AUCTORES
Globalize your Research
Case Series
*Corresponding Author: Elisa Paolin, Department of Public Health, Experimental and Forensic Medicine, Human Anatomy Unit, University of Pavia, Italy.
Citation: Emiko Asai, and Elisa Paolin, (2024), Resolution of Non-Diabetic Intractable Wounds with Severe Comorbidities Using Autologous Micrografts, International Journal of Clinical Case Reports and Reviews, 19(3); DOI:10.31579/2690-4861/571
Copyright: © 2024, Elisa Paolin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 07 October 2024 | Accepted: 17 October 2024 | Published: 31 October 2024
Keywords: micrografts; non-healing wounds; wound healing; ulcers; autologous micrografts
The wound healing process is a complex mechanism related to many factors, such as the patient’s comorbidity, blood flow, and nutritional status. This study aimed to retrospectively evaluate patients affected by various disease states and treated with Rigenera® technology, a standardized micrograft preparation system. A total of 8 patients were treated with a suspension of autologous micrografts obtained from mechanical disaggregation of small pieces of skin tissue. The micrografts suspension was applied alone to the wound or in combination with porcine collagen scaffolds directly onto the wound as described in the text. Across all cases, there was marked improvement in wound healing, with a mean healing time of 103 days and a reduction of the diameter of the wounds by 54%. Notably, the use of micrografts resulted in an overall higher quality of life for the patients. This simple and low-cost micrografting procedure that can promote wound healing of different kinds of chronic non-healing wounds and does not require postoperative rest.
As wound healing professionals, plastic surgeons deal with various conditions of wounds daily. While recent technological advancements, including new wound dressing, Negative Wound Pressure Therapy (NPWT), and Negative Pressure Wound Therapy with instillation therapy and dwelling time (NPWT-id) devices [1-3], and growth factor formulations have facilitated the cure of previously refractory cases, there are still many cases that continue to be a challenge [4-8]. That is because the wound healing process is influenced by a multitude of factors such as the patient’s comorbidities, nutritional status, and age [9-11].
Autologous micrografts (AMGs), which were initially described by Pérez in 2010, have been proven to be efficacious in tissue repair through numerous studies [12]. The Rigenera® technology represents an innovative and standardised micrograft preparation system. Progenitor cells expressing surface markers of mesenchymal stem cells and ex-tracellular matrix are contained within the suspension, supporting the regenerative potential of micrografting [13-16]. AMGs have been reported to have a size of around 80µm, consisting of endothelial and progenitor cells, as well as extracellular matrix (ECM) ingredients, which can aid in reducing the inflammatory process and support wound healing [13,15,17]. The Rigenera® technology is an innovative and standardized micrograft preparation system that involves mechanical disaggregation of small pieces of tissues using the Rigeneracons medical device, a biological disruptor of human tissues, to obtain AMGs. The suspension of AMGs can then be immediately injected for grafting procedures without further processing. This protocol is easy and safe to use, and there is substantial evidence demonstrating its application for the tissue repair of post-operative and post-traumatic wounds [14, 18–20], and chronic ulcers [15, 21–24]. Early evidence also shows the applicability of AMGs in orthopedics [25,26] and cardiac fields [27–30].
In this context, we present a retrospective evaluation of eight patient cases showing non-diabetic intractable wounds with severe comorbidities. All patients underwent as-sociated and previous clinical procedures followed by treatment with the Rigenera® micrografting technology. This retrospective analysis aims to provide further evidence of the potential effectiveness and safety of this innovative and standardized micrograft preparation system in treating non-diabetic intractable wounds.
An overall total of eight patients, comprising of four males and four females, with a mean age of 60.1 ± 18.9 years, were treated and retrospectively evaluated. Prior to the administration of the AMGs treatment, all cases underwent an evaluation for systemic and local diseases. Table 1 summarizes the clinical characteristics of each patient, including the type of disease, wound diameter, and comorbidities. It is noteworthy that none of the patients exhibited diabetes as a comorbidity.
It is also important to mention that all patients provided written informed consent before each treatment.
| Patients | Age/Sex (F=Female M=Male) | Type of Disease/ Anatomical Site | Comorbidities | Diameter of Wound (cm)/ Site |
| 1 | 38/F | Skin defect after excision of pyoderma/ bilateral groin region | Obesity | Left 8x6.5 Right 7.5x6 |
| 2 | 46/F | Pressure sore / left foot | Sensory disorders | 7x8 |
| 3 | 37/M | Pressure sore /sacral region | Lower body paralysis, obesity | After 3rd surgery: 7×6×5(d) |
| 4 | 79/M | Skin ulcer after necrotizing fasciitis / hip | Lower body paralysis, hypertension, epilepsy | 15×7 hip suture wound 3×3.5 thigh skin ulcer 18×8 lower leg suture wound |
| 5 | 61/M | Pressure sore around the scar / sacral region | Paraplegia | 2x1 1.5x1 |
| 6 | 88/F | Burn ulcer / left lower leg | Lymphedema, heart disease, hypertension | 12x10 |
| 7 | 73/F | Abdominal wall defect | Mixed connective tissue disease (MCTD), Sjögren's syndrome, interstitial pneumonia, and hypertension | 15x18 |
| 8 | 59/M | Skin ulcer on the precordium | Cardiac disease | 15x7 |
Table 1: The clinical characteristics of the selected patients were comprehensively evaluated and recorded. The data includes the sex and age of each patient, the type of disease, the anatomical site of the wounds, comorbidities, and diameter of the wounds prior to the AMG treatment.
2.1. Rigenera® Technology
The tissue size to be harvested for producing AMGs is calculated based on the wound area that needs to be covered. The tissue collection is approximately 1:400, meaning that a skin sample of 2x2x2 mm was collected from healthy skin to cover a wound area of 4 cm2. The donor site is sutured after tissue collection. The sample is then divided into its epithelial and dermal components, and each is cut into small pieces. They are separately put into a Rigeneracons device with adequate saline solution and disaggregated for approximately 4 minutes [31]. The AMGs suspension obtained from the dermal component is injected into the wound margins (0.1ml/1cm intervals apart). At the end, to cover the ulcer surface, AMGs from the epithelial component are sprayed onto the wound surface using a syringe equipped with a mist adapter. When required, AMGs components are used to infiltrate a porcine collagen scaffold (Pelnac Gplus, Gunze Medical Limited, Japan) and subsequently applied onto the ulcer surface [15].
2.2. Case 1:
The patient was presented with multiple pyoderma that extended from both sides of the groin area to the external genitalia. After the resection of the external genitalia lesion, a wide skin defect was left, which was reconstructed with bilateral gluteal fold flaps. Due to the unavailability of a pedicled flap around the groin area for reconstruction, skin grafting would have been the standard procedure. However, due to the patient's obesity, AMG treatment was chosen to avoid the risk of venous thrombosis caused by obesity and postoperative bed rest. The AMGs were obtained by collecting a small piece of tissue with a scalpel (2.5 cm in diameter), and they were injected into the wound site and sprayed on the surface after resection. Since the need for early mobilization prohibited the placement of a suction drain under the bilateral gluteal fold flap, NPWT was used for exudative suction after surgery. The reconstruction of the external genitalia with a bilateral gluteal fold flap was successful, and no complications were reported. The skin defect in the bi-lateral groin region that underwent AMG treatment was steadily reduced, and epithelialization was confirmed during the re-examination on the 69th postoperative day, as shown in Table 2 and Figure 1.
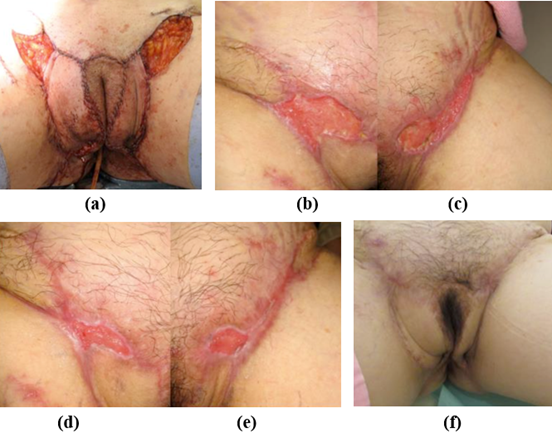
Figure 1: Condition at the end of surgery (a), at 4 weeks (b-c, right and left groin), at 8 weeks (d-e right and left groin), at 1 year after the micrograft procedure (f).
2.3. Case 2
The patient was presented with an intractable foot ulcer that was difficult to treat due to a congenital sensory disorder. Despite the small size of the ulcer, it had formed a large pocket internally. It showed no signs of contraction even after preservation treatment through irrigation and application of topical agents. A decision was made to perform a surgical procedure, considering the option of free skin flap reconstruction after debridement. However, the latter option was ruled out due to the lengthy operation and the patient's sensory disorder. There was a risk of recurrent ulceration caused by the postoperative bulkiness of the flap, which could compromise the outcome and restrict mobility, preventing the patient from wearing fitted shoes.
Therefore, the chosen method for wound contraction was the AMG treatment. The wound pocket was opened to clean and debride, and AMGs were injected inside the pocket as much as possible. The partially and surgically incised wound area was cleansed, followed by applying of a topical agent. Minimal walking was required as part of the standard post-operative procedure. The pockets healed and disappeared within 2 days after the operation. The open wound was then treated with continual cleaning and topical agents, and epithelialization was confirmed on the 108th day after the operation (refer to Table 2 and Figure 2). The patient could walk wearing custom-made shoes im-mediately after healing, and no recurrence was observed thereafter.
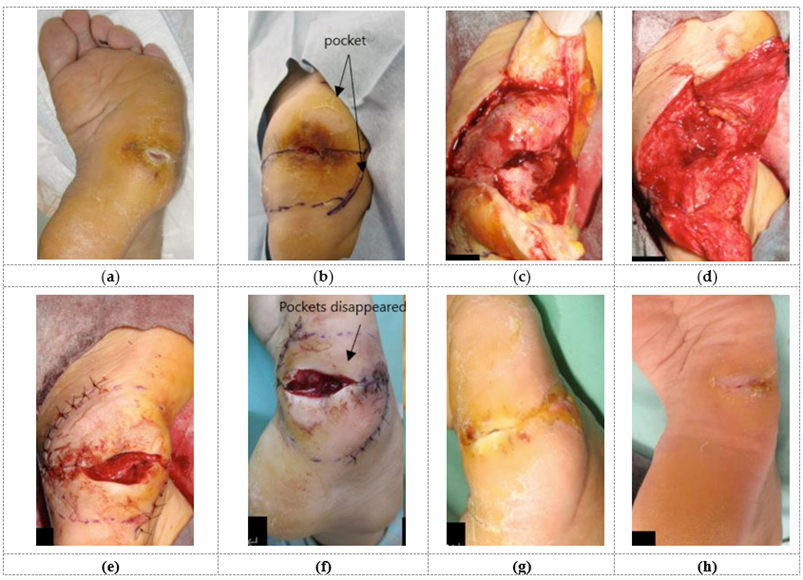
Figure 2: A 46-year-old-female with pressure sore caused by sensory disorder on the right foot. Preoperative condition (a), skin incision design (b), condition after pocket incision (c), after removal of inflammatory granulation tissue during operation (d), after sutures (e). The pocket dis-appeared two days after the operation (f), at 4 weeks (g), completely dried up 16 weeks after operation (h).
2.4. Case 3
The patient presented with lower body paralysis due to spinal cord injury and was diagnosed with clinical obesity. A shallow ulcer near the anus, measuring about 2 cm in diameter with a healthy granulation color, was managed through conservative treatment consisting of wound cleaning and application of growth factors such as fibroblast spray, as well as using povidone-iodine to suppress biofilms which showed no signs of improvement.
The first AMG treatment was administered five months after onset of the ulcer, but no effect was observed. Subsequently, the AMG treatment was repeated ten months after onset, and the ulcer showed signs of gradual reduction. The donor tissue, measuring 2 cm in diameter, was harvested using a scalpel. The AMG suspension was injected and sprayed onto the wound area, and infused into an artificial dermis (Pelnac Gplus, Gunze Medical Limited, Japan) before being applied onto the wound.
However, a pocket formation was observed toward the sacral on the ulcer head side one month after the operation. Reconstruction after debridement by pedicled flap was considered, but it was deemed more appropriate to perform colostomy expansion in advance to prevent postoperative infection. The patient’s obesity made this difficult to do, and it was regarded that postoperative bed rest could cause further weight gain. As a result, reconstruction by pedicled flap was abandoned, and a third AMG treatment was performed in combination with collagen sponges (Pelnac Gplus, Gunze Medical Limited, Japan), to optimize the efficacy of AMG injection at the pocket region. In the third treatment, the main aim was weight management to prevent future pressure ulcer recurrence by continuing rehabilitation while cleaning the wound and treating it with topical agents.
We observed that the shallow ulcers were epithelialized about 2 weeks after surgery. The pocket opening showed good granulation growth and a gradual shrinking. After the operation, wound management based on cleaning and topical agents continued, as well as bed rest, except for occasional wheelchair movement during rehabilitation. Although the ulcer has been reduced, it has not achieved complete epithelialization four months after the operation, as shown in Figure 3 and under Table 2.
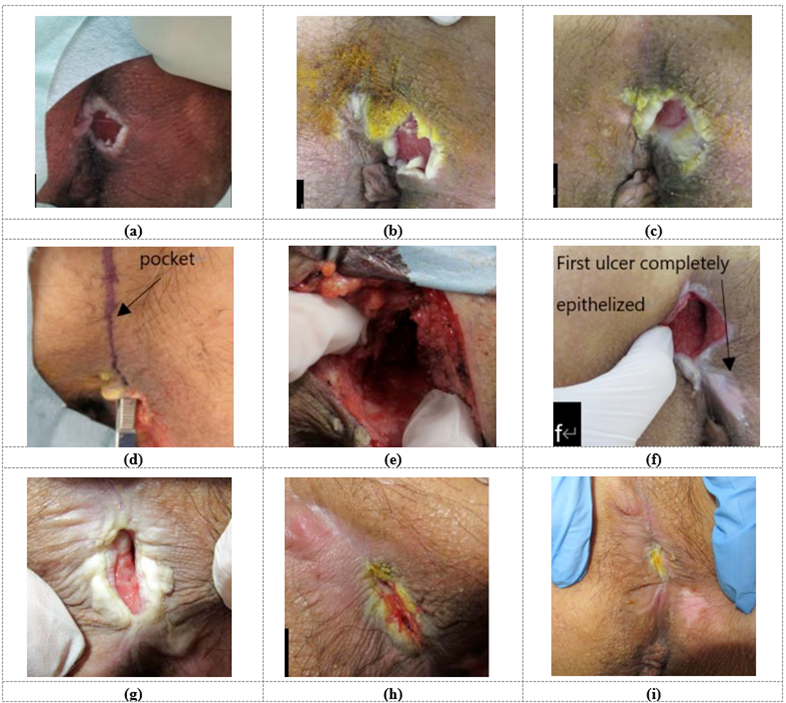
Figure 3: The images depict the progression of a pressure sore on the sacral part of a 37-year-old- male with lower body paralysis. The photos show the condition before the first operation (a), be-fore second operation (b), 4 weeks after the second operation (c), before the third operation (d), af-ter the pocket incision during operation (e), at 4 weeks (f), at 16 weeks (g), at 24 weeks (h), and 8 months after the third operation (i).
2.5. Case 4
The patient suffered from lower body paralysis as because of a spinal cord injury, which was further complicated by the presence of pressure ulcers and necrotizing fasciitis. The skin ulcer initially developed from Fournier's gangrene and pressure ulcers in the right gluteal region, gradually spreading to include the groin, genitalia, gluteal region, and greater trochanter. Cleansing with NPWT-id was performed to prepare the wound for closure. The unloaded areas, such as the genitalia and greater trochanter, were simply sutured, while the sciatic part was closed by transferring a pedicled posterior thigh flap. The thigh area, where the flap was taken from, was closed with sutures. Partial necrosis and dehiscence were observed on the posterior thigh flap that was transplanted onto the buttock. These areas were operated on under local anesthesia. The sciatic ulcer was sutured after debridement. However, tension at the time of suture caused partial necrosis and the development of a skin ulcer at the flap's donor site.
To improve blood flow, promote adhesion, and soften scar tissue, AMG was injected subcutaneously immediately after suturing the wounds. Additionally, a collagen scaffold (Pelnac Gplus, Gunze Medical Limited, Japan) was immersed in an AMG suspension and applied to the skin ulcer on the thigh and the donor site of the flap after the AMG injection. Approximately 10 days after the operation, all sutures dehisced, and partial necrosis of the lower leg flap occurred. However, further operations were not performed due to the patient's age. The patient was transferred to another hospital for ongoing conservative treatment 1.5 months after the initial operation. At the time of re-examination, favorable granulation growth was observed, and the lower leg had epithelialized three months after surgery. While increased granulation was present at the sciatic region, reaching the same height as the skin surface, it had not yet epithelialized even eight months after surgery. Table 2 provides information about the size of the ulcer before surgery or at the time of flap elevation, which was then compared to the size of postoperative dehiscence of the ulcer. Similar evaluations were conducted for the parts that were not sutured wounds.
2.6. Case 5
The patient experienced paraplegia resulting from a spinal cord injury and had been dealing with two small ulcers for four years. Despite undergoing skin chip grafting two years after the ulcers first appeared, there was only a slight size reduction, and no complete epithelialization was achieved.
To address this issue, the patient received treatment with AMG using donor tissue of approximately 2 cm in diameter, harvested using a scalpel. The patient showed significant progress despite the AMG treatment being administered four years after the ulcers first appeared. Complete epithelialization was achieved after 104 days from the treatment, indicating that the therapy was effective in promoting wound healing.
2.7. Case 6
The patient presented with an ulcer resulting from a burn wound along with lymphedema. Initially, conservative treatment was administered as an outpatient using irrigation and topical agents. Surgical intervention was then performed once sufficient granulation had developed. While skin grafting was considered, the patient's advanced age and muscle weakness from bed rest posed risks to the activities of daily living (ADL) and the potential for delirium due to hospitalization.
Instead of traditional approaches, the patient received outpatient treatment with AMG without the need for postoperative bed rest. Since the skin defect extended to the fascia, the initial rate of contraction was slow. However, the healing process accelerated over time, and epithelialization was confirmed during an examination on the 107th day after surgery.
2.8. Case 7
The patient was a seventy-three-year-old female with a medical history of mixed connective tissue disease (MCTD), Sjögren's syndrome, interstitial pneumonia, and hypertension. She was currently receiving treatment for thrombocytopenic purpura with antiplatelet agents, immunosuppressants, and steroids. In April 2022, she was admitted to the hospital’s surgical department for a gastrointestinal perforation that necessitated an emergency colostomy. Postoperatively, the patient experienced anastomotic leakage and incomplete fusion of the peristomal skin with the intestine.
On May 24th, 2022, the patient was referred to the Department of Plastic Surgery, Fukushima, Red Cross Hospital. Upon initial examination, a 15 x 18 cm abdominal wall defect with granulation tissue atop the intestine and separation of the intestine from the peristomal skin were noted. Given the patient’s poor condition and bedridden state, traditional abdominal wall reconstruction under general anesthesia was deemed unsuitable.
On June 8th, 2022, an AMG treatment was performed under local anesthesia. Following debridement of the wound edges around the abdominal wall defect and artificial anus, micrografts were injected sub-dermally on the artificial anus area and the lesion was resutured. The adbominal wall defect was treated with dermal micrograft injection and in combination with collagen sponges (Pelnac Gplus, Gunze Medical Limited, Japan). The treatment was well-tolerated, and significant progress was observed.
Complete epithelialization of the abdominal wall area was achieved 96 days from the AMG, and the peristomal skin had fully integrated. The patient was subsequently transferred to another hospital the following day. Despite the absence of photographic evidence of full epithelialization, the AMG treatment demonstrated remarkable efficacy in this complex case, providing a less-invasive alternative to conventional surgical methods, as shown in Figure 5 and under Table 2.
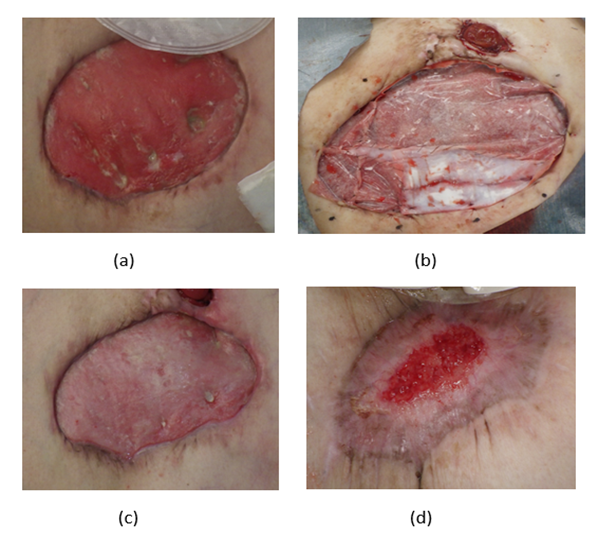
Figure 4: Clinical progression of abdominal wall defect treatment in a seventy-three-year-old female with multiple comorbidities. (a) depicts the patient's initial condition at the time of diagnosis, (b) shows the condition after AMG treatment combined with collagen sponge (c) shows the progression of the wound after 2 weeks from the use of the Rigenera® technology (d) displays the wound condition after 11 weeks from the use of the Rigenera® technology.
2.9. Case 8
The patient, a 59-year-old male, underwent cardiac bypass surgery in July 2022. The following year in August, complications arose when wire breakage and screw displacement led to sub-sternal dehiscence. All fixation devices were removed under general anesthesia, followed by debridement and the application of NPWT-id. Despite these measures, the patient presented with a significant skin ulcer measuring 15 x 7 cm on the precordium, with the ulcer bed exposing the mediastinum.
Referred to our department in August 2023, the patient underwent a bilateral pectoralis major myocutaneous flap procedure the following month, under general anesthesia to close the wound after further debridement. However, post-surgical complications included partial necrosis at the bottom edge of the right flap and subcutaneous hemorrhage at the precordium. Even with NPWT-id, the wound pocket persisted.
The wound pocket was fully opened in October, and continuous cleansing was initiated. Subsequently, on November 22nd, 2023, debridement and micrograft treatment in combination with collagen sponges (Pelnac Gplus, Gunze Medical Limited, Japan) were performed under local anesthesia. Rapid epithelialization was observed, except for a small fistula tract of approximately 1 cm caused by a bone sequestrum that took longer to heal. Further debridement the following year in March involved the removal of a sequestrum beneath the ulcer, followed by resuturing. Complete healing was observed by April, with all sutures removed, as shown in Figure 6 and under Table 2.
This case highlights the complexity of treating sub-sternal dehiscence after cardiac surgery and the effectiveness of micrograft treatment in promoting rapid wound epithelialization.
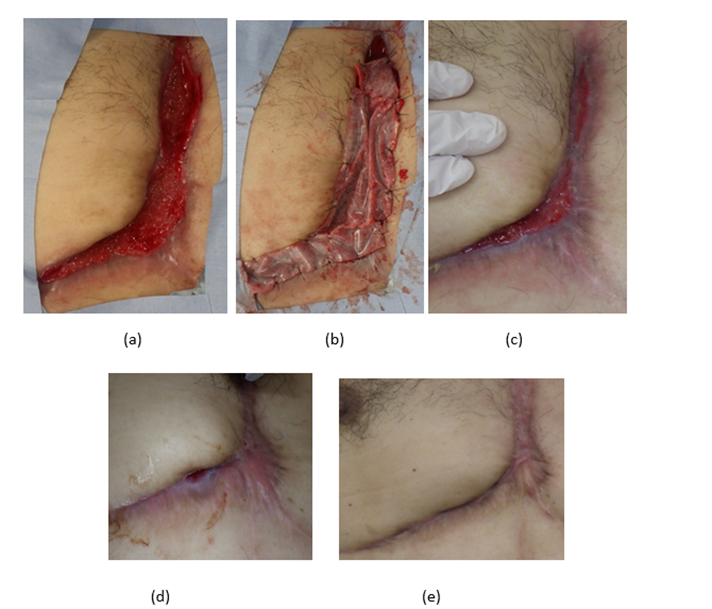
Figure 5: Images showing the progression of a skin ulcer in a 59-year-old male on the precordium following cardiac bypass surgery. (a) depicts the patient's initial condition at the time of diagnosis, (b) shows the condition after AMG treatment combined with collagen sponge (c) shows the progression of the wound after 1 month from the use of Rigenera® technology (d) displays the wound condition two months before complete re-epithelialization. (e) complete closure.
Figure 5: Images showing the progression of a skin ulcer in a 59-year-old male on the precordium following cardiac bypass surgery. (a) depicts the patient's initial condition at the time of diagnosis, (b) shows the condition after AMG treatment combined with collagen sponge (c) shows the progression of the wound after 1 month from the use of Rigenera® technology (d) displays the wound condition two months before complete re-epithelialization. (e) complete closure.
| Case No. | Duration Before Treatment | % Diameter Reduction At Week 4 | Healed At Week 8 | Healed At Week 16 | Healing Time After AMG (days) | Dermal Substitute |
| 1 | 0 week | 30 | No | Yes | 69 | / |
| 0 week | 50 | No | Yes | 69 | / | |
| 2 | 7 weeks | 90 | No | Yes | 108 | / |
| 3 | 52 weeks | 20 | No | No | 245 | Pelnac Gplus |
| 4 | 2 weeks | 50 | No | No | Decreased | / |
| 2 weeks | 40 | No | Yes | 100 | Pelnac Gplus | |
| 39 weeks | 60 | No | Yes | 100 | / | |
| 5 | 199 weeks | 50 | No | Yes | 104 | / |
| 204 weeks | 30 | No | Yes | 104 | / | |
| 6 | 5 weeks | 60 | No | Yes | 107 | / |
| 7 | 2 weeks | 70 | No | Yes | 96 | Pelnac Gplus |
| 8 | 16 weeks | 100 | No | Yes | 33 | Pelnac Gplus |
| Average | 54 | 89 | ||||
| STDEVP | 24 | 24 |
Table 2: The averages were obtained by applying Dixon's Q test with a 95% confidence interval to eliminate outliers.
The present evaluation aimed to assess the effectiveness and safety of the Rigenera® micrografting technology in treating non-diabetic intractable wounds with severe comorbidities. The study included eight patient cases with various etiologies of wounds, and the outcomes were analyzed to provide further evidence of the therapeutic benefits of this micrograft preparation system. The use of AMGs derived from the Rigenera® technology allowed for applying a suspension containing progenitor cells and extracellular matrix components to the wound site. This approach has been previously shown to support tissue regeneration and promote wound healing in various clinical scenarios [27,28, 32–42].
Our data indicate that the reduction in wound diameter at four weeks was 52% without the use of the dermal substitute (Pelnac Gplus), compared to 60% with Pelnac. This suggests that the effectiveness of Rigenera® micrografts is independent of the presence of Pelnac.
Besides, our findings suggest that applying AMG is a beneficial approach to accelerate the healing process of non-diabetic wounds that are unresponsive to previous treatments.
Previous studies have demonstrated that AMGs contain progenitor cells showing pluripotent mesenchymal stem cell markers, growth factors, and extracellular matrix constituents [31]. Furthermore, AMGs have been shown to promote the movement of fibroblasts and keratinocytes by activating the extracellular signal-reproduced kinase (ERK) signaling pathway [43]. They also stimulate expression of transforming growth factor-beta1 (TGFβ-1) early in granulation tissue and increase the expression of alpha-smooth muscle actin (αSMA), indicating the existence of myofibroblasts and angiogenesis [17,44]. Additionally, AMG injection is a straightforward procedure that requires no complex introduction methods and has minimal impact on the donor site.
In our study, AMGs have also been used with NPWT and NPWT-id for faster healing and wound bed preparation before implantation [19,43].
In both Case 1 and Case 2, AMG treatment facilitated wound contraction and healing, underscoring its potential as an effective alternative to traditional surgical approaches.
The effectiveness of AMG treatment is not limited to chronic wounds, as it has also been applied to deficient wounds after lesion resection and surgical sutures. This treatment approach is particularly well-suited for elderly patients, as it allows for outpatient treatment without the risk of muscle weakness or delirium associated with hospitalization, as demonstrated in Case 3, 6 and 7.
Furthermore, Niimi et al. [32] reported a clinical case where AMGs were successfully utilized with the dermal collagen substitute for wound treatment. This case highlighted the versatility and potential synergies of combining AMG treatment with advanced wound care products, emphasizing the importance of exploring various therapeutic approaches
for optimal outcomes. The collaborative use of AMGs showcases a promising strategy for addressing challenging wounds and underscores the significance of a multifaceted treatment approach in wound management. In the referenced study, micrografts within the scaffold
were verified through immunostaining assessment, revealing the presence of non-epithelial-derived cells, including collagen fibers, vascular
endothelial cells, and neutrophils. These cell types exhibited a gradual increase over the observation period. The histological evaluation suggested the integration of micrografts into the scaffold, collectively contributing to the advancement and facilitation of the wound healing process.
Another study conducted by Li et al. [45] delved into the concurrent application of artifical dermis and micrografts in a murine wound animal model. This investigation serves to enhance our comprehension of the efficacy of artificial scaffolds when utilized alongside micrografts in the context of wound management.
Our study also included a case involving a patient with sensory disorder. In Case 2, the pocket completely disappeared two days after the procedure. In some cases, involving paralysis, the regenerative response to AMG treatment appeared to be slower than other cases. However, it is important to note that these cases cannot be generalized as the application sites differ.
In Case 4, we did not observe a significant change after the initial AMG treatment. A study by Jimi et al (2017) suggests an optimal density or tissue suspension for the wound healing in mice, hypothesizing that a high concentration of AMGs injected into a small area may trigger a down-regulation [46]. Still, given the human body mass this seems unlikely. In this case, it may be more relevant to take into consideration the patient's condition after flap transfer, including factors such as muscle weakness, weight gain, and pressure ulcer recurrence.
AMG technology has proven effective in promoting wound healing across a variety of complex cases, including late-stage interventions, patients with multiple comorbidities, and challenging wound management scenarios, as showed in Case 5,6,7, and 8. These cases collectively reinforce the therapeutic benefits of AMG technology in accelerating wound healing, reducing hospitalization risks, and improving patient outcomes, suggesting its promising role in modern wound care practices.
Including cases with different etiologies and complexities strengthens the findings of this study and provides valuable insights into the applicability of the Rigenera® micrografting technology across diverse wound scenarios. The study’s retrospective nature limits the ability to draw definitive conclusions or establish direct comparisons with control groups. However, the observed data supports that the effects of Rigenera® micrografts are not age-related and the consistent positive outcomes in the cases support further investigation through prospective studies or clinical trials. Further research with larger number of patients, randomized controlled trials, and longer follow-up periods is warranted to validate and expand upon the findings of this study.
In conclusion, our study emphasizes the effectiveness and versatility of AMGs as a straightforward and cost-effective therapeutic approach for managing non-diabetic, intractable wounds, particularly in patients with severe comorbidities. The results demonstrate that AMGs can significantly enhance wound healing, irrespective of the presence of adjunctive therapies like Pelnac Gplus. This indicates that Rigenera® micrografts offer a robust therapeutic option that is not affected by the dermal substitute.
The procedure’s minimal impact on the donor site makes it a practical choice for a diverse range of patients, including those who are elderly or obese.
Further investigations, including larger patient cohorts and randomized controlled trials, are necessary to validate these findings and explore the full potential of this innovative treatment approach.
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, E.A.; methodology, E.A. and E.P.; validation, E.A. and E.P.; formal analysis, E.A. and E.P.; investigation, E.A.; resources, E.A. and E.P.; Data curation, E.A. and E.P.; writing—original draft preparation, E.A. and E.P.; writing—review and editing, E.P.; visualization, E.P.; supervision, E.A. and E.P.
This research received no external funding.
Institutional Review Board Statement: The study was conducted in accordance with and ap-proved by the Internal Review Board of Fukushima Red Cross Hospital.
Informed consent was obtained from all subjects involved in the study.
EP is affiliated with the R&D department of HBW srl, the company that owns the Rigenera® Micrografting Technology. All other authors declare that the research was conducted independently and without any financial or commercial relationships that could be perceived as a potential conflict of interest.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner
