AUCTORES
Globalize your Research
Research article
*Corresponding Author: Shikha Dhawan, Society for Health Allied Research and Education (SHARE) INDIA, Telangana, India.
Citation: Manjula Singh, Pankaj Ghatbandhe, Gaurav Mehta, Farida Khatun, Seevan Asad, et al., (2023), Baseline Assessments of TB Detection Centres in Selected Public and Private Laboratories in India and Need for Introducing Quality Management Systems, Clinical Research and Clinical Trials. 7(2) ; DOI:10.31579/2693-4779/122
Copyright: 2022 Shikha Dhawan, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 February 2023 | Accepted: 13 February 2023 | Published: 23 February 2023
Keywords: quality management systems; national tuberculosis elimination program; TB detection centres; designated microscopy centres; ISO 15189
Quality is sine qua non of healthcare services. Laboratory driven Quality Management Systems (QMS) are key to provide quality diagnosis. Missed diagnosis due to laboratory lacunae stymies Tuberculosis (TB) control measures, amplifies morbidity, mortality and, disease transmission. Hence, a pilot was conceptualized with Central TB Division, National TB Elimination Program (NTEP), Ministry of Health and Family Welfare, Government of India and Indian Council of Medical Research (ICMR) to perform baseline assessments for QMS at four laboratories from public and private centers at Delhi and Odisha. Data was collected using a standardized checklist. Scores were compiled for compliance to quality standards for attributes assessed across seven thematic domains. The assessment helped identify current status in terms of performance, processes followed and, areas requiring improvement. TB laboratories were found lacking in human resources and, backup staff that disrupted service delivery. Other gaps included quality aspects in documents, records, process controls in smear microscopy, optimum resource utilization and laboratory safety. Adage for missed TB diagnosis included laboratory errors, failure to document, collect and, test repeat samples for Nucleic Acid Amplification Tests (NAAT), lack of patient centric laboratory signages, equipment handling and, maintenance. Baseline assessments of quality at TB laboratories mainly focused attention on areas of greatest need to ameliorate the health system challenges and, develop roadmaps to accelerate improvement. By adapting to local context, QMS can be enacted through a shared vision in quality care to positively impact TB case finding and, optimize utilization of NAAT for diagnosis thereby setting stage for nationwide adoption and, scaleup.
TB: Tuberculosis
NTEP: National Tuberculosis Elimination Program
ICMR: Indian Council of Medical Research
QMS: Quality Management System
IRL: Intermediate Reference Laboratory
NRL: National Reference laboratory
DMC: Designated Microscopy Centre
NAAT: Nucleic Acid Amplification Test
UDSCT: Universal Drug Susceptibility Testing
EQA: External Quality Assurance
ISO: International Organization for Standardization
SOP: Standard Operating Procedure
CQI: Continuous Quality Improvement
PPE: Personal Protective Equipment
Tuberculosis (TB) disease is as old as the history of mankind, still relevant and, devastating. TB diagnostic laboratories are critical to case finding, improving patient and, public health outcomes. Smear microscopy for TB case detection is plagued with poor sensitivity that may miss TB case diagnosis and, does not ascertain drug resistance (Chopra & Singh, 2020). Rapid molecular TB diagnostics like GeneXpert MTB/Rif and Truenat MTB have made inroads in National TB Control Programs across the globe even in peripheral laboratories (Li et al., 2021).A strong laboratory quality management system (QMS) is critical to ensuring the quality of testing and, for better health system outcomes (Centres for Disease Control and Prevention, n.d.). The consequences of low-quality diagnosis and, care is manifold and, include treatment delays, increased drug resistance, morbidity, mortality and, ongoing TB transmission (Li et al., 2021). It also impacts on the links between clinical and, laboratory service delivery points affecting prompt diagnosis and, initiating effective treatment timely. Reducing TB mortality and, incidence is contingent on quality assured coverage of TB services across the entire care continuum (Reid & Goosby, 2020).
India continues to have the highest TB burden in the world and, accounts more than one fourth (27%) of the globally TB reported cases (TB India Report, 2022). India’s National TB Elimination Program (NTEP) has envisaged microbiological confirmation of all TB cases and, upfront rapid molecular diagnostics as part of universal drug susceptibility testing (UDST) supported by a three-tiered laboratory network. The laboratory network includes six apex National Reference Laboratory (NRL), 27 nodal Intermediate Reference Laboratory (IRL), 21820 Designated Microscopy Centres (DMCs) (now TB Detection Centres) or the peripheral laboratories at the bottom-most laboratory tier along with 3760 NAAT (Nucleic Acid Amplification Test) laboratories with Truenat MTB and, GeneXpert MTB deployed at district and sub-district level (TB India Report, 2022). NRLs and IRLs play a critical role in ensuring quality TB diagnostic services throughout the network by onsite supervisions, proficiency testing and, external quality assurance (EQA) (TB India Report, 2022).
Under NTEP, sputum smear microscopy is routinely implemented via the decentralized, peripheral DMCs, often located within primary health centres, community health centres, hospitals, specialty clinics, medical colleges, non-government organizations and, private sector. In addition, to meet the goals of UDST, rapid molecular diagnostics have been deployed at NAAT laboratories. Battery operatable and, portable Truenat MTB meets the criterion of smear replacement technology at peripheral laboratories while GeneXpert are strategically located at District TB Centres, TB units (sub-districts) and, medical colleges (TB India Report, 2022). A decentralized approach using NAATs increases access in primary care settings and, is poised to reduce diagnostic delays with the potential for same-day diagnosis and, treatment initiation. As shown by a recent study, onsite rapid molecular TB testing and streamlined workflow led to 56% increase in treatment initiation (Cattamanchi et al., 2021). NTEP has mandated EQA for sputum smear microscopy to produce accurate, reliable and, reproducible laboratory results and is in the process of expanding EQA for molecular diagnostics (TB India Report, 2022). However, except EQA, comprehensive QMS, being cost intensive, have not been attempted for DMCs and NAAT laboratories. An error in any part of the laboratory testing cycle can produce a poor laboratory service. Quality issues cannot be solved by EQA alone and, mandate the whole repertoire of standards and, controls in resources and documents.
Poor quality sputum microscopy services may result in failure to detect persons with active TB or unnecessary anti-TB treatment for non-TB cases (Parsons et al., 2011). Manual or automated molecular diagnostics can miss TB cases as they are subject to sub-optimum utilization due to inaccuracy associated with lack of operator competence or failure to adhere to standard test procedures or consumable stock-out. There are also other sources of error, such as incorrect reagent storage or expiration and machinery malfunction leading to non-conclusive test results (errors/invalids/indeterminates). Rapid molecular diagnostics are particularly prone to inaccurate pipetting, cross-contamination or the mix-up of samples (Parsons et al., 2011). Errors can also be introduced during sample collection, labeling and, transportation, registration at the laboratory and, delivery of results. Combined, these errors can lead to significant variance in the accuracy of the reported result, potentially leading, in some cases, to incorrect or, delayed diagnosis, inappropriate treatment which predisposes the development of drug resistant or leaves patient shopping for health and, transmitting TB (Li et al., 2021). Laboratory services in peripheral laboratories may suffer many operational challenges including poor infrastructure, limited resources, inadequate human resource capacity, supply chain and, equipment maintenance capacity, staff resistance and, weak underlying health systems (Parsons et al., 2011; Cattamanchi et al., 2021). QMS provides a comprehensive overview of laboratory quality systems and, quality assurance. Quality assured TB diagnostics builds a sanguine and robust health system, that can foster the goals of TB elimination.
We undertook a pilot study of a phased implementation approach starting with baseline assessments to identify gaps in order to introduce and, implement QMS in four NTEP DMCs, two each in high burden states of the country, stratified for both public and, private run facilities. Learnings from the study will not only provide evidence to improve laboratory quality but also to plan focused strategies on quality improvement and, country wide scale up.
Study Design: Laboratory driven quality systems should ensure better TB case finding, diagnosis and, service delivery as aligned with India’s National Strategic Plan. In a pilot mode, we assessed the already existing QMS at public and, private DMCs for smear microscopy and, rapid molecular diagnostics.
Study Site: Four DMCs were selected across two provinces/states. The sites consisted of one public and, one private sector DMC each at Delhi, a metropolitan city located in north-central India and at Odisha, a province in north-eastern India. All the study sites were referred with codes. The sites in Delhi were private DMC-RKM and, public DMC-KCC. The sites in Odisha were public DMC-CHC and, private DMC-KIMS. With regards to structural and, functional profile, all public and, private DMCs selected for the study are integrated with laboratory services, accessibility and, EQA for smear microscopy as per NTEP requirements.
Assessment Tool: A standard checklist was used to assess and, validate NTEP policies and, procedures as aligned to DMCs. The checklist was prepared based on ISO 15189 (International Organization for Standardization (2012); Guidelines for Quality Assurance of smear microscopy for diagnosing tuberculosis (2005); and TB Microscopy Network Accreditation, Global Laboratory Initiative, Stop TB Partnership (2013). The checklist comprised of seven domains embedded in 12 Quality System Essentials for laboratories, World Health Organization (2011) (Table 1).
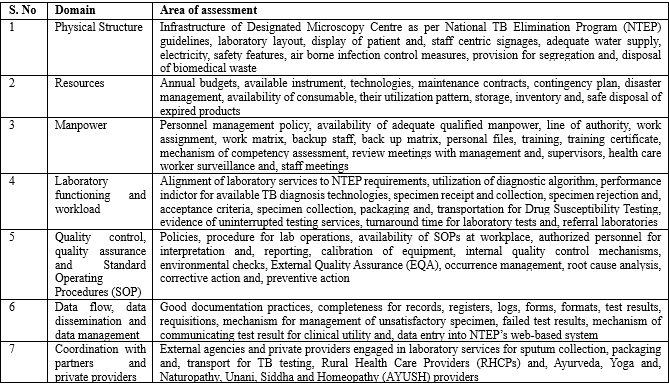
Table 1: Key areas of baseline assessment for Quality Management Systems
Multiple attributes were assigned as questions to each domain to assess different components of QMS. Each question was assigned a score of two (2) for compliance, one (1) for partial compliance and, zero (0) for non-compliance.
Evaluation Process: The baseline assessment was conducted by the project team at four sites from two regions in June 2021. The project team was trained to utilize the checklist by microbiologists from NRL, IRL and, study team comprising of public health experts with prior knowledge of laboratory standards and, quality assessments. On-site, two-day assessments were conducted at each DMC to physically verify, review, validate policies, procedures and, documentation processes. The date of assessments was pre-decided in discussion with the DMCs to ensure the availability of management, staff, and, supervisors. The process of baseline assessment is outlined in Table 2.
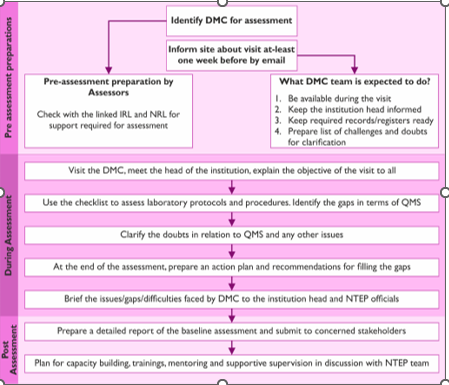
Table 2: Process of baseline assessment at Designated Microscopy Centre (DMC)
Assessment was conducted by direct observation, review of records and, staff interview. To ensure the compliance with NTEP guidelines and, standards, a specimen was followed from collection, registration, work distribution, testing, result verification, trouble shooting of errors, recording, reporting and, communication of test results. The strength of the laboratory’s systems and, operations were determined for smear microscopy and, optimum utilization of rapid molecular diagnostics wherever available.
Data collection and scoring: Based on the assessment checklist and, evidence provided by the DMCs, the data was captured manually in real time. The sites were assessed for a maximum possible score of 100% and were further graded for the level of compliance based on the obtained scores. Above average (75-89%), average (55-74%), below average (30-54%) and, poor (0-29%) provided a one-point reflection of performance which helped the sites to plan improvement. The assessment scores and, emergent issues were summarized to measure the level of compliance to the evaluation checklist. This also provided insights for the level of capacity building required.
Data Analysis: Analysis was done using excel. All the attributes and scores were rechecked and, validated for analysis before importing the data into excel. Data were summarized by graphs, charts and, tables to show laboratory QMS implementation status domain wise.
We used data from laboratory operational processes. No patient information was used; thus, there was no ethics review required for this study.
As quality management principles, with exception of EQA, have never been introduced at DMCs in India, we undertook a pilot study to understand the requirements and, utility of establishing such systems. This was also the need of the hour as India is scaling up rapid molecular diagnostics at districts and, sub-districts that need to be encompassed in quality diagnostics. A total of four TB laboratories were included in the study and, evaluated. The laboratories at Odisha scored “above average” in QMS performance as compared to “average” performance by Delhi DMCs. The benchmark for QMS implementation was found to be at par for both public and private DMCs at Delhi, while the public DMC at Odisha performed better as compared to its private DMC. Delhi site KCC scored 71% (average), RKM scored 72% (average); DMCs at Odisha-CHC scored 84 % (above average) and, KIMS obtained 78% (above average) rating as shown in Figure 1
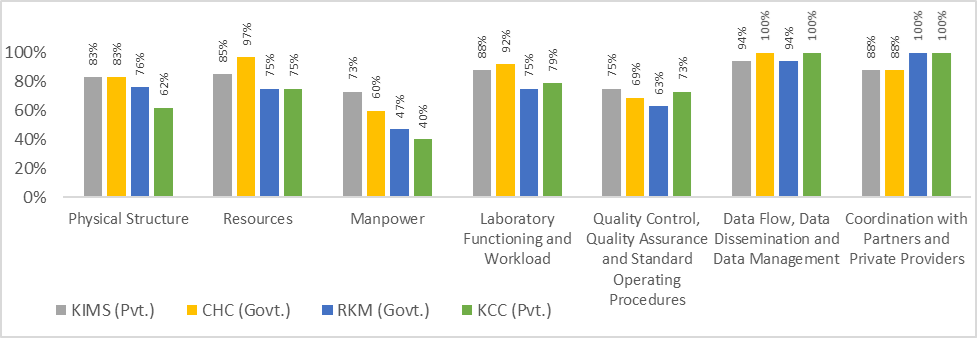
KIMS; CHC; RKM; KCC: Codes for study sites
Pvt.: Private laboratory
Govt.: Government laboratory
Figure 1: Cumulative domain wise scores obtained by TB laboratories in baseline assessments for quality management systems
Based on key domains assessed, it was observed that all four laboratories lacked knowledge about the laboratory QMS procedures, healthcare worker surveillance, adequate documentation process, manpower was not trained in biosafety, fire safety, spill management, troubleshooting for equipment malfunctioning and, inventory management for laboratory consumables. In addition, availability and, implementation of Standard Operating Procedures (SOPs) was inadequate at all sites. Measures for continuous quality improvement (CQI) were also found lacking at all sites. Routine quality control in analytical phase was lacking to identify CQI activities. Storage and, temperature monitoring of heat labile laboratory consumables for NAAT was not done. Lack of equipment spare parts was observed that may lead to laboratory downtime on equipment malfunction.
Major gaps that could impact diagnostic services and, quality care for TB patients included lack of back up staff and, replacement matrix for staff on leave or, assigned other duties. At both the private laboratories, dedicated manpower to perform TB tests were lacking leading to delay in diagnosis for patients, their continued misery and, continued shopping for health. Delhi laboratories lacked mechanisms for recording specimen rejection, request for repeat specimen for testing ensuing missed TB case diagnosis; also, failure to report critical results was responsible for delay in clinical management of the patient and, their treatment initiation. Moreover, it was even difficult to ascertain laboratory turnaround time as time of sample receipt were not documented. At both the DMCs at Delhi and, private laboratory at Odisha, display of patient centric signages were absent. Lack of signages displayed at DMC as well as at the referring outpatient departments hindered TB suspected individuals from accessing TB services as they were not aware exactly where they have to go for TB diagnosis.
Gaps unique to private laboratory at Odisha included lack of impermeable and, chemical resistant work bench and, sink for staining for smear microscopy and, biomedical waste management as per guidelines. The laboratory was pending visit from the higher laboratory for EQA and, onsite supervision. The public laboratory at Delhi lacked distinct specimen receipt and, testing areas, sharps were not disposed in leak proof containers and, there were frayed electric wires. Personal protective equipment (PPE) was either not available or not utilized appropriately and, consistently at Odisha laboratories. Annual Maintenance Contract for instruments were also found lacking at private laboratories at Delhi and, Odisha.
Based on the gap analysis, a recommendation report and, action plan was prepared and, submitted to each laboratory for introduction of QMS. The blueprint to introduce and, implement QMS standards was prepared to address the gaps obtained during the baseline assessment and, shared with all the stakeholders.
Enhancing accountability towards diseases of public health relevance has shown to improve health system outcomes and, reduce disparity in last mile service delivery. Sanguine workforce equipped with skill, supported by efficient tools and, data can deliver quality ensured healthcare. Peripheral laboratories need to be augmented to be the strongest link in health system delivery by tackling issues in services, infrastructure, safety, staffing and, funding. QMS can provide a lucrative avenue to address laboratory inadequacies and, strengthen it as a robust pillar to deliver patient centric care.
We conducted a pilot to ascertain if investments made in introducing QMS can be dovetailed to increasing TB case finding, optimize utilization of NAATs and, reducing the burden of missing TB cases. Our baseline assessments were useful to highlight key lacunae in the health system and, identify areas of improvement to create a blueprint of laboratory driven quality management system
We assessed existing QMS of DMCs in Delhi and, Odisha. In all four DMCs, the compliance score for each domain assessed showed key areas which needed improvement. Gaps were identified in laboratory physical structure, safety measures, equipment handling and, maintenance, specimen rejection and, repeat testing, management of laboratory commodity and, supplies, quality processes, documentation & display of patient centric Information Education and Communication (IEC) resource for awareness, cough etiquettes and, specimen collection area.
The best practices that were brought under India’s NTEP was in 2012 with an innovative and visionary electronic recording and reporting system called Nikshay (Central TB Division, Ministry of Health and Family Welfare, Government of India) that digitally collates records of TB patients, including notifications from private providers; has stood the test of time. This offers a unique, undisputed opportunity to match performance against targets, retrospection and eventually plan corrective actions (Balakrishnan et al., 2021). This is the major reason for robust data flow and dissemination of programmatic data across states and country, also observed during baseline assessments. Another positive aspect noted during baseline assessments was the sync between private and, public laboratories and, coordination with partners and, private providers. In Odisha, the private laboratory was only performing sputum microscopy while the government facility was doing rapid molecular diagnostics, culture and, drug susceptibility testing as per NTEP’s UDST theme for the private laboratories.
If laboratory signages and, functional hours are not displayed appropriately, it is difficult for patient to locate laboratories as also noted by Cattamanchi et. al., 2015. This was much evident in the baseline assessments and, may potentially affect the service and programmatic objective. Therefore, focused attention is required to display laboratory location, timing and services to improve patient experience and, satisfaction. This has also been advocated globally by public health professionals to bring forth quality aspects in a TB free world (Reid & Goosby, 2020).
Lack of back up staff and, replacement matrix that hampers laboratory functioning was evident in the baseline assessment. This has also been cited as the main cause for not conducting TB testing for willing people seeking diagnostic services at these laboratories (Lisboa, et al., 2020; Bankole & Ajayi, 2022; Der et al., 2022). Trained, competent and motivated back up staff and, replacement matrix is essential to ensure uninterrupted services and maintaining the quality of all laboratory services and, correct TB diagnosis at first patient visit with scale up of NAATs. TB work force multitasking and, being overworked is also reported in other Indian studies (Pai et al., 2017; Arinaminpathy et al., 2019; Bhardwaj, 2020). Staff motivation and, continual professional developments can be crucial to obtain accurate and reliable results (Kruk et al., 2018). As noted in baseline assessment, the inadequate quality control procedure in all phases of lab operation including lack of CQI procedures and, backup/replacement staff can serve as the barriers to receiving quality care by patients. These are the major element of QMS and, require concerted effort at all levels of a public health program.
Our TB QMS assessment gave a fair and, easily replicable tool to evaluate the quality systems in laboratories looking into TB diagnosis and care. It helps sets targets and, also segregate into gaps identified in thematic domains making them addressable or seek alternative reparative measures. The quality of TB care is important. With careful planning, robust process for CQI and, capacity building many roadblocks can be removed. Based on the lessons learned from the pilot study, a blue print for QMS implementation has been developed (Figure 2). The blueprint dovetails QMS with CQI keeping in view of recommendations for adoption and, national scale up. It encompasses a user-friendly tool and, cost-effective capacity building processes using IRLs and NRLs. At inception the DMCs will be assessed for the baseline to understand the current situation, gaps and, challenges. This will facilitate development of institution specific action plans for capacity building, training and, mentoring. The progress on implementation of QMS will be monitored through follow up assessments. The sites securing >90 % in follow up assessment will be awarded for certificate for quality compliance. Facilities will undergo improvement workshops where the efforts will be concentrated on the identified weaknesses and, areas that require improvements. Subsequent to resolution of action points from last assessment, sites will undergo follow up assessment until the certification. This blue print also envisages engaging the existing lab network of IRLs and NRLs to monitor the certified sites for maintenance of certification and, CQI using standard assessment checklist and, capacity building tools.
To summarize, processes in strengthening peripheral TB diagnostic laboratories can be challenging, intricate and, cost intensive. It is a complex system, involving many factors including varied phases of laboratory procedures, processes, management and, linkages. Some of the key factors in this system include the laboratory leadership, management, structure, environment, competent staff, quality control procedures, communications, good documentation practices, good-quality reagents and, equipment. When entire laboratory system is organized into an understandable and, workable structure, the opportunity to ensure quality increases. Therefore, the QMS model, which looks at the entire laboratory system, is very important for achieving quality assured services and, patient care. Our study showed that QMS should be introduced at peripheral laboratories and, adequately supported with human and financial resources. With QMS as the backbone, access to TB care can be increased and, simultaneously ensuring that the care provided is of sufficiently high quality. Systematic mentorship and, regular follow will be effective and, continuous evaluation of QMS for compliance will maximize the benefit to the National TB Programs by improving quality, increasing the case finding and, justify cost effectiveness of introducing and, scaling up new diagnostic technologies. The study recommends that there should be step wise QMS implementation at peripheral TB diagnostic laboratories with inbuilt monitoring mechanism for ensuring compliance to improve quality patient diagnostic and, the ensuing care and, services to the affected people.
Acknowledgement
Indian Council of Medical Research, New Delhi is acknowledged for the funding support
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
