) (Orozco et al., 2010). The bio-Pd NPs can also be fixed on electrodes to increase the electroconductivity of the electrochemical cell. The electro-active bacteria used for the bio-Pd production and Pd will increase the electro-conductivity. This improved electro-conductivity results in an increased electron transfer and thus a higher catalytic activity (Quan et al., 2015a Quan et al., 2015b; Cheng et al., 2017). Some recent reviews have mainly underlined the potential use of green synthesis for metal NP production with their advantages and general applications (Kumari et al., 2019; Fahmy et al., 2020; Singh et al., 2020). However, the overview of the mechanisms and optimisation of production methods specific for bio-Pd NPs with their novel applications such as in electrochemical systems are missing. A summary of different methods specific to how to increase the catalytic activity of the produced bio-Pd NPs is also present. There is a clear need to acquire a better understanding of the role of bio-Pd NPs in electrochemical cells as the pathway of the microbial production.
In this study, biogenic-palladium nanoparticles (bio-Pd NPs) with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalys was examined during catalytic degradation process in the efficient removal of Ofloxacin (OFX) and Doxycycline (DOX) micropollutants from pharmaceutical industry wastewater plant, İzmir, Turkey. Different pH values (3.0, 4.0, 6.0, 7.0, 9.0 and 11.0), increasing micropollutants (OFX and DOX) concentrations (5 mg/l, 15 mg/l, 30 mg/l and 45 mg/l), increasing Bio-Pd NPs concentrations (5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l, 40 mg/l and 60 mg/l), different Bio-Pd NPs/cell dry weight (CDW) mass ratios (5/5, 6/4, 7/3, 8/2, 9/1, 1/9, 2/8, 3/7 and 4/6), increasing recycle times (1., 2., 3., 4., 5., 6. and 7.) was operated during catalytic degradation process in the efficient removals of OFX and DOX micropollutants in pharmaceutical industry wastewater. The characteristics of the synthesized NPs were assessed using DRS, EDX, FESEM, FTIR, ICP-MS, TEM, XRD and XPS analyses, respectively. The catalytic degradation mechanisms of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalysts and the reaction kinetics of OFX and DOX micropollutants were evaluated in pharmaceutical industry wastewater during catalytic degradation process. ANOVA statistical analysis was used for all experimental samples.
2.1. Characterization of Pharmaceutical Industry Wastewater
Characterization of the biological aerobic activated sludge proses from a pharmaceutical industry wastewater plant, İzmir, Turkey was performed. The results are given as the mean value of triplicate samplings (Table 1).
| Parameters | Unit | Concentrations |
| Chemical oxygen demand-total (CODtotal) | (mg/l) | 4000 |
| Chemical oxygen demand-dissolved (CODdissolved) | (mg/l) | 3200 |
| Biological oxygen demand-5 days (BOD5) | (mg/l) | 1500 |
| BOD5 / CODdissolved | 0.5 | |
| Total organic carbons (TOC) | (mg/l) | 1800 |
| Dissolved organic carbons (DOC) | (mg/l) | 1100 |
| pH | 8.3 | |
| Salinity as Electrical conductivity (EC) | (mS/cm) | 1552 |
| Total alkalinity as CaCO3 | (mg/l) | 750 |
| Total volatile acids (TVA) | (mg/l) | 380 |
| Turbidity (Nephelometric Turbidity unit, NTU) | NTU | 7.2 |
| Color | 1/m | 50 |
| Total suspended solids (TSS) | (mg/l) | 250 |
| Volatile suspended solids (VSS) | (mg/l) | 187 |
| Total dissolved solids (TDS) | (mg/l) | 825 |
| Nitride (NO2-) | (mg/l) | 1.7 |
| Nitrate (NO3-) | (mg/l) | 1.91 |
| Ammonium (NH4+) | (mg/l) | 2.3 |
| Total Nitrogen (Total-N) | (mg/l) | 3.2 |
| SO3-2 | (mg/l) | 21.4 |
| SO4-2 | (mg/l) | 29.3 |
| Chloride (Cl-) | (mg/l) | 37.4 |
| Bicarbonate (HCO3-) | (mg/l) | 161 |
| Phosphate (PO4-3) | (mg/l) | 16 |
| Total Phosphorus (Total-P) | (mg/l) | 40 |
| Total Phenols | (mg/l) | 70 |
| Oil & Grease | (mg/l) | 220 |
| Cobalt (Co+3) | (mg/l) | 0.2 |
| Lead (Pb+2) | (mg/l) | 0.4 |
| Potassium (K+) | (mg/l) | 17 |
| Iron (Fe+2) | (mg/l) | 0.42 |
| Chromium (Cr+2) | (mg/l) | 0.44 |
| Mercury (Hg+2) | (mg/l) | 0.35 |
| Zinc (Zn+2) | (mg/l) | 0.11 |
Table 1: Characterization of Pharmaceutical Industry Wastewater
The M9 medium used for washing the bio-Pd NPs was prepared based on the recipe and Na2HPO4, NaCl, KH2PO4 and NH4Cl purchased from Merck (Merck, Germany). The KOH tablets used as electrolyte was purchased from Merck (Merck, Germany). For Inductively coupled plasma mass spectrometry (ICP-MS) analysis, ultra-pure water (Resistivity > 18.2 MΩ cm, Millipore, France) was used. The Na2PdCl4 powder (98%), phosphate-buffered saline (PBS) tablets, LB broth used for bio-Pd NPs production, and methyl orange for the activity test were purchased from Sigma-Aldrich (Sigma-Aldrich, Germany). Pro analysis purity level 14 mol/l HNO3 (Sigma-Aldrich, Germany), further purified by sub-boiling distillation, and 9.8 mol/l H2O2 (Sigma-Aldrich, Germany) were used for acid digestion. 1 g/l single-element standard solutions of Pd and Rh (Merck, Germany) were used for method development and calibration purposes, and for internal standardization, respectively.
The production of bio-Pd NPs was carried out as described by De Windt et al. (2005). Shewanella oneidensis MR-1 cells were grown in LB medium overnight at 28°C on a shaker, and harvested by centrifuging at 10000 rpm for 10 min. The cells were washed three times with M9 medium and resuspended in M9 medium to a final optical of OD610 = 0.5 with Spectronic 200 (Thermofischer, USA). The Na2PdCl4 was added to the resuspended solution at a concentration of 50 mg/l Pd+2 and this solution was incubated overnight on a shaker at 20°C. Production of different types of bio-Pd NPs was performed in a custom-built experimental setup. Corresponding controls were prepared in the same manner but were not exposed to H2(g). The bio-Pd NPs produced and controls were washed three times with PBS and stored at 5°C.
1 gram wet cells was added to 200 ml of PdCl2 solution (200 mg/l), the pH of the solution was adjusted to 2.0, and the container was then sealed. The solutions were incubated under anaerobic conditions by flushing with N2(g) for 5 min. After incubation at 30oC for 60 min to allow for Pd+2 biosorption, the bio-Pd NPs was harvested and collected by centrifugation (8000 rpm, 8 min) and resuspended in deionized water. The Bio-Pd(II) was transferred to a 100 ml three-necked flask. Then, the three-necked flask was flushed with H2(g) and left at 25oC with stirring (150 rpm) in H2(g) atmosphere. After 5 h, the bio-Pd NPs used in the next experiment was collected by centrifugation (8000 rpm, 8 min) and washed twice with deionized water. 50 ml PdCl2 solution at pH=2.0 was added to a 100 ml round-bottomed flask. 100 ml round-bottomed flask was flushed with H2(g), and left at 25oC with stirring (150 rpm) in H2(g) atmosphere, collected by centrifugation (8000 rpm, 8 min) and washed twice with deionized water after 5 h to preparation Pd(0) without Shewanella oneidensis MR-1.
An experimental reactor setup (in triplicate) containing an electrochemical cell (EC) connected to a glass column was used to produce bioPd NPs. A two-compartment EC (dimensions: 25 cm x 10.0 × 3.0 cm) made from two Perspex® frames was used to separate the cation exchange membrane (CEM) (Membrane International Inc., USA) from the electrodes. A stainless-steel electrode was used as the cathode (dimensions: 5 × 20 cm) and an iridium (Ir) electrode was used as the anode. Between the compartments and CEM, rubber sheets were placed to create a liquid-tight seal, and the frames were bolstered. A pump at 255 ml/min flow rate was used to recirculate the KOH electrolyte. The current of the EC was controlled by a power supply, Faraday’s Law was used to calculate the amount of H2(g) produced based on the current applied. The EC was connected to a glass column (wrapped in aluminum foil to prevent light penetration), in which the microorganisms/Pd+2 solution was transferred in, after incubation. The top of the glass column was connected to a gascounter. The bottom of the column contained a two-way connector between the EC and a gasbag for N2(g) flushing, both regulated by a valve.
2.6. Characterization of the bio-Pd NPs
2.6.1. Transmission Electron Microscopy (TEM) Analysis
The obtained bio-Pd NPs was collected and harvested by centrifugation (8000 rpm, 5 min), washed twice with deionized water, and resuspended in ethanol (C2H6O) and dripped onto a carbon-coated copper (Cu) Transmission Electron Microscopy (TEM) grid. Vacuum drying then occurred to the biological Pd(0) for 24 h at 25oC room temperature. The dry samples on the Cu grid were viewed and examined by TEM Analysis recorded in a JEOL JEM 2100F, Japan under 200 kV accelerating voltage. The size and structure of the bio-Pd NPs samples were identified with TEM analysis.
2.6.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-Ray (EDX) Spectroscopy Analysis
The morphological features and structure of the bio-Pd NPs was determined by Field Emission Scanning Electron Microscopy (FESEM) (FESEM, Hitachi S-4700). The elements on the surface of the bio-Pd NPs were analysed using energy dispersive X-ray analysis (EDX) with EDX spectrometry device (TESCAN Co., Model III MIRA). The biological Pd NPs on Shewanella oneidensis MR-1 were harvested and collected by centrifugation (8000 rpm, 5 min), resuspended in C2H6O and dripped onto a thin film. The samples of the biological Pd NPs on Shewanella oneidensis MR-1 were dried overnight in a vacuum drying oven at 30oC and analysed by FESEM and EDX.
2.6.3. X-Ray Diffraction (XRD) Analysis
Powder XRD patterns were recorded on a Shimadzu XRD-7000, Japan diffractometer using Cu Kα radiation (λ = 1.5418 Å, 40 kV, 40 mA) at a scanning speed of 1o /min in the 10-80o 2θ range. Raman spectrum was collected with a Horiba Jobin Yvon-Labram HR UV-Visible NIR (200-1600 nm) Raman microscope spectrometer, using a laser with the wavelength of 512 nm. The spectrum was collected from 10 scans at a resolution of 2 /cm. The zeta potential was measured with a SurPASS Electrokinetic Analyzer (Austria) with a clamping cell at 300 mbar.
2.6.4. X-Ray Photoelectron Spectroscopy (XPS) Analysis
The valence state of the biogenic palladium nanoparticles was investigated and was analyzed using XPS (ESCALAB 250Xi, England). XPS used an Al Ka source and surface chemical composition and reduction state analyses was done, with the core levels recorded using a pass energy of 30 eV (resolution approximately 0.10 eV). The peak fitting of the individual core-levels was done using XPS-peak 41 software, achieving better fitting and component identification. All binding energies were calibrated to the C 1s peak originating from C–H or C–C groups at 284.6 eV.
2.6.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
Bulk ICP-MS, Agilent 8800 ICP-MS instrument (Agilent Technologies, Japan), analysis was performed to determine the concentration of Pd in the different bio-Pd NPs samples. For bulk analysis, the bio-Pd NPs samples were acid digested in closed Savillex® PFA beakers prior to ICP-MS. 5 ml of suspended bio-Pd in PBS was centrifuged at 10000 ×g for 30 min, after which the supernatant was removed. The resulting pellet was acid digested with 1.5 ml of 14 mol/l HNO3 and 0.5 ml of 9.8 mol/l H2O2. The closed beakers were heated to 115°C overnight on a hot plate. After complete mineralization, the digestates were evaporated at 90°C until dryness, then redissolved in 2.0 ml of 0.35 mol/l HNO3. This solution was further diluted with 0.35 mol/l HNO3 and rhodium (Rh) was added as the internal standard (final concentration = 2 μg/l) to compensate for potential matrix effects and/or signal instability.
2.6.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The FTIR spectra of experimental samples was recorded using the FT-NIR spectroscope (RAYLEIGH, WQF-510).
2.6.7. Diffuse Reflectance UV-Vis Spectra (DRS) Analysis
DRS Analysis in the range of 200–800 nm were recorded on a Cary 5000 UV-Vis Spectrophotometer from Varian. DRS was used to monitor the OFX and DOX concentrations in experimental samples.
A certain amount of OFX was dissolved in ultrapure water acidified with glacial acetic acid (CH3COOH) (5 ml/l), and the solution was adjusted to pH=3.2 with hydrochloric acid (HCl) (1 mol/l). 30 mg biological Pd NPs was resuspended in 20 ml of this OFX solution. Catalytic removal of OFX using this biological Pd NPs was carried out in the dark at 25oC with stirring at 150 rpm. 3 ml of the reaction mixture was removed every 5 h with a syringe and passed through a syringe filter with a pore size of 0.22 mm to remove biological Pd(II) or biological Pd(0). An ultraviolet (UV) spectrophotometer was used to detect the residual content of OFX in the supernatant at 420 nm, and the removal of OFX was calculated by comparison with a standard curve. When the removal of OFX was conducted under anaerobic conditions, 30 mg biological Pd NPs was transferred into the OFX solution, and then H2(g) was bubbled into the suspension for at least 10 min from the bag, and a hose was used to connect a balloon of H2(g) and the reactor vessel.
A certain amount of DOX was dissolved in ultrapure water acidified with CH3COOH (5 ml/l), and the solution was adjusted to pH=3.2 with HCl (1 mol/l). 30 mg biological Pd NPs was resuspended in 20 ml of this DOX solution. Catalytic removal of DOX using this biological Pd NPs was carried out in the dark at 25oC with stirring at 150 rpm. 3 ml of the reaction mixture was removed every 5 h with a syringe and passed through a syringe filter with a pore size of 0.22 mm to remove biological Pd(II) or biological Pd(0). An ultraviolet (UV) spectrophotometer was used to detect the residual content of DOX in the supernatant at 240 nm, and the removal of DOX was calculated by comparison with a standard curve. When the removal of DOX was conducted under anaerobic conditions, 30 mg biological Pd NPs was transferred into the DOX solution, and then H2(g) was bubbled into the suspension for at least 10 min from the bag, and a hose was used to connect a balloon of H2(g) and the reactor vessel.
The catalytic activity of bio-Pd NPs was evaluated by the removal of 100 mg/l Methyl Orange (in biological triplicates), in serum flasks. Suspended bio-Pd was centrifuged at 10000 x g for 10 min, and the pellet of centrifuged bio-Pd corresponded to 80.95 μg Pd. The pellet was resuspended in 19.2 ml deionized water and 0.8 ml Methyl Orange stock solution (7.63 mmol/l) was added. The serum flasks were flushed with 100% N2(g) for 20 cycles, subsequently, 120 ml of 100% H2(g) was added. 1 ml sample was taken at 0 min, 10 min, 20 min, 40 min, 60 min, 80 min, 100 min and 120 min and was filtered using a 0.20 μm filter. The absorbance was measured at a λmax = 465 nm using a Cary 5000 UV-Vis Spectrophotometer from Varian. Deionized water and (filtered) suspended bio-Pd in deionized water were used as a control for the absorbance measurements. Suspended microorganisms without Pd were used as a control for the catalytic activity test.
Chemical oxygen demand-total (CODtotal), chemical oxygen demand-dissolved (CODdissolved), total phosphorus (Total-P), phosphate phosphorus (PO4-3-P), total nitrogen (Total-N), ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3--N), nitrite nitrogen (NO2--N), biological oxygen demand 5-days (BOD5), pH, Temperature [(oC)], total suspended solids (TSS), total volatile suspended solids (TVSS), total organic carbon (TOC), Oil, Chloride (Cl-), total phenol, total volatile acids (TVA), disolved organic carbon (DOC), total alkalinity, turbidity, total dissolved solid (TDS), color, sulfide (SO3-2), sulfate (SO4-2), bicarbonate (HCO3-), salinity, cobalt (Co+3), lead (Pb+2), potassium (K+), iron (Fe+2), chromium (Cr+2), Mercury (Hg+2) and zinc (Zn+2) were measured according to the Standard Methods (2017) 5220B, 5220D, 4500-P, 4500-PO4-3, 4500-N, 4500-NH4+, 4500-NO3-, 4500-NO2-, 5210B, 4500-H+, 2320, 2540D, 2540E, 5310, 5520, 4500-Cl-, 5530, 5560B, 5310B, 2320, 2130, 2540E, 2120, 4500-SO3-2, 4500-SO4-2, 5320, 2520, 3500-Co+3, 3500-Pb+2, 3500- K+, 3500-Fe+2, 3500-Cr+2, 3500- Hg+2, 3500-Zn+2, respectively (Baird et al., 2017).
Total-N, NH4+-N, NO3--N, NO2--N, Total-P, PO4-3-P, total phenol, Co+3, Pb+2, K+, Fe+2, Cr+2, Hg+2, Zn+2, SO3-2, and SO4-2 were measured with cell test spectroquant kits (Merck, Germany) at a spectroquant NOVA 60 (Merck, Germany) spectrophotometer (2003).
The measurement of color was carried out following the methods described by Olthof and Eckenfelder (1976) and Eckenfelder (1989). According these methods, the color content was determined by measuring the absorbance at three wavelengths (445 nm, 540 nm and 660 nm), and taking the sum of the absorbances at these wavelengths. In order to identify the color in pharmaceutical industry wastewater (25 ml) was acidified at pH=2.0 with a few drops of 6 N HCl and extracted three times with 25 ml of ethyl acetate. The pooled organic phases were dehydrated on sodium sulphate, filtered and dried under vacuum. The residue was sylilated with bis(trimethylsylil)trifluoroacetamide (BSTFA) in dimethylformamide and analyzed by gas chromatography–mass spectrometry (GC-MS) and gas chromatograph (GC) (Agilent Technology model 6890N) equipped with a mass selective detector (Agilent 5973 inert MSD). Mass spectra were recorded using a VGTS 250 spectrometer equipped with a capillary SE 52 column (HP5-MS 30 m, 0.25 mm ID, 0.25 μm) at 220°C with an isothermal program for 10 min. The initial oven temperature was kept at 50oC for 1 min, then raised to 220oC at 25oC/min and from 200 to 300oC at 8oC/min, and was then maintained for 5.5 min. High purity He (g) was used as the carrier gas at constant flow mode (1.5 ml/min, 45 cm/s linear velocity).
The total phenol was monitored as follows: 40 ml of pharmaceutical industry wastewater was acidified to pH=2.0 by the addition of concentrated HCl. Total phenol was then extracted with ethyl acetate. The organic phase was concentrated at 40°C to about 1 ml and silylized by the addition of N,O-bis(trimethylsilyl) acetamide (BSA). The resulting trimethylsilyl derivatives were analysed by GC-MS (Hewlett-Packard 6980/HP5973MSD).
Methyl tertiary butyl ether (MTBE) was used to extract oil from the water and NPs. GC-MS analysis was performed on an Agilent gas chromatography (GC) system. Oil concentration was measured using a UV–vis spectroscopy fluorescence spectroscopy and a GC–MS (Hewlett-Packard 6980/HP5973MSD). UV–vis absorbance was measured on a UV–vis spectrophotometer and oil concentration was calculated using a calibration plot which was obtained with known oil concentration samples.
2.11. Statistical Analysis
ANOVA analysis of variance between experimental data was performed to detect F and P values. The ANOVA test was used to test the differences between dependent and independent groups, (Zar, 1984). Comparison between the actual variation of the experimental data averages and standard deviation is expressed in terms of F ratio. F is equal (found variation of the date averages/expected variation of the date averages). P reports the significance level, and d.f indicates the number of degrees of freedom. Regression analysis was applied to the experimental data in order to determine the regression coefficient R2, (Statgraphics Centurion XV, 2005). The aforementioned test was performed using Microsoft Excel Program.
All experiments were carried out three times and the results are given as the means of triplicate samplings. The data relevant to the individual pollutant parameters are given as the mean with standard deviation (SD) values.
3.1. Bio-Electrochemical Cell Mechanism of Bio-Pd NPs Production
Each bacteria is different, bio-Pd NPs synthesis mainly depends on the outer membrane c-type cytochrome and hydrogenase enzyme (De Windt et al., 2005; Fredrickson et al., 2008; Deplanche et al., 2010; De Corte et al., 2012; Hou et al., 2017). The c-type cytochrome is responsible for transporting the electrons across the bacterial cell to the metals (Ng et al., 2013a; Ng et al., 2013b). At the same time, the hydrogenase contains redox proteins that are important for reducing metals and consequently contribute to the formation of metal particles. Here, the Pd+2 will be converted to Pd0 ; hence, the formed particles can be found on the cell wall, inside the cell membranes, or cytoplasm (Fredrickson et al., 2008; Hennebel et al., 2011; Bucking et al., 2012; Ng et al., 2013a; Ng et al., 2013b; Dundas et al., 2018). The electroactive bacterium, Shewanella oneidensis MR-1, is a well-established microorganism for the production of metal NPs. It has the characteristics to perform the biological dissimilatory metal reduction process (DMR). In this biological process, various metal (loid)s, outside the microorganism, are used as a terminal electron acceptor. Energy is conserved by oxidising an (in)organic matter and reducing a metal (Lovley, 1993; Shi et al., 2009; Dundas et al., 2018; Xiong et al., 2018). The mechanism used by the microorganisms depends on the type of metal used for NP production (Lovley, 1993; Marshall et al., 2006; Mikheenko et al., 2008; Shi et al., 2009; Deplanche et al., 2010). The production process of bio-Pd NPs can be in general described in three steps: (1) biological adsorption (biosorption) on the cell surface, (2) bioreduction of Pd+2 to Pd0in- and outside the cell wall, and (3) autocatalytic reduction, of Pd+2 to Pd0 which is not enzymatic and clusters onto the Pd NPs of the cell wall (De Corte et al., 2012; Deplanche et al., 2014; Xu et al., 2018). The production of bio-Pd NPs by Shewanella oneidensis MR-1 will be discussed in detail here, due to the general and beneficial character of the microorganisms for the production of bio-Pd NPs. First, biosorption of Pd+2 on the cell wall can occur by ion exchange, electrostatic interaction, complexation, and physical adsorption which was shown in FTIR analysis by Xu et al. (2018). Independent of the type of biosorption process, the Pd+2 is converted to Pd0, by chemical or autocatalytic conversion (De Corte et al., 2012; Ng et al., 2013a; Ng et al., 2013b; Deplanche et al., 2014). When ion exchange, electrostatic interaction, or complexation occurs, the functional groups of the cell surface have an essential role and are responsible for attaching the Pd+2 to the cell wall. Nevertheless, it is also possible to have physical adsorption, where the functional groups do not intervene in attaching the Pd+2 to the cell wall (Xu et al., 2018). However, besides the biosorption, the cell wall can convert the Pd+2 to Pd0 which was observed in scanning electron microscopic energy-dispersive X-Ray spectroscopy (SEM-EDX) and transmission electron microscopic (TEM) images (Xu et al., 2018). This indicates that bioreduction can be achieved through the hydroxyl group present on the cell surface. Interestingly, Yang et al. (2020) found that this bioreduction of Pd+2 to Pd0 occurs through the c-type cytochrome on the cell wall, more specific Cym A (such as OmCA and MtrC). Furthermore, the production of bio-Pd NPs inside the cells is also present. Yang et al. (2020), suggested that the NADH-dehydrogenase, [FeFe]-hydrogen (HydA), and [NiFe]-hydrogenase (HyaB) are essential for the bio-Pd NPs production, as they influence the size, distribution, and the number of bio-Pd NPs (Figure 1).
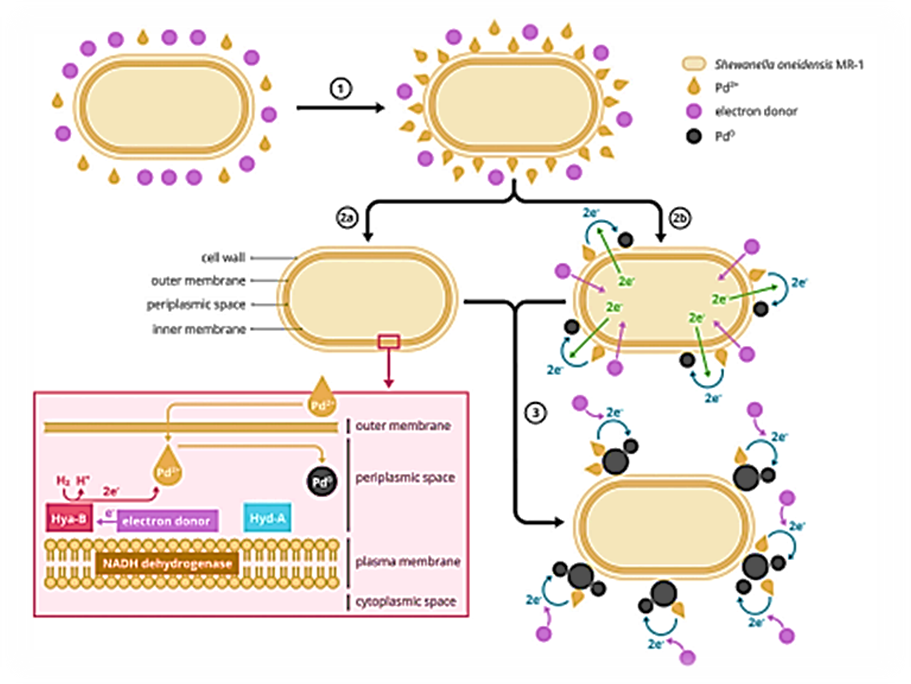
Figure 1: Production mechanism of bio-Pd NPs inside and outside the microorganisms: (1) Adsorption of Pd+2 on the cell wall, (2a) conversion of Pd+2 to Pd0 inside the cell, (2b) on the cell wall, and (3) autocatalytic conversion of Pd+2 to Pd0 outside the cell. The figure was inspired by Deplanche et al. (2010), Ng et al. (2013a), and Yang et al. (2020).
These enzymes are mainly responsible for the formation of bio-Pd NPs inside and outside the cell membrane (Yang et al., 2020). Whereas [NiFe]-hydrogenase produces hydrogen from formate and oxidises hydrogen, [FeFe]-hydrogenase works together with formate dehydrogenases in the form of formate-hydrogen lyase that generates hydrogen from formate (Yang et al., 2020). Dundas et al. (2018), stated that the hydrogenase enzymes play an essential role in facilitating the reduction process of Pd+2 by Shewanella oneidensis MR-1. It was observed that this process followed the pseudo-first-order kinetic model (Ng et al., 2013a; Dundas et al., 2018). Furthermore, it was found that [NiFe]-hydrogenase had greater activity than [FeFe]-hydrogenase; therefore, [NiFe]-hydrogenase is more involved in the synthesis of bio-Pd NPs (Yang et al., 2020). Yang et al. (2020), stated that bio-Pd NPs on the outer membrane was produced by NADH-dehydrogenase, while production in the periplasm was provided by [FeFe]-hydrogenase and [NiFe]-hydrogenase (Yang et al., 2020). The hydrogenase mechanism of Bio-Pd NPs in the Shewanella oneidensis MR-1 bacteria cells was shown in detail at Figure 2.
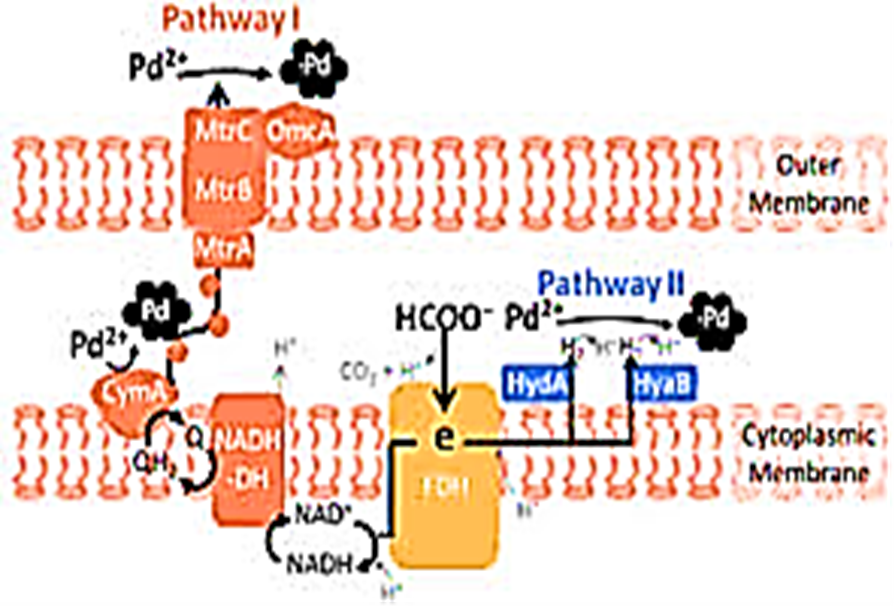
Figure 2: Hydrogenase mechanism of Bio-Pd NPs in the Shewanella oneidensis MR-1 bacteria cells
Deplanche et al. (2010) provided evidence that mainly [NiFe]-hydrogenase, localised in the periplasm, is essential for facilitating the production of bio-Pd NPs. This was supported by other researchers as well (Mikheenko et al., 2008; Deplanche et al., 2010; Shi et al., 2011; De Corte et al., 2012; Ng et al., 2013; Yang et al., 2020). This [NiFe]-hydrogenase enzyme can form and oxidate H2(g) in a bidirectional function. When oxidation of H2(g) occurs, electrons are released and used to reduce Pd+2 (Courtney et al., 2016; Yang et al., 2020). Anaerobic conditions are often required before reducing Pd+2 as only under these conditions [NiFe]-hydrogenase is expressed (Shi et al., 2011; Courtney et al., 2016). This enzyme can release electrons by oxidation of H2(g), H2(g) also needs to be added as an electron donor for the conversion (Ng et al., 2013a). The production of bio-Pd NPs does not only come from [NiFe]-hydrogenase, 50% of the converted Pd+2 comes from other enzymes (Ng et al., 2013a). This was due to the similarities between Ni+2 and Pd+2, according to Deplanche et al. (2014). The Ni+2 is a key component for many metalloenzymes, that are located in the cytoplasm and will be simultaneously transported by the Ni+2 “trafficking system” through the cell membrane. Due to the chemical similarities Pd+2 is transported across the membrane in the same way (Deplanche et al., 2014). The Pd+2 taken up by the cytoplasm is deposited as NPs, however, this cannot substitute for the Ni+2 function (Gomez-Bolivar et al., 2019a; Gomez-Bolivar et al., 2019b). Nevertheless, after the uptake and deposition of the bio-Pd NPs, the metabolic activity could be detected through flow cytometry (Omajali et al., 2019). Once the Pd+2 is taken upinside the cytoplasm the Pd+2 starts forming Pd0 “seeds”, the formation of these “seeds” results in a loss of cell viability (Gomez-Bolivar et al., 2019a; Gomez-Bolivar et al., 2019b). Besides the biological reduction of Pd+2, the chemical conversion also appears in this production mechanism. The added electron donor can also be used for the chemical reduction of Pd+2 without the use of microorganisms. These chemical-produced NPs are going to attach to the existing bio-Pd NPs, causing particle aggregation (De Windt et al., 2006; Deplanche et al., 2014). Chemically produced NPs have a negative zeta potential and attract the Pd+2, by the van der Waals forces, and finally attach to the newly formed bio-Pd NPs (Berg et al., 2009; Sathishkumar et al., 2009). The particle aggregation negatively influences the catalytic activity (De Windt et al., 2006). The bio-Pd NPs are often detected as NPs in spherical form, where the size can vary abundantly (Sahin and Gubbuk, 2022). Besides this, recentlyit was found that microbes were able to dissolve Pd0 which is an intermediate form of Pd+2 towards Pd NPs during microbial reduction by Shewanella oneidensis MR-1 cells (Zheng et al., 2022). Furthermore, 2D structured nanomaterials of Pd were also discovered; nevertheless, the 2D structured Pd NPs are not synthesised with biological carriers, but are chemically produced (Xu et al., 2021).
The presence of metals, like Pd, in microorganisms has a significant impact on the viability of the cells because of the high toxicity. Once the NPs are formed, the bacteria die (Suja et al., 2014). However, the toxicity of the metal to the microorganisms is strongly dependent on the type of metal, concentration, and microorganisms. In some cases, the presence of metals can enhance cellular viability which is strongly dependent on the concentration of the metal, by enhancing the decomposition of reactive oxygen species (ROS) (De Windt et al., 2006; Wu et al., 2011; Sakimoto et al., 2016; Ji et al., 2018; Chen and Chen, 2021). When high concentrations of metals are present, microorganisms tend to produce ROS due to the stress caused by the presence of the heavy metals (Yin and Gao, 2011; Chen and Chen, 2021; Joudeh et al., 2021). It was suggested that Shewanella oneidensis also produces ROS when heavy metals are present, causing damage to essential proteins responsible for transport and respiration inside the cell (Yin and Gao, 2011). The presence of ROS damage proteins which directly reduces the ability of the cells to survive or thrive. Second, it also damages proteins that can release irons in the cultures, resulting in a higher stimulation of ROS production and is more fatal because it occurs when the damaged cells are recovering (Yin and Gao, 2011). De Windt et al. (2006) observed that for 50 mg/l Shewanella oneidensis MR-1 the cells were inviable at a Pd+2 concentration of 125 mg/l (De Windt et al., 2006; Chen and Chen, 2021). It was also found that bio-Pd NPs were more toxic for Gram-positive than Gram-negative bacteria (Adams et al., 2014). Nevertheless, depending on the type of metal and bacteria biological conversion can still occur independently of the viability of the bacterial cell due to the presence of specific organic functional groups on the cell wall (Vijayaraghavan and Ashokkumar, 2017). This differential expression also occurred for the functional genes, whereof the highest changes can be found for genes responsible for amino acid transport and metabolism, carbohydrate transport and metabolism, transcription, post-translational modification, protein turnover, and chaperons. Compared to other heavy metals, Pd caused higher protein damage due to cross-linking, disruption of the 3D structures, and allosteric movements. It was found that the microorganism was still metabolic active, and hence viable after exposure to 100 µM sodium tetrachloropalladate, but not culturable. This is due to the downregulation of the repair genes and up-regulation of carbohydrate metabolism genes (Joudeh et al., 2021). However, it was found that higher resistance toward the toxicity of Pd can be solved by genetic modification. Nevertheless, genetic modification is expensive and labour-intensive.
3.2. Bio-Pd NPs Production Methods
Bio-Pd NPs production can be performed by using microorganisms, plants, and anaerobic sludge, respectively. Microorganisms are often used for production due to their lower cost and environmental sustainability (Mughal et al., 2021). The use of Shewanella oneidensis and Desulfovibrio desulfuricans are well known for their highly active production of nanocatalysts. However, after the production, an extra washing step is needed to remove the produced H2S which is poisonous to the catalyst (Lloyd et al., 1998; Wu et al., 2015; Courtney et al., 2016). In contrast, the toxification of the catalyst from H2S can be prevented by using Escherichia coli and Rhodobacter sphaeroides (Redwood et al., 2008; Deplanche et al., 2010). The production of bio-Pd NPs with Shewanella oneidensis MR-1 was explained to detail in Figure 3.

Figure 3: Production of bio-Pd NPs with Shewanella oneidensis MR-1
The formation of the bio-Pd NPs by Gram-negative or Gram-positive microorganisms is different due to cell wall differences which play an essential role by influencing the metals’ binding onto the surface (Adams et al., 2014; Deplanche et al., 2014). Various Gram-negative and Gram-positive strains have been tested for the production of different-sized bio-Pd NPs, where a significant difference in size was found. The bio-Pd NPs produced by Gram-negative bacteria tend to outcome small NPs equally distributed around the cell wall. While in contrast, Gram-positive bacteria produce a few large NPs (Deplanche et al., 2014). This difference was not caused by the distinct metabolic activity, but potentially by biosorption. Sorption of Pd+2 was higher for Gram-negative strains than for Gram-positive strains. Resulting in higher concentrations of Pd+2 in the solution for Gram-positive bacteria. Subsequently, more chemical conversions are appearing, hence the generation of larger NPs (Deplanche et al., 2014).
Plants are also often used for the production of bio-Pd NPs. Different plants and forms – (e.g., powder or extract) and complete parts of plants (e.g., stem, leaf, root, fruit, flower, and seed) can be used (Sathishkumar et al., 2009; Siddiqi and Husen, 2016; Vijayaraghavan and Ashokkumar, 2017). They have the capability of accumulating metal ions that can be reduced to NPs (Bali et al., 2006; Vijayaraghavan and Ashokkumar, 2017). However, the use of entire plants is prevented, as this resultsin the production of NPs of different sizes and shapes throughout the plant. This is caused by the difference in penetration and localisation of the metal ions in the plant due to the diverse reduction capacities of the plant parts (Sharmila et al., 2017). Complex methods are needed to extract, purify, and increase the recovery of the particles (Bali et al., 2006; Sharmila et al., 2017; Vijayaraghavan and Ashokkumar, 2017). Therefore, plant extracts are often preferred in general due to the instant conversion of metals into NPs, absence of penetration, higher recovery, and less variation in size and shape (Makarov et al., 2014; Siddiqi and Husen, 2016; Shaik et al., 2017; Md Ishak et al., 2019). Thisis due to the functioning of biomolecules from the extracts as reducing and stabilising agents (Shaik et al., 2017; Vijayaraghavan and Ashokkumar, 2017; Sarmah et al., 2019).
A new approach for bio-Pd NPs production can be obtained through anaerobic granular sludge which is mainly used to treat wastewater (Pat-Espadas et al., 2016; Quan et al., 2020). The benefit of using anaerobic granular sludge is (1) treatment of wastewater, (2) recovery of Pd+2 from wastewater, and (3) the catalytic activity of the NPs. This is without any additional cost and energy (Quan et al., 2015a; Quan et al., 2015b; Quan et al., 2019). However, the reduction of Pd+2 through anaerobic sludge is strongly dependent on the type of electron donor. Hence, different types of electron donors were tested. Nonetheless, only H2(g) and formate showed a biological and chemical reduction of Pd+2 which was not found for pyruvate, lactate, acetate, and ethanol (Pat-Espadas et al., 2016). A part of the Pd+2 present in the wastewater remained unconverted both biologically and chemically. Besides, the latter two mechanisms, the third mechanism of conversion occurred through biosorption of the metal ion which is the bonding between the Pd+2 and sludge, by chemisorption (Pat-Espadas et al., 2016). Extracellular ligands induced this binding with weak acid organic functional groups associated with organomonometallic complexation (Gould and Genetelli, 1978; Alibhai et al., 1985; Pat-Espadas et al., 2016). This biosorption was also caused by the interaction between the organic functional groups on the cell wall (e.g., amine, amide, phosphoryl, and carboxyl groups) and the Pd+2 (Fahmy et al., 2006; Rotaru et al., 2012; Pat-Espadas et al., 2016). However, an inhibitory effect was also observed on the microbial community due to the exposure of bacteria to Pd. The bio-Pd NPs affected the bacterial and archaeal community as well as the produced total volatile fatty acids (Pat-Espadas et al., 2016; Quan et al., 2020). When an increase in volatile fatty acids is present, this indicates a failure of the anaerobic sludge, more specifically the inhibition of the microbial community present in the sludge (Appels et al., 2008; Franke-Whittle et al., 2014). Volatile fatty acids are normally produced and used by the microorganisms present in the anaerobic sludge (Ahring et al., 1993; Appels et al., 2008). However, when there is an increase in the volatile acids, this indicates that some of the microorganisms are inhibited, consequently resulting in a decrease in pH and an inhibition of the other microorganisms that are present (Zaher et al., 2004). Hence, this inhibitory effect on the anaerobic sludge is undesired, as the sludge is responsible for treating the wastewater. This toxic effect is different for each microbial community in the granular sludge due to their characteristics and spatial organisation in the sludge. The bacterial community is present in the outer layer, while the methanogens are in the granular sludge’s core (Hulshoff Pol et al., 2004). However, contradictory results were obtained regarding the inhibition effect on the different methanogenic archaeal communities–acetoclastic and hydrogenotrophic methanogens.
The disruption of the biological conversion of Pd+2 can also be affected by the binding of Pd onto proteins or by the replacement of essential metals for the function of the proteins. This replaced or bound Pd can disrupt the enzyme function and structure which results in the loss of their essential functional proteins (Vallee BL, Ulmer, 1972; Quan et al., 2020).
3.3. The Catalytic Activity of Bio-Pd NPs
Reductions are needed to trigger the well-known catalytic activity of bio-Pd NPs such as dehalogenation or hydrogenation reactions. Therefore, different compounds can be used, such as pyruvate, lactate, acetate, formate, and H2(g) (Pat-Espadas et al., 2016; Cheng et al., 2017). Based on research, H2(g) is the most efficient electron donor to activate the dehalogenation or hydrogenation processes by the catalytic activity of the bio-Pd NPs. The Pd adsorbs H2(g) and converts it into atomic hydrogen which is very reactive as a reduction agent (Van Nevel et al., 2011; De Gusseme et al., 2012). The hydrogen radicals adsorbed onto the NPs contribute significantly to the high catalytic activity of bio-Pd NPs (De Windt et al., 2006; Hennebel et al., 2011). However, the catalytic efficiency of bio-Pd NPs can be influenced by (1) the size and distribution of the NPs on the cell wall, and (2) the activation method of the bio-Pd NPs (Hou et al., 2017; Xiong et al., 2018).
Small bio-Pd NPs can be obtained without using extreme conditions and toxic chemicals through the presence of the biological carrier (De Windt et al., 2006). The size of the NPs has a direct influence on the catalytic activity (Zhou et al., 2006). Therefore, small NPs give a high ratio of surface area/volume which results in high surface energy combined with high reactivity (Hennebel et al., 2009a; Hennebel et al., 2009b; Leso and Iavicoli, 2018). This catalytic activity can be optimised by changing the size and distribution of the NPs. The aforementioned optimisation depends on the Pd/cell dry weight (CDW) ratio which affects the viability of the biological carrier, hence the microorganism (De Windt et al., 2006; Zhou et al., 2006; Gomez-Bolivar et al., 2019a; Gomez-Bolivar et al., 2019b). According to De Windt et al. (2006), and Hou et al. (2017), a lower Pd/CDW ratio gives a higher biological conversion of Pd+2 into NPs. This results in small and uniformly distributed NPs throughout all the cells. On the contrary, a high ratio gives large NPs due to the abrupt death of cells because of the high Pd+2 concentration, which is toxic, compared to the CDW (De Windt et al., 2006). Hence, a low ratio is desired when bio-Pd NPs are produced (De Windt et al., 2006; Hou et al., 2017).
Dehalogenation and hydrogenation by the catalytic activity of bio-Pd NPs occurs mainly through the addition of H2(g) as an electron donor. This can be optimised by changing the activation method (De Windt et al., 2006; Hennebel et al., 2009a; Hennebel et al., 2009b; Hennebel et al., 2010). Activation agents such as potassium hydroxide (KOH) and sodium hydroxide (NaOH) are often used as bioinorganic catalysts, due to their reaction ability with inert carbon materials that create desired pores in the carbon precursor (Świątkowski, 1999; Linares-Solano et al., 2012). Optimised catalytic activity of bio-Pd NPs is established by applying KOH as an activation agent. This method is altering the shape of microorganisms and Pd NPs, as the composition of the Pd NPs, by the thermal treatment of KOH. This thermal treatment carbonises the cells and starts the production of porous structures by guiding the reaction between KOH and the carbon (Świątkowski, 1999; Xiong et al., 2018). Two reaction mechanisms contribute to the formation of the large porous structure: (1) decomposition of functional groups of microorganisms resulting in a high porosity and surface area combined with the release of some volatiles [e.g. H2O(liq), CO2(g) and CO(g)]. Furthermore, (2) the release of H2O(liq) and CO2(g) is contributing as well, through the physical activation of CO2(g) and steam. The dispersed Pd NPs are spread throughout the carbon hybrid material. This stabilises the Pd NPs and facilitates the diffusion of the target compounds and electron donors to the catalyst’s active sites. The elevated temperature also influences the Pd NPs by increasing the crystallinity of the NPs which showed a substantial effect on the catalytic performance. The advantages of this method are (1) an increase in dispersion of small NPs on the carrier, (2) having more binding sites and surface anchoring groups, and (3) enhancing the catalytic activity by improving the hydrophilicity of the nanostructures (Xiong et al., 2018). It is also possible to use hydrothermal processes to increase the activity, when producing alloys, such as PdAu alloys which contributes to the heteroatom doping of Pd and Au. To maintain and prevent aggregation from occurring, graphene oxide was used. The benefits of this process are (1) an increase in catalytic activity and (2) higher durability compared to commercial Pd/C NPs (Liu et al., 2016).
3.4. The Effect of Bio-Pd NPs on Electrochemical Systems
Fuel cell technologies presentlow carbon energy, high energy-density, zero-emission of CO2(g), and relatively easy production (Aki et al., 2005; Orozco et al., 2010; Quan et al., 2015a; Quan et al., 2015b). Two types of cell technologies are commonly used, hence electrochemical cells:(1) MFC and (2) Microbial Electrolysis Cell (MEC). Lately, research has been focusing on the use of these cells combined with catalysts due to their higher efficiency. One of the commonly used elements for this is Pd (Saravanakumar et al., 2016; Wang et al., 2019; Tahernia et al., 2020). It has been proven that the use of Pd can enhance the reaction rate, conductivity, and charge transfer, hence the generation of current (Cheng et al., 2017; Wang et al., 2019). The bio-Pd NPs have also been applied in electrochemical cells (Cheng et al., 2017).
The MFC is a bio-electrochemical system that uses microorganisms to transform chemical energy into electricity (Lovley, 2008; Chaturvedi et al., 2016). Only electroactive bacteria can be used through the process, as they serve as biocatalysts, hence generating bio-energy (Kumar et al., 2016; Thapa et al., 2022). These microorganisms transfer electrons, directly (DET) or mediated (MET), to the electrode, by oxidising organic compounds, e.g., glucose, formate, and acetate, which serve as carbon sources (Kumar et al., 2016). By oxidising the organic compounds, microorganisms make electrons available. This is transferred to the anode, and from there to the cathode through a circuit where they reduce the oxidant (Allen et al., 1993; Tahernia et al., 2020). Hence, the efficiency of this transfer from chemical to electricity dramatically depends on the anode. The anode is the primary location where the microorganisms attach (Watanabe et al., 2008; Quan et al., 2015a; Quan et al., 2005b). Therefore, a variety of carbon-based materials enhance bacterial attachment and hence the transfer of electrons (Quan et al., 2015a; Quan et al., 2005b). Recently, attention is gained to the use of bio-Pd NPs to cover the anode. This increases the performance of the MFC, by enhancing the generation of current. The combination of bio-Pd NPs with the anode establishes the use of the MFC for electro-oxidation, here, Pd present in the anode is electrocatalytic active (Saravanakumar et al., 2016). The bio-Pd NPs serve as an electroactive catalyst that enables the degradation of organic compounds which increases the number of electrons (Quan et al., 2015a; Quan et al., 2005b).
This improves the transfer of electrons and reduction in resistance of charge transfer in the electrochemical systems (Figure 4). It was found that a higher loading of bio-Pd NPs could increase the Coulombic efficiency from 14% (1 mg bio-Pd/cm2) to 31% (2 mg bio-Pd/cm2) compared to the anode without bio-Pd NPs (Quan et al., 2015a). Matsena et al. (2020), also found an increase in bio-energy generation from 31.1% to 59.6%, with 2 to 4 mg bio-Pd/cm2, respectively. This was compared to the electrode without bio-Pd NPs (Matsena et al., 2020).
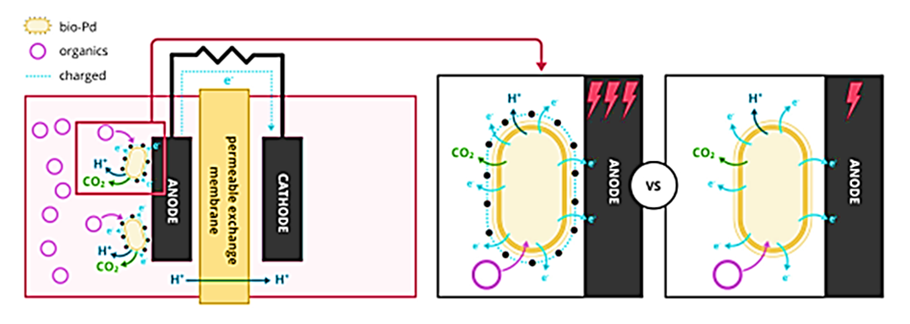
Figure 4: Coating anode with bio-Pd in the microbial electrochemical fuel cell (MFC) system to increase the generation of energy. The figure was adapted from Hou et al. (2017).
Besides, the loading of bio-Pd NPs on the electrode is the type of organic compound also essential for improving the generation of bio-energy. Quan et al. (2015a), and Matsena et al. (2020) reported the inadequate use of glucose to improve bio-energy generation. This is due to the absence of direct electro-oxidation by bio-Pd NPs and hindered utilisation from the microorganisms to generate power (Wu et al., 2011; Quan et al., 2015a; Wu et al., 2018; Matsena et al., 2020; Tahernia et al., 2020). Hence, it is important to use a suitable organic compound for the electro- and microbial oxidation. The advantage of having bio-Pd NPs in the electrochemical systems are (1) the presence of microorganisms to oxidise the organic compounds and (2) bio-Pd NPs to catalyse the oxidation of the organic compounds further which improves the energy generation (Yong et al., 2002; Quan et al., 2015a; Quan et al., 2005b; Matsena et al., 2020).
While energy is produced in MFC, MEC partially reverses the process using the energy for the production of H2(g) or degradation of contaminants. The H2(g) is considered an alternative to fossil fuels (Shafiee and Topal, 2009). The advantage of this system is that there is no emission of CO2(g), clean end-products, and has high-energy-density (Orozco et al., 2010; Abdalla et al., 2018). The MEC principles are based on the hydrogen evolution reaction (HER) which is a cathodic reaction that electrochemically split water in the cathode (Seh et al., 2017). The HER process is a combination of hydrogen adsorption and desorption from the electrode surface where an optimal level of bonding strength between catalytic surface and hydrogen atoms needs to be achieved aiming not to favour one step over the other. If the bonding between hydrogen and the catalytic surface is weak, adsorption is inadequate, and the overall efficiency would suffer. Besides, if the bonding between hydrogen and the electrocatalytic surface is too strong it is not desorbed from the surface effectively, lowering overall efficiency (Safizadeh et al., 2015). To increase this reaction, a catalyst can be used, e.g. platinum and Pd, by coating the metal onto an electrode (Yuan and He, 2017). The Pd is often used because of having the best properties in hydrogen-storage, under suitable conditions Pd adsorbs more than 900 times its volume of hydrogen (Dekura et al., 2019; Wang et al., 2019; Hou et al., 2020). It also plays an important role in the redox reaction. The use of bio-Pd accelerates the reaction as it serves as a catalyst which increases the HER and, consequently, removes contaminants. This is due to the higher concentration of electron donors which can be used for catalytic activity (Hou et al., 2016; Hou et al., 2017). However, the characteristics of the bio-Pd NPs to achieve a high catalytic activity are different for electrochemical systems and in suspension (De Windt et al., 2006; Hou et al., 2017). In electrochemical systems, increased conductivity is obtained by interconnecting all bio-Pd NPs, and hence, smaller NPs in size are not crucial. This results in the activation of every particle in the electrochemical system and thus an enhanced catalytic activity. Therefore, the electrochemical active surface area (ECSA) is an important parameter in conductivity and is directly influencing the catalytic performance (Hou et al., 2017). However, the size does play an important role in the catalytic activity of bio-Pd NPs in suspension (De Windt et al., 2006). The highest catalytic activity is obtained when small NPs are present. This is due to the ratio in size of NPs versus uncovered areas of the cell with Pd (Hou et al., 2017). Hou et al. (2017), observed the difference in catalytic activity between bio-Pd in electrochemical systems and suspensions. The bio-Pd NPs with Pd/CDW ratio=6/1 showed the biggest NPs of 54.3±16.4 nm, while 25.8 ±7.8 nm particles were obtained for the 3/1 ratio. As indicated by De Windt et al. (2006), the ratio of Pd/CDW determines the size, extracellular distribution, and coverage of the Pd NPs in the cell. Nevertheless, the highest current densities were obtained due to the high ECSA, and hence, favourable catalytic activity in the electrochemical system was reached with the NPs produced with Pd/CDW ratio=6/1 (De Windt et al., 2006; Hou et al., 2017). However, the highest catalytic activity of bio-Pd in suspension was obtained for Pd/CDW ratio=3/1. Hence, high coverage of Pd NPs on cells can negatively impact the catalytic activity depending on the use (Hou et al., 2017).
3.5. Bio-Pd NPs Characteristics
3.5.1. The Results of Transmission Electron Microscopy (TEM) Analysis
The TEM images of bio-Pd NPs at the Shewanella oneidensis MR-1 cell surface was observed in micromorphological structure level in bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters (Figure 5).
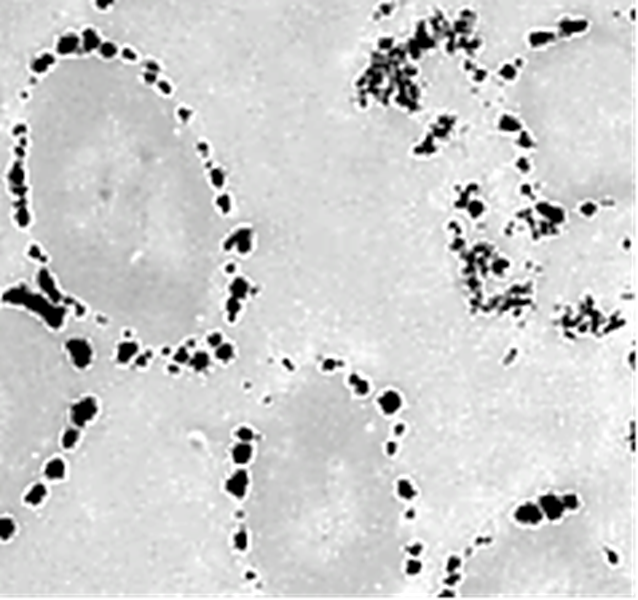
Figure 5: TEM images of bio-Pd NPs at the Shewanella oneidensis MR-1 cell surface in micromorphological structure level in bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters.
3.5.2. The Results of Field Emission Scanning Electron Microscopy (FESEM) Analysis
The morphological features of pure g-C3N4 NPs, pure CeO2 NPs and g-C3N4/CeO2 NCs were characterized through FE-SEM images (Figure 6). The FESEM images of untreated pure Shewanella oneidensis MR-1 bacteria was shown in pharmaceutical industry wastewater (Figure 6a). The FESEM images of bio-Pd NPs loaded with Shewanella oneidensis MR-1 cell surface were observed in bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX micropollutants in pharmaceutical industry wastewaters (Figure 6b). The FESEM images of bio-Pd NPs loaded with Shewanella oneidensis MR-1 cell surface were observed in bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of DOX micropollutants in pharmaceutical industry wastewaters (Figure 6c).
 |  |
| (a) | (b) |
 | |
| (c) | |
Figure 6: FESEM images of (a) untreated pure Shewanella oneidensis MR-1 bacteria, (b) after bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX micropollutants and (c) after bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of DOX micropollutants in pharmaceutical industry wastewaters
3.5.3. The Results of Energy Dispersive X-Ray (EDX) Spectroscopy Analysis
The EDX analysis of bio-Pd NPs was also performed to investigate in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters (Figure 7).

Figure 7: EDX spectrum of bio-Pd NPs in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters
The Results of X-Ray Diffraction (XRD) Analysis and X-Ray Photoelectron Spectroscopy (XPS) Analysis
The results of XRD analysis was observed to bio-Pd NPs, in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters (Figure 8a). The characterization peaks were found at 2θ values of 39.25o, 46.10o, 68.17o, 80.07o and 84.46o, respectively, and which can also be indexed as (111), (200), (220), (311) and (222), respectively, implying g-C3N4/CeO2 NCs in pharmaceutical industry wastewater with photocatalytic degradation process for OFX antibiotic removal (Figure 8a). The XPS analysis of bio-Pd NPs was also performed to investigate in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters (Figure 8b).
 |
| (a) |
 |
| (b) |
Figure 8: (a) XRD spectra of bio-Pd NPs and (b) XPS spectra of bio-Pd NPs in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
The ICP-MS analysis of bio-Pd NPs was also performed to investigate in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters (Figure 9). After 70 min bio-in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria was shown in Figure 9a. The main peaks of ICP-MS spectrum for bio-Pd NPs NPs was observed at 696.28 1/cm, 1047.32 1/cm, 1095.34 1/cm, 1651.02 1/cm, 3014.66 1/cm and 3357.95 1/cm wavenumber, respectively (Figure
 |
| (a) |
 |
| (b) |
Figure 9: ICP-MS spectra of (a) after 70 min bio-in the bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria, and (b) for the catalytic removal of OFX and DOX micropollutants in pharmaceutical industry wastewaters
3.5.6. The Results of Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The FTIR spectrum of OFX and DOX micropollutans was obtained after catalytic removals with Bio-Pd NPs in Shewanella oneidensis MR-1, respectively, in pharmaceutical industry wastewater (Figure 10). The main peaks of FTIR spectrum for OFX catalytic removal with Bio-Pd NPs in Shewanella oneidensis MR-1 (red spectrum) was observed at 3800 1/cm, 3150 1/cm, 2880 1/cm, 2250 1/cm, 1800 1/cm, 1500 1/cm, 1400 1/cm, 1320 1/cm, 1170 1/cm, 1000 1/cm, 980 1/cm, 970 1/cm, 820 1/cm, 750 1/cm and 500 1/cm wavenumber, respectively (Figure 10a). The main peaks of FTIR spectrum for DOX catalytic removal with Bio-Pd NPs in Shewanella oneidensis MR-1 (blue spectrum) was obtained at 3750 1/cm, 3100 1/cm, 2800 1/cm, 2740 1/cm, 2400 1/cm, 1750 1/cm, 1580 1/cm, 1500 1/cm, 1360 1/cm, 1290 1/cm, 1000 1/cm and 750 1/cm wavenumber, respectively (Figure 10b).

Figure 10: FTIR spectrum of (a) OFX catalytic removal with Bio-Pd NPs in Shewanella oneidensis MR-1 (red spectrum), (b) DOX catalytic removal with Bio-Pd NPs in Shewanella oneidensis MR-1 (blue spectrum), respectively, in pharmaceutical industry wastewater.
The Results of Diffuse reflectance UV-Vis Spectra (DRS) Analysis
The DRS spectrum of pure Bio-Pd NPs and bio-Pd NPs with Bio-Pd NPs in Shewanella oneidensis MR-1 cells was observed after catalytic removals of OFX and DOX micropollutants in pharmaceutical industry wastewater (Figure 11). First, the DRS spectra of pure Bio-Pd NPswere obtained in the wavelength range from 250 nm to 500 nm using diffuse reflectance UV-Vis spectra (Figure 11a). Absorption peaks were observed at wavelengths of 310 nm for pure bio-Pd NPs (red pattern) (Figure 11a), 300 nm for bio-Pd NPs in Shewanella oneidensis MR-1 cells (blue pattern) (Figure 11b), respectively, after catalytic removals of OFX and DOX micropollutants in pharmaceutical industry wastewater
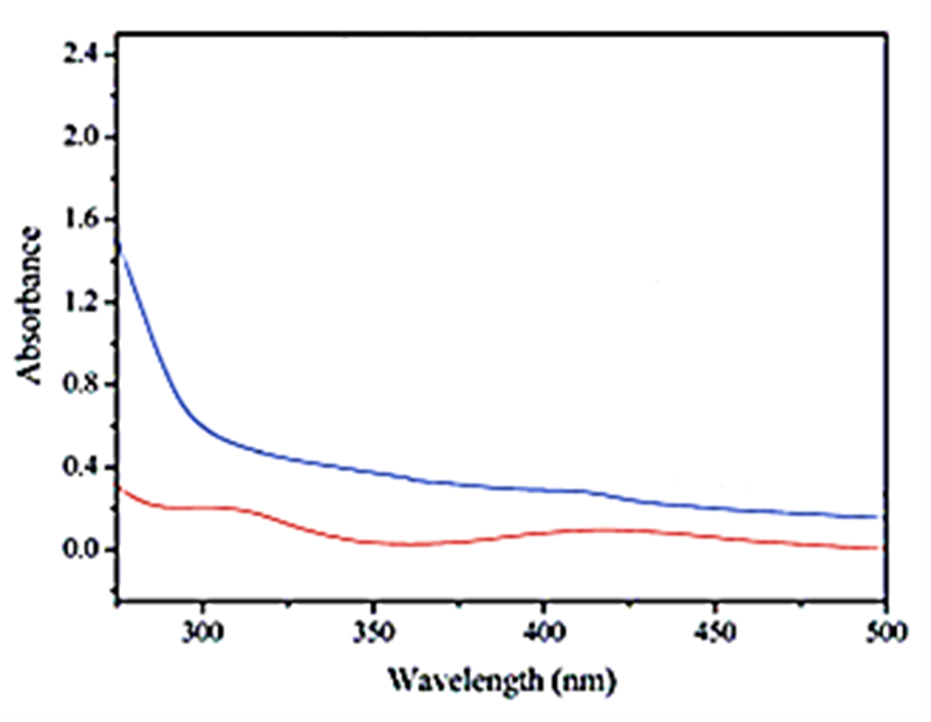
Figure 11: The DRS spectrum of (a) pure Bio-Pd NPs (red pattern) (b)bio-Pd NPs with Bio-Pd NPs in Shewanella oneidensis MR-1 cells (blue pattern), respectively, catalytic removals of OFX and DOX micropollutants in pharmaceutical industry wastewater.
The Reaction Kinetics of OFX Antibiotic
The reaction kinetics OFX were investigated using the Langmuir–Hinshelwood first-order kinetic model, expressed by Eddy et al. (2015), as following Equation (1):
 (1)
(1)
where; ro: denotes the initial catalytic degradation reaction rate (mg/l.min), and k: denotes the rate constant of a first-order reaction. At the beginning of the reaction, t = 0, Ct = C0, the equation can be obtained after integration as following Equation (2):
 (2)
(2)
where; C0 and C : are the initial and final concentration (mg/l) of OFX; the solution at t (min) and k (1/min) are the rate constant.
The correlation coefficients had R2 values greater than 0.9, as a result, the first-order kinetic model fit the experimental data well. The first-order rate constants (k) were determined from the slope of the linear plots.
3.7. The Reaction Kinetics of DOX Antibiotic
DOX micropollutants removal using catalytic degradation can be described using a pseudo first-order reaction kinetic model (Equation 3) (Al-Hamadani et al., 2016; Villegas-Guzman et al., 2017; Monteagudo et al., 2018). However, a complete linear relationship was not observed for a first-order kinetic law and pollutants elimination could not be characterized by a single rate constant.
 (3)
(3)
where; r: is the initial degradation rate (μmol/l.min), k0: is the pseudo-rate constant (1/min) and C: is the pollutant initial concentration (μmol/l).
A Langmuir type mechanism model indicates that organic molecules adsorb and desorb from the liquid interface layer surrounding of the cavitation bubble, reaching a pseudo-steady state, and the degradation rate (r) can be represented by Equation 4 (Okitsu et al., 2005):
 (4)
(4)
where; r: is the initial degradation rate (μmol/l.min), k0: is the pseudo-rate constant (μmol/l.min), C: is the pollutant initial concentration (μmol/l) and K: is the equilibrium constant of the target compound at the interfacial region (l/μmol). [
Thus, the degradation rate is the sum of the rates in the bulk and in the interface layer and can be expressed by the Equation 5 (Serpone et al., 1994).
 (5)
(5)
where; kb: is a constant representing the rate of decomposition in the bulk liquid (μmol/l.min), r: is the initial degradation rate (μmol/l.min), k0: is the pseudo-rate constant (μmol/l.min), C: is the pollutant initial concentration (μmol/l) and K: is the equilibrium constant (l/μmol) (Serpone et al., 1994).
Data indicates that the experimental results fit better ( > 0.98 higher coefficient of determination R2) with the model proposed by Serpone et al. (1994) in comparison with the Okitsu et al. (2005) model and the pseudo first-order kinetics model.
3.8 Effect of Increasing pH values for OFX and DOX Catalytic Removals with Bio-electrochemical Cell Assisted Production of Bio-Pd NPs with Shewanella oneidensis MR-1 Bacteria in Pharmaceutical Industry Wastewater
Increasing pH values (pH=3.0, pH=4.0, pH=6.0, pH=7.0, pH=9.0 and pH=11.0, respectively) was examined during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater for OFX catalytic removal (Figure 12). 66%, 84%, 95%, 55% and 43% OFX removal efficiencies was measured at pH=3.0, pH=4.0, pH=7.0, pH=9.0 and pH=11.0, respectively, after 24 h catalytic degradation time, at 25oC (Figure 12). The maximum 99% OFX removal efficiency was obtained during photocatalytic degradation process in pharmaceutical industry wastewater, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 12).
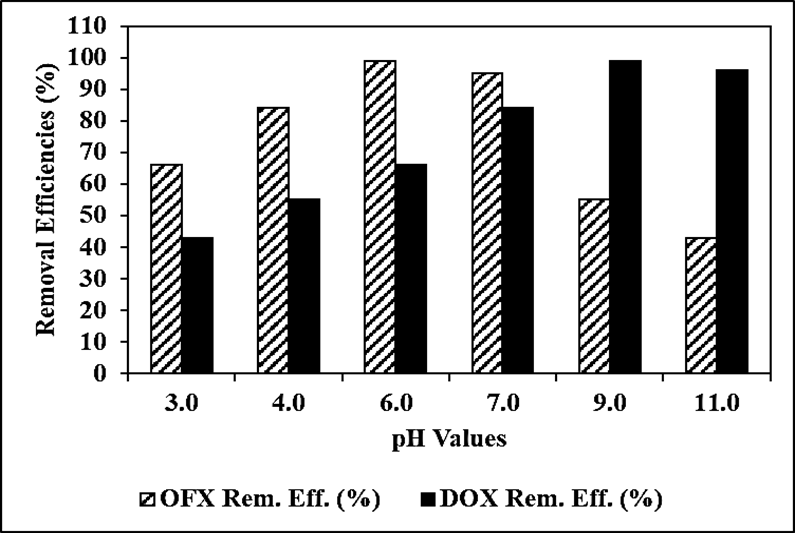
Figure 12: Effect of increasing pH values for OFX and DOX catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater after 24 h catalytic degradation time, at 25oC, respectively.
Increasing pH values (pH=3.0, pH=4.0, pH=6.0, pH=7.0, pH=9.0 and pH=11.0, respectively) was examined during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater for DOX removal (Figure 12). 43%, 55%, 66%, 84% and 96% DOX removal efficiencies was measured at pH=3.0, pH=4.0, pH=6.0, pH=7.0 and pH=11.0, respectively, after 24 h catalytic degradation time, at 25oC (Figure 12). The maximum 99% DOX removal efficiency was obtained during photocatalytic degradation process in pharmaceutical industry wastewater, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 12).
3.9. Effect of Increasing OFX and DOX Concentrations for OFX and DOX Catalytic Removals with Bio-electrochemical Cell Assisted Production of Bio-Pd NPs with Shewanella oneidensis MR-1 Bacteria in Pharmaceutical Industry Wastewater
Increasing OFX concentrations (5 mg/l, 15 mg/l, 30 mg/l and 45 mg/l) were operated during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at pH=6.0, after 24 h catalytic degradation time, at 25oC, respectively (Figure 13). 84%, 93% and 75% OFX removal efficiencies were obtained to 5 mg/l, 15 mg/l and 45 mg/l OFX concentrations, respectively, after 24 h catalytic degradation time, at pH=6.0 and at 25oC (Figure 13). The maximum 99% OFX removal efficieny was found with catalytic degradation process in pharmaceutical industry wastewater, at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 13).
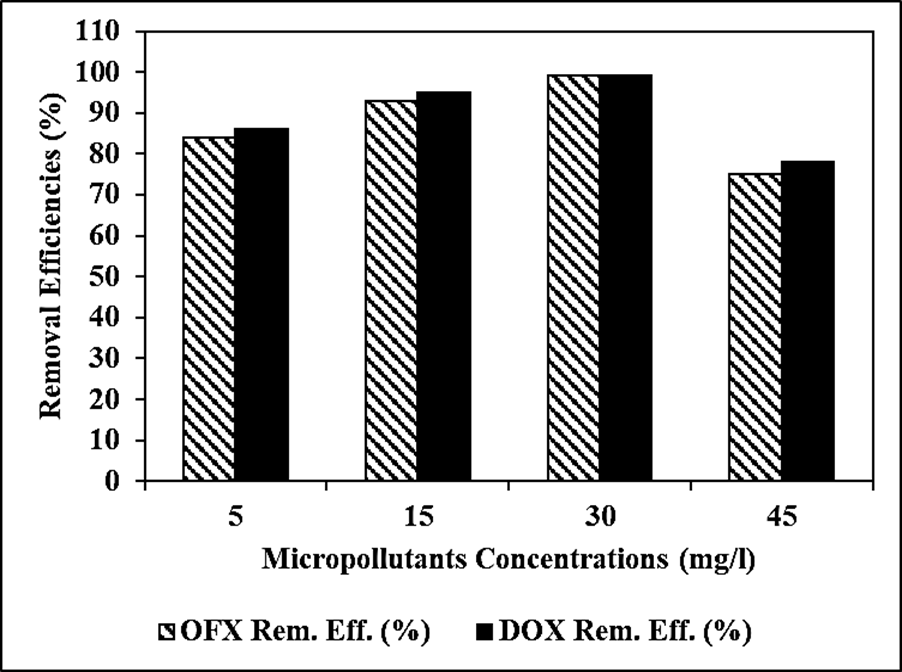
Figure 13: Effect of increasing OFX and DOX concentrations for OFX and DOX catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater after 24 h catalytic degradation time, at 25oC, respectively.
Increasing DOX concentrations (5 mg/l, 15 mg/l, 30 mg/l and 45 mg/l) were operated during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at pH=9.0, after 24 h catalytic degradation time, at 25oC, respectively (Figure 13). 86%, 95% and 78% DOX removal efficiencies were obtained to 5 mg/l, 15 mg/l and 45 mg/l DOX concentrations, respectively, at pH=9.0 and at 25oC (Figure 13). The maximum 99% DOX removal efficieny was found with catalytic degradation process in pharmaceutical industry wastewater, at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 13).
Effect of Increasing Bio-Pd NPs Concentrations for OFX and DOX Catalytic Removals with Bio-electrochemical Cell Assisted Production of Bio-Pd NPs with Shewanella oneidensis MR-1 Bacteria in Pharmaceutical Industry Wastewater
Increasing Bio-Pd NPs concentrations (5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l, 40 mg/l and 60 mg/l) were operated during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0, at 25oC, respectively (Figure 14). 53%, 67%, 74%, 86% and 91% OFX removal efficiencies were obtained to 5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l and 60 mg/l Bio-Pd NPs concentrations, respectively, at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0, at 25oC, respectively (Figure 14). The maximum 99% OFX removal efficieny was measured to 40 mg/l Bio-Pd NPs with catalytic degradation process in pharmaceutical industry wastewater, at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 14).
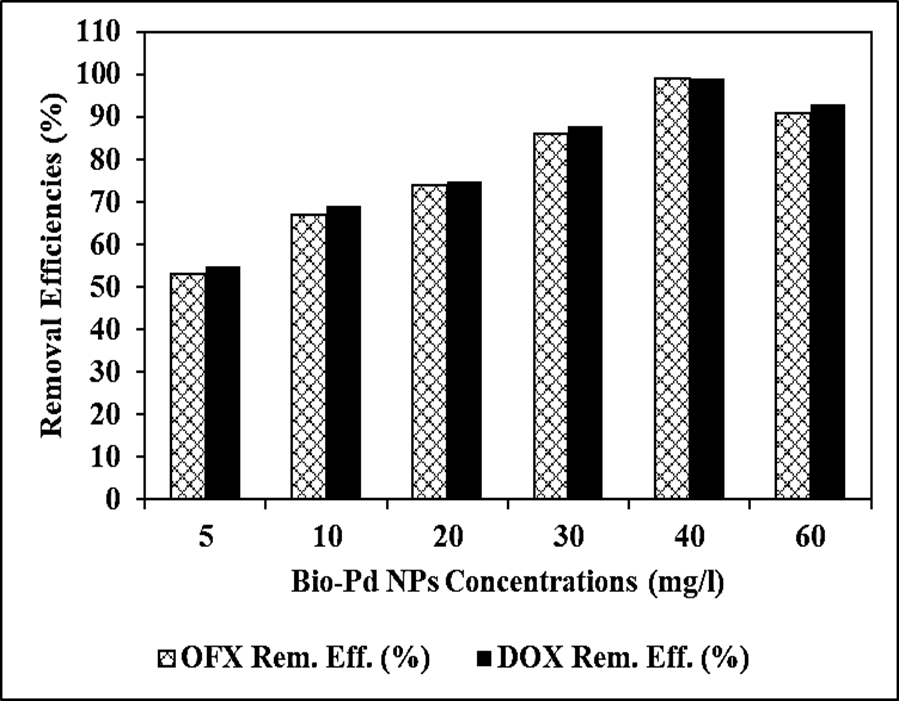
Figure 14: Effect of increasing Bio-Pd NPs concentrations for OFX and DOX catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater after 24 h catalytic degradation time, at 25oC, respectively.
Increasing Bio-Pd NPs concentrations (5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l, 40 mg/l and 60 mg/l) were operated during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0, at 25oC, respectively (Figure 14). 55%, 69%, 75%, 88% and 93% DOX removal efficiencies were obtained to 5 mg/l, 10 mg/l, 20 mg/l, 30 mg/l and 60 mg/l Bio-Pd NPs concentrations, respectively, at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0, at 25oC, respectively (Figure 14). The maximum 99% DOX removal efficieny was measured to 40 mg/l Bio-Pd NPs with catalytic degradation process in pharmaceutical industry wastewater, at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 14).
Effect of Different Bio-Pd NPs/Cell Dry Weight (CDW) Mass Ratios for OFX and DOX Catalytic Removals with Bio-electrochemical Cell Assisted Production of Bio-Pd NPs with Shewanella oneidensis MR-1 Bacteria in Pharmaceutical Industry Wastewater
Different Bio-Pd NPs/CDW mass ratios (5/5wt, 6/4wt, 7/3wt, 8/2wt, 9/1wt, 1/9wt, 2/8wt, 3/7wt and 4/6wt, respectively) were examined during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l OFX, at 40 mg/l Bio-Pd NPs, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 15). 79%, 83%, 76%, 61%, 47%, 54%, 63% and 77% OFX removal efficiencies were measured at 5/5wt, 7/3wt, 8/2wt, 9/1wt, 1/9wt, 2/8 wt, 3/7wt and 4/6wt Bio-Pd NPs/CDW mass ratios, respectively, at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 15). The maximum 99% OFX removal efficiency was measured at 6/4 wt Bio-Pd NPs/CDW mass ratios at 30 mg/l OFX, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 15).
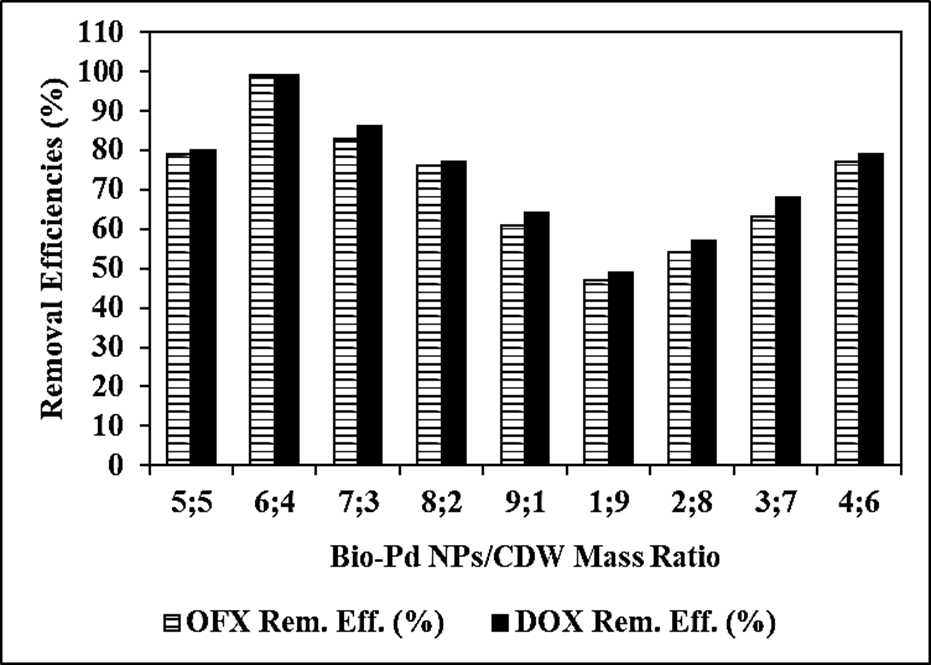
Figure 15: Effect of different Bio-Pd NPs/CDW mass ratios for OFX and DOX catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater after 24 h catalytic degradation time, at 25oC, respectively.
Different Bio-Pd NPs/CDW mass ratios (5/5wt, 6/4wt, 7/3wt, 8/2wt, 9/1wt, 1/9wt, 2/8wt, 3/7wt and 4/6wt, respectively) were examined during catalytic degradation process with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 15). 80%, 86%, 77%, 64%, 49%, 57%, 68% and 79% DOX removal efficiencies were measured at 5/5wt, 7/3wt, 8/2wt, 9/1wt, 1/9wt, 2/8wt, 3/7wt and 4/6wt Bio-Pd NPs/CDW mass ratios, respectively, at 30 mg/l DOX after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 15). The maximum 99% DOX removal efficiency was measured at 6/4wt Bio-Pd NPs/CDW mass ratios at 30 mg/l DOX, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 15).
Effect of Different Recycle Times for OFX and DOX Catalytic Removals with Bio-electrochemical Cell Assisted Production of Bio-Pd NPs with Shewanella oneidensis MR-1 Bacteria in Pharmaceutical Industry Wastewater
Different recycle times (1., 2., 3., 4., 5., 6. and 7.) were operated for OFX catalytic removal with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l OFX, 40 mg/l Bio-Pd NPs, at 6/4 wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at 25oC, respectively (Figure 16). 90%, 87%, 84%, 81%, 78%, 76% and 72% OFX removal efficiencies were measured after 2. recycle time, 3. recycle time, 4. recycle time, 5. recycle time, 6. recycle time and 7. recycle time, respectively, at 30 mg/l OFX, 40 mg/l Bio-Pd NPs, at 6/4wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 16). The maximum 99% OFX removal efficiency was measured in pharmaceutical industry wastewater during catalytic degradation process, after 1. recycle time, at 30 mg/l OFX, 40 mg/l Bio-Pd NPs, at 6/4wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at pH=6.0 and at 25oC, respectively (Figure 16).
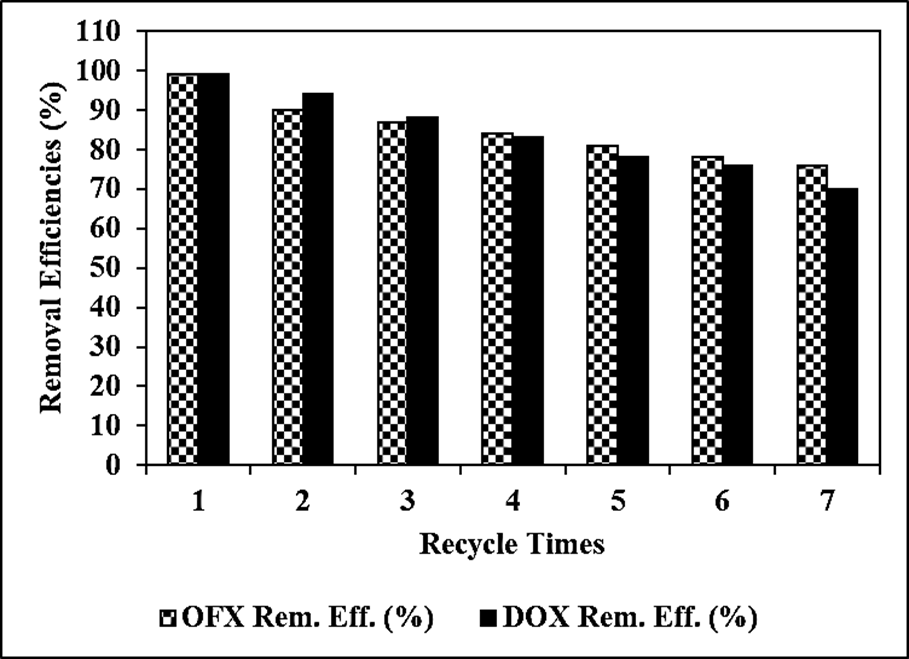
Figure 16: Effect of recycle times for OFX and DOX catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater after 24 h catalytic degradation time, at 25oC, respectively.
Different recycle times (1., 2., 3., 4., 5., 6. and 7.) were operated for DOX catalytic removal with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l DOX, 40 mg/l Bio-Pd NPs, at 6/4wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 16). 94%, 88%, 83%, 78%, 76% and 70% DOX removal efficiencies were measured after 2. recycle time, 3. recycle time, 4. recycle time, 5. recycle time, 6. recycle time and 7. recycle time, respectively, at 30 mg/l DOX, 40 mg/l Bio-Pd NPs, at 6/4wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 16). The maximum 99% DOX removal efficiency was measured in pharmaceutical industry wastewater during catalytic degradation process, after 1. recycle time, at 30 mg/l DOX, 40 mg/l Bio-Pd NPs, at 6/4wt Bio-Pd NPs/CDW mass ratio, after 24 h catalytic degradation time, at pH=9.0 and at 25oC, respectively (Figure 16).
The Comparison with other Scientific Studies in the Literature
Comparison of our study “Bio-electrochemical cell assisted production of biogenic palladium nanoparticles with Shewanella oneidensis MR-1 bacteria for the catalytic removal of ofloxacin (OFX) and doxycycline (DOX) micropollutants in pharmaceutical industry wastewaters” with other scientific studies in the literature is summaried at Table 2.
| Micropollutants | Experimental Conditions and Results | References |
| Diclofenac | Continuous removal in MEC systems, with 57% lower removal efficiency | (De Gusseme et al., 2012) |
| Ciprofloxacin | According to Martins et al. (2017) was the removal negligible as 35 and 49% of ciprofloxacin was removed by active- and non-active catalyst, respectively | (He et al., 2020) |
| Sulfamethoxazole | 85% Removal was accomplished after 24 h, with a removal rate of 0.110 1/h | (Martins et al., 2017) |
| Ibuprofen | No removal of the painkiller by bio-Pd produced by D. vulgaris; however, it was removed by the bacterium | (Martins et al., 2017) |
| 17β-estradiol | Low removal of the compound, 35 and 11%, by catalytic activity and adsorption, respectively | (Martins et al., 2017) |
| İbuprofen, sulfamethoxazole, naproxen, furosemide, citalopram, diclofenac, atorvastatin, lorazepam | ibuprofen (69.5%) < sulfamethoxazole> 94.3%) < lorazepam> | (Law et al., 2023) |
| ciprofloxacin | the reductive removal of ciprofloxacin with Bio-Pd NPs catalyst, 87.70% ciprofloxacin removal at 25oC | He et al., 2020 |
| Ofloxacin (OFX) and Doxycycline (DOX) | Bio-electrochemical cell assisted production of biogenic palladium nanoparticles with Shewanella oneidensis MR-1 bacteria for catalytic removal in pharmaceutical industry wastewater 99% OFX removal efficiency, at pH=6.0, after 24 h catalytic degradation time, at 30 mg/l OFX, 40 mg/l Bio-Pd NPs, Bio-Pd NPs/CDW mass ratio=6/4, at 25oC. 99% DOX removal efficiency, at pH=9.0, after 24 h catalytic degradation time, 30 mg/l DOX, 40 mg/l Bio-Pd NPs, Bio-Pd NPs/CDW mass ratio=6/4, at 25oC. | This study |
Table 2: The Comparison with other Scientific Studies in the Literature
The maximum 99% OFX removal efficiency was obtained catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l OFX concentration, 40 mg/l Bio-Pd NPs concentration, Bio-Pd NPs/CDW mass ratio=6/4, after 24 h catalytic degradation time, at pH=6.0, at 25oC, respectively.
The maximum 99% DOX removal efficiency was observed catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a bio-electrochemical cell catalyts in pharmaceutical industry wastewater, at 30 mg/l DOX concentration, 40 mg/l Bio-Pd NPs concentration, Bio-Pd NPs/CDW mass ratio=6/4, after 24 h catalytic degradation time, at pH=9.0, at 25oC, respectively.
As a result, OFX and DOX micropollutants catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalysts in pharmaceutical industry wastewater was stable in harsh environments such as acidic, alkaline, saline, and then was still effective process. When the amount of micropollutants was increased, the Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts during catalytic degradation process performance was still considerable. The synthesis and optimization of Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria heterostructure bio-electrochemical cell catalysts provides insights into the effects of preparation conditions on the material’s characteristics and performance, as well as the application of the effectively designed bio-electrochemical cell catalyst in the removal of antibiotics, which can potentially be deployed for purifying wastewater, especially pharmaceutical industry wastewater.
In hhis study provides strong support for the use of Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts with to efficiently remove micropollutants (OFX and DOX) from the environment and the possible applicability to wastewater treatment plants as a tertiary treatment system. In addition to, before applying Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts in real wastewater treatment systems, more investigation is required. H2(g) can be submitted by implementing a bio-electrochemical cell system into the treatment plant or more research can be performed on finding an alternative electron donor for the reductive dehalogenation of halogenated micropollutants by the catalytic activity of the Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts. It is also important to recover Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts for economical feasibility. The recovery of Pd+2 ions from the supernatant solution, after production of Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts, can be achieved by the addition of Shewanella oneidensis MR-1 to the solution, as the matrices of the cells and the supernatants are the same. It is also possible to recover leaching Pd NPs from the bio-Pd NPs, by chemical reaction, such as by the addition of nitric acid.
The catalytic removals with bio-electrochemical cell assisted production of bio-Pd NPs with Shewanella oneidensis MR-1 bacteria as a heterostructure bio-electrochemical cell catalysts method can also be applied to other waste metal ions to prepare the biogenic metals, facilitate their recovery and reuse in degrading micropollutants in pharmaceutical industry wastewater. Finally, the combination of a simple, easy operation preparation process, excellent performance and cost effective, makes this Bio-Pd NPs with Shewanella oneidensis MR-1 bacteria bio-electrochemical cell catalyts a promising option during catalytic degradation process in pharmaceutical industry wastewater treatment.
This research study was undertaken in the Environmental Microbiology Laboratories at Dokuz Eylül University Engineering Faculty Environmental Engineering Department, Izmir, Turkey. The authors would like to thank this body for providing financial support.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.
