AUCTORES
Globalize your Research
Case Report
*Corresponding Author: Noa Lina Eleni Aegerter, Clarunis, University Centre for Gastrointestinal and Liver Diseases, 4002, Basel, Switzerland.
Citation: Noa L. E. Aegerter, Georg Henniger, Gabriel F. Hess, Alexandar Tzankov, Simone Muenst, et al., (2024), Radioactive Seed-guided Resection of Cholangiocellular Carcinoma in Cirrhotic Patients; Case Report, International Journal of Clinical Case Reports and Reviews, 20(3); DOI:10.31579/2690-4861/567
Copyright: © 2024, Noa L. E. Aegerter. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 04 October 2024 | Accepted: 24 October 2024 | Published: 09 December 2024
Keywords: radioactive seed-guided resection; cholangiocellular carcinoma; combined cholangio-hepatocellular carcinoma
The most common cause for hepatocellular carcinoma (HCC) is cirrhosis. Furthermore, cirrhosis is a risk factor for cholangiocarcinoma (CC). Detection of HCC and CC can be challenging in both radiologic imaging and during surgical resection, especially in patients with an underlying parenchymal liver disease such as cirrhosis. Therefore, radioactive seed-guided resection of these tumors, analogously to breast cancer, could be an interesting approach. We present two cases of cirrhotic patients in whom this method of tumor labeling was used.
The first case was a patient suffering from suspected HCC. Although the targeted lesions had been verified by intraoperative ultrasound before non-anatomical resection, histological examination of the specimen showed no tumor. Three months later, tumor progression was seen on liver imaging. Therefore, the patient underwent interventional labeling of the tumor with a radioactive seed followed by open resection. Complete removal of a combined HCC-CC of the liver in segment VII was achieved.
In the second patient, seed-guided resection was recommended due to underlying cirrhosis. Furthermore, the two lesions were rather small and the localization were surgically difficult to reach. Both lesions were pre-labelled with help of TACE and Lipiodol the day before. Subsequently, two radioactive seeds were placed in the lesions, and resection was performed successfully.
This report emphasizes the difficulties, which surgeons and radiologists may face in tumor entities that are difficult to identify both macroscopically, by palpation and intraoperative imaging techniques. It also highlights the successful adaption of a procedure commonly used for breast cancer surgery for liver surgery.
AASLD : American Association for the Study of Liver Diseases
AFP : alpha-fetoprotein
ASH : Alcoholic Steatohepatitis
CC : Cholangiocarcinoma
CEUS : contrast-enhanced ultrasound
cHCC-CC : combined cholangio-hepatocellular carcinoma
HCC : Hepatocellular Carcinoma
125I : Iodine-125
NASH : Non-alcoholic Steatohepatitis
TACE : Transarterial Chemoembolization
Cirrhosis as a consequence of alcoholic liver disease is the most common cause for hepatocellular carcinomas (HCC) as well as a risk factor for cholangiocarcinoma (CC) [1,2], in western countries [3]. Both CC and HCC can be detected by contrast enhanced ultrasound with a sensitivity almost similar to multiphasic CT or MRI [4]. For all suspected primary liver tumors, the routine diagnostic procedures include a triphasic CT or an MRI [5]. For all imaging modalities the detection of these tumors in cirrhotic livers can be difficult due to the altered liver parenchyma background signals. Moreover, in cirrhotic patients, interpretation of intraoperative palpation and ultrasound imaging can be impeded by dense liver tissue and small tumor size [6]. Therapeutically, the removal of the tumor by non-anatomical liver resection is a widely accepted method for individuals with cirrhosis to preserve as much functional liver capacity as possible [7].
Nevertheless, in daily practice this approach can be complex, especially if the tumors are difficult to visualize with ultrasound and can only be seen in CT/MRI [8].
Radioactive seed-guided resection is a commonly used method in breast cancer surgery [9,10]. The Iodine-125 (125I) seeds can be placed in the tumor by an interventional radiologist up to 8 days before surgery [10]. This represents the period during which enough radiation is being emitted for successful intraoperative localization with a handheld gamma probe [11]. Up to date, only interventional implantation of 125I seeds for treatment of unresectable HCC and CC has been described [12-14]. Thus, the two cases presented here are the first to present evidence for the utility of radioactive seed-guided resection of intrahepatic CC/cHCC-CC.
The aim of the seed-guided liver resection was to improve tumor detection in patients with underlying cirrhotic liver disease, where tumor localization could not be achieved through the use of standard imaging.
A 71-year-old male patient was referred to the Clarunis Center for Abdominal Surgery at the University Hospital Basel for resection of a suspected malignant lesion in the liver segment VII. The obese patient was under regular gastroenterologic surveillance for alcoholic liver disease with a liver cirrhosis Child-Pugh class A, which had been diagnosed 11 years previously. His medical history included one episode of acute upper gastrointestinal bleeding due to oesophagal varices grade I, diabetes type 2, peripheral neuropathy with sensory gait abnormality and generalized atherosclerosis. Furthermore, the patient suffered from coronary heart disease and had undergone mitral valvuloplasty. Atrial fibrillation had been treated by implantation of a left atrial appendage occlusion device since the patient had a HASBLED score of 7 points and oral anticoagulants were contraindicated. The patient was also affected by chronic kidney disease and had a baseline eGFR of 58 mL/min/1.7, corresponding to a creatinine of 129 µmol/L.
Diagnostic access
In December 2019, ultrasound imaging of the liver showed typical signs of cirrhosis. However, these findings could not account for the rise of alpha-fetoprotein (AFP) to 75 ng/mL. MRI was performed and showed a tumor formation of 2 cm in size, which was located in the liver segment VII. The most probable diagnosis at presentation was a HCC (CHILD A). Simultaneous rise of AFP, a primary tumor marker for HCC, and typical morphology of the neoplasm with hyperintensity in arterial phase and washout during portal/late phase in multiphasic MR imaging [4], supported this presumptive diagnosis. No vascular invasion or intra-/extrahepatic metastases were seen. Tumour size was quantified as 20 mm, which equaled a stage cT1b cN0 cM0. First line treatment by surgical removal of the neoplasm was chosen over ablative techniques in compliance with the current guideline for the treatment of HCC, published by the American Association for the Study of Liver Diseases (AASLD) in 2018 [15]. In patients with cirrhosis, preserving liver function, e.g. sparing as much healthy parenchyma as possible, is important. Therefore, atypical liver resection was chosen over anatomical liver segment resection [7].
Preoperative assessment included cardiologic examination of the patient, based on which the patient was decreed fit for surgery.
Treatment
The patient underwent open cholecystectomy and non-anatomical resection of liver segments VI/VII and V after verification of the non-palpable lesions with intraoperative ultrasound (B-mode). Postoperatively, the patient was admitted to the ICU for further monitoring and transferred to the surgical ward in stable condition the following day.
Five days after surgery, the patient had to be readmitted to the ICU due to altered neurological status requiring advanced pharmacological intervention and surveillance. Additionally, the patient showed signs of postoperative ileus. Serum-ammonia levels of 139 µmol/L indicated an episode of hepatic encephalopathy successfully treated with lactulose, sodium benzoate and arginine. The paralytic ileus improved significantly after the insertion of a nasogastric tube and by administering additional stool-regulating drugs.
Histological examination of the surgical specimen showed only cirrhotic tissue with multiple regenerative nodules but no malignant tumor. The case was discussed in the multidisciplinary tumor board, which recommended watchful waiting with serial imaging and tumor marker testing.
In line with this plan, a scheduled MRI three months later showed slight progression of a subcapsular tumor in segment VII, which grew to 24 x 19 mm in size (Figure 1 A B). In addition, a rise of AFP to 97 ng/mL was seen. Thus, the tumor board recommended repetitive surgery, which concurred with the patient’s wishes.
Considering the unexpected outcome of the first surgery, proper intraoperative identification of the lesion was deemed crucial for successful resection. In a two-step approach, the interventional radiologist first marked the tumor by CT-guided implantation of radioactive seeds (Figure 1 C D)
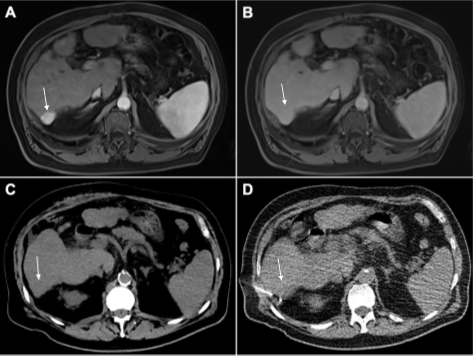
Figure 1: Primary liver tumor in liver segment VII (arrow). (A) Hyperintensity is seen in arterial phase and (B) washout in portal phase of multiphasic MRI of the liver (both marked by arrows). Both are typical features of hepatocellular carcinoma. (C) Native CT of the slightly hypodense lesion in segment VII (arrow). (D) Percutaneous implantation of the 125I seed.
according to anatomical landmarks (arrow). Subsequently, re-laparotomy and atypical resection of the marked lesion was conducted. Iodine-125 radiation of the seed with peak values in the resected tissue could be identified using a gamma probe. Intraoperative X-ray of the specimen verified complete removal of the seed (FIGURE 2).
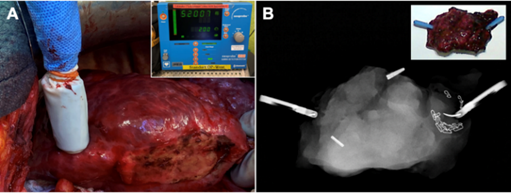
Figure 2: (A) Intraoperative localization of the 125I seeds was achieved with a handheld gamma radiation probe. Peak values of approximately 52000 units could be detected. (B) Intraoperative X-ray of the specimen confirmed extraction of both radioactive seeds, which had been implanted before surgery.
Nevertheless, pathologic examination again did not reveal any signs of malignancy in the sample. Possible displacement of the radioactive seed due to technical difficulties and the inherent problems of landmark-guided interventions was suspected during the reevaluation of the case with the interventional radiologist. Two days later, the procedure was repeated. This time, precise localization and surgical removal of the tumor could be achieved by using late arterial phase in contrast-enhanced CT for guiding the seed’s implantation (FIGURE 3).

Figure 3: Multiphasic CT-guided implantation of a 125I seed. (A) Native CT. (B) Arterial phase with hyperdensity of the lesion in liver segment VII (arrow). (C) Washout is seen during portal phase (arrow). (D) Implanted seed (arrow).
Histological workup showed a combined cholangio-hepatocellular carcinoma (cHCC-CC) with cholangiocellular stem cell features, which represents a rare tumor entity and, therefore, is not part of standard diagnostic regimens in most hospitals [16,17]. After an uneventful postoperative clinical course, the patient was discharged one week later.
Outcome and follow-up
Postoperative discussion of the case in the multidisciplinary tumor board led to the suggestion of regular tumor follow-up by scheduled clinical examination and liver MRI every six months. The patient recuperated very well from surgery. Upon discharge from the hospital, the patient started intensified outpatient physiotherapy and was admitted to an oncologic rehabilitation facility two months later. Here, the patient mainly worked on improving his general fitness and reducing the risk of falling due to his sensory gait abnormality.
Case Presentation 2
The second patient who underwent radioactive seed labelling of a liver tumor was a 77-year-old male referred to the Clarunis Center in January 2021 for further investigation of a suspected HCC. In a follow-up ultrasound during of a possible liver cirrhosis and advanced liver fibrosis, detected in 2017, two focal intrahepatic lesions were found. An MRI in December 2020 emphasized the suspicion of a possible HCC in segment VII with a diameter of 19 mm.
Additionally, the patient had a history of alcohol abuse for several years, oesophagal varices graded I, arterial hypertension, obesity, hyperferritinemia without genetic evidence of hemochromatosis, bullous pemphigoids and had undergone a hernia repair.
Diagnostic access
Due to the suspected liver cirrhosis, an ultrasound with contrast medium as well as a second liver biopsy were performed. The ultrasound showed a hypoechogenic lesion without contrast enhancement, which was hyperintense in the arterial phase and isointense in the portal venous phase, but no wash-out. Liver biopsy showed a chronic sclerosing steatohepatitis and clear signs of fibrosis, which was compatible with alcoholic steatohepatitis (ASH) or non-alcoholic steatohepatitis (NASH). The steatosis and fibrosis had increased compared to the last biopsy. A lesion biopsy could not be performed because of its localization and difficulties with visualization.
Levels of The patient was presented at the multidisciplinary tumor board in February 2021. Primary resection of the lesion was recommended with a preoperative localization using a radioactive seed.
Additionally, a CT scan of the abdomen and pelvis with contrast medium was performed, which showed the lesion in segment VII with a size of 1.6x1.1 cm (LIRADS 4 in synopsis with the MRI), signs of liver cirrhosis, a splenomegaly, no enlarged lymph nodes and a normal liver vessel anatomy with plaques in the coeliac artery.
Treatment
Due to our experience in the first case and the even more difficult anatomical situation for an image-guided puncture, we decided to used Lipiodol-CT as a well approved tool to visualize HCC lesions in CT independently from the contrast agent application also during CT fluoroscopy [18].
The patient underwent a transarterial chemoembolization (TACE) with application of Doxorubicin/Lipiodol, which was afterwards controlled with a DynaCT. After the injection, two lesions were visible, one in segment IV/V and one in segment VII/VIII. Two Iodine-125 seeds were placed under CT guidance the following day, one into each lesion.
The patient underwent atypical resection of liver segments IV/VIII, segment VII and a simultaneous cholecystectomy on the same day. The seeds could be easily located intraoperatively with the gamma probe and ultrasound. Resection was performed using CUSA, bipolar device and clip ligation. Both seeds were present in the 2D and 3D radiography. A blood loss of 450 ml occurred and initially the patient needed 15 mcg/min norepinephrine. Furthermore, creatinin was 143 umol/l postoperatively but regressed under fluid substitution.
Postoperatively, the patient was transferred to the ICU. The day after the surgical intervention, the patient showed sudden signs of a sinus bradycardia which subsequently aggravated into a pulseless electric activity. Successful reanimation of 4 minutes was performed. The additional workup was unremarkable except for a transiently raised troponin level (peak 73 ng/l). There were no additional abnormalities, and the patient was transferred to the surgical ward in a stable state after two days. Ultrasound and echocardiography showed no signs of right heart strain or acute heart ischemia.
Over the course of the hospitalization, the patient showed signs of paralytic ileus, which was confirmed by abdominal x-ray and successfully treated conservatively with placement of a nasogastric tube.
On the seventh postoperative day, the patient developed a cough and increasing inflammatory levels. A chest x-ray showed an infiltrate in the left lower lobe. Antibiotic treatment with intravenous Piperacillin/Tazobactam was initiated for suspected hospital acquired pneumonia. After his symptoms resolved and the inflammatory parameters decreased, antibiotic treatment was switched to oral medication and continued for a total duration of 14 days. Due to presence of ascites, a diuretic treatment with Spironolactone was added. The intraoperatively placed drains were removed promptly. The gastric tube was removed on day 7 after surgery.
Histologically, a poorly differentiated CC with a size of 17 mm was found in segment IV/VIII, corresponding to TNM stage pT1a, pNx, (V0, L0), G3, and R0.
Segment VII showed no tumor but a regenerative nodule of 3mm. Furthermore, the liver parenchyma showed incomplete cirrhotic remodeling.
Outcome and follow-up
In the postoperative multidisciplinary tumor board, an adjuvant chemotherapy with capecitabine was recommended.
11 days after the surgery, the patient could be discharged home in good general condition. The patient underwent eight cycles of chemotherapy with capecitabine, the dosage of which had to be reduced due to the appearance of a hand-foot syndrome in cycle eight. At six months' follow-up, the patient presented in good overall condition, and the MRI showed no signs of tumor recurrence.
Intraoperative identification of CC and HCC can be difficult, especially in cirrhotic liver tissue. The difficulties in intraoperative tumor identification encountered in the first case could be due to the relatively small tumor size as well as the cirrhotic liver parenchyma remodeling, which is rare in the case of CC [5,8].
In intraoperative ultrasound, regenerative nodules can be mistaken for HCC, as both can present as iso- to slightly hypoechoic lesions in B-mode sonography [19]. Intraoperative application of contrast-enhanced ultrasound (CEUS), which is not a standard procedure in our hospital, might have been helpful in these cases, since regenerative nodules show isoenhancement in arterial and portal/late-phases [19].
In contrast, HCC typically shows hyperenhancement in arterial phase and washout/hypo enhancement during portal phases [20]. However, it should be noted that well differentiated HCC can also present as hypo vascular lesions in B-mode, retaining hypoechoic behavior in arterial phase and turning isoechoic in portal phase [21]. However, the possibility of definite identification of the first patient’s lesions by intraoperative ultrasound remains questionable, considering the final pathological diagnosis of cHCC-CC. These rare carcinomas appear mostly non-specific as a hypoechoic lesion in ultrasound examination, and may resemble a regenerative nodule [17]. Considering the sometimes-overlapping appearances of primary tumors and regenerative nodule of the liver, intraoperative employment of CEUS could have been an asset for the treatment of our two patients. Still, it would not have guaranteed successful tumor resection in our first case. A hybrid operating theater with an MRI system could overcome those difficulties and would have been of great benefit, but is not currently available in our hospital.
Being an established method in breast surgery and often used in our hospital in this context, implantation of an Iodine-125 seed to label the lesions was conducted under native CT guidance [9,10]. In the first patient, it remains elusive why the seed implantation based on anatomical landmarks went wrong, since this technique is also quite approved and reliable for diagnostic CT-guided biopsies of liver tumors, which cannot be visualized or accessed by ultrasound-guided measures. Taking the successful second attempt of seed implantation into account, general usage of contrast-enhanced CT or even better Lipiodol-CT as in the second case seems advisable for CT-guided interventional labelling of the tumor before surgery, due to the more serious consequences of misplacement (as compared to a diagnostic liver biopsy). However, the risk of the Lipiodol TACE has to be weighed against the probability of seed misplacement.
To implement this method for tumor localization into daily clinical practice, certain requirements regarding radiation protection have to be fulfilled, depending on the local regulatory obligations. In Switzerland, complete seed extraction has to be confirmed intraoperatively by specimen radiography and by ensuring no residual source of radiation is traceable in the patient with the gamma probe system. However, given proper seed placement, the tumor detection by handheld gamma probe system and its resection are straightforward. Thus, this method could represent a feasible way of preoperative tumor labelling in cirrhotic liver patients.
Radioactive seed-guided resection can be an asset to intraoperative localization and successful resection of suspected malignant lesions in cirrhotic liver tissue.
For preoperative radioactive seed marking of primary liver tumors, localization based solely on anatomical landmarks should not be performed, but instead at least multiphasic CT or even Lipiodol CT be used to target the lesion for the puncture clearly.
Usage of contrast enhanced ultrasound (CEUS) can be helpful for tumor localization in cirrhotic liver tissue. Further studies are needed to analyze its possible benefit in an intraoperative setting.
Ethics approval and consent to participate
The study was approved by the local ethics committee (ref. no 2019-02118) and conducted according to the Swiss federal act on research involving human beings (Human Research Act, HRA) and the guidelines of good clinical practice (GCP).
Consent of both patients was obtained.
Each author has completed the Conflicts of Interest Disclosure Statement, which are uploaded together with the manuscript. There is no conflict of interest.
No funding was used.
Authors contributions
Concept: PD Dr. med. Savas D. Soysal, Prof. Dr. med. Otto Kollmar
Supervision: PD Dr. med. Savas D. Soysal, Prof. Dr. med. Otto Kollmar
Writing: Noa Lina Eleni Aegerter, Dr. med Georg Henniger
Literature search: Noa Lina Eleni Aegerter, Dr. med Georg Henniger
Data collection: Noa Lina Eleni Aegerter
Critical review: Dr. med. Georg Henniger, Dr. med. Gabriel F. Hess, Prof. Dr. Alexandar Tzankov, PD Dr. med. Simone Muenst, Dr. med Noemi Schmidt, Prof. Dr. med. Christoph J. Zech, Prof. Dr. med. Otto Kollmar, PD Dr. med. Savas D. Soysal
Availability of data and materials
All data was collected of the clinical documentation system ISMED, which is used at the University hospital of Basel. Further inquiries can be directed to the corresponding author.
Acknowledgements
We thank Dr. med. Philipp Sedlaczek (Clarunis Clarunis, University Centre for Gastrointestinal and Liver Diseases, 4002, Basel, Switzerland who has presented our work at the Deutscher Krebskongress in February 2024.