AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2640-1053/149
Riggs pharmaceutical University of Karachi Pakistan.
*Corresponding Author: Rehan Haider, Riggs pharmaceutical University of Karachi Pakistan.
Citation: Rehan Haider, (2023), Wilson's Disease, J. Cancer Research and Cellular Therapeutics, 7(3); DOI:10.31579/2640-1053/149
Copyright: © 2023, Rehan Haider. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 12 June 2023 | Accepted: 20 June 2023 | Published: 30 June 2023
Keywords: ceruloplasmin; copper metabolism; diagnosis; wilson's disease
In 1912, Kinnear Wilson was the first to describe an inherited fatal disease associated with progressive lenticular degeneration, chronic liver disease and cirrhosis (Wilson 1912) [1]. In the same year, Kayser and Fleischer found that patients with Wilson's disease (WD) often had brownish deposits of copper on the cornea, now called Kayser-Fleischer rings (Fleischer 1912) [2]. WD is an autosomal recessive metabolic disorder. Its ATP7B gene encodes a copper-transporting ATPase (Bull 1993, Tanzi 1993, Petrukhin 1993, Yamaguchi 1993) [3,4,5,6]. A genetic defect in the ATP7B protein reduces Biliary excretion of copper leads to accumulation of copper in the cornea and various organs, including the liver, brain, and kidneys. Alteration of the ATP7B protein also reduces copper incorporation into ceruloplasmin. The corresponding presence of ceruloplasmin APO (ceruloplasmin without copper incorporation) results in decreased circulating levels of ceruloplasmin due to the shortened half-life of the APO protein. Thus, despite the accumulation of copper in many organs, circulating levels of copper and ceruloplasmin are reduced in most patients with WD. The prevalence of WD is rare, estimated at 3 per 100,000 in the general population (Friedman 1990) [7]. The clinical picture may vary. Some patients with WD are diagnosed with liver problems, while others have neurological or psychiatric symptoms; many patients show both liver and neurologic disease (Figure 1). Episodes of hemolysis and renal abnormalities may also occur. WD typically affects children and younger. Adults and is rarely seen in adults over 40 years of age. WD is fatal if not treated appropriately. Medications to treat WD are copper chelators. such as penicillamine and trientine (Walshe 1956) [8]. Lately, zinc has been. it is used to reduce the intestinal absorption of copper and to detoxify free circulating copper. Patients with fulminant liver failure or decompensated cirrhosis. may need a liver transplant (LTX) to cure WD
Medical photograph Screening for WD is useful simplest in households with an affected member. In all other circumstances, diagnostic techniques are done only while signs and findings are suggestive of WD. these include liver sickness, neurological signs and symptoms, renal abnormalities, and episodes of hemolysis. WD is identified in the giant majority of sufferers among the ages of five and 35. There are rare reports of patients diagnosed at 3–5 years of age (Kalac 1993, Wilson [9,10]. and as much as approximately 60 years of age (Gow 2000) [11]. late-onset WD is a frequently ignored situation (Ferenci, 2007) [12]. Diagnostic workup does not rely upon a single check, but consists of identification of Kayser Fleischer corneal earrings, reduced serum ceruloplasmin and copper, in addition to quantitative willpower of liver copper concentration (Scheinberg 1952, Walshe 1956, Saito 1987, Stremmel 1991, Roberts 2003). [13,14,15,16,17]. (Parent 2) Genetic testing is generally executed only in spouse and children of sufferers with confirmed WD. it is easy to diagnose WD in subjects with liver cirrhosis, usual neurological manifestations, and Kayser-Fleischer jewelry; lots of these patients’ gift between the whole of 5 and 35 and feature decreased serum copper and ceruloplasmin (Sternlieb 1990). however, a substantial range of patients with WD have only liver disease and might not have Kayser-Fleischer jewelry or decreased serum ceruloplasmin stages (Steindl 1997) [18]. In these instances, the prognosis can be difficult; the dimension of 24-hour urinary copper excretion often helps to support the suspicion of WD. A liver biopsy with measurement of quantitative copper attention must be executed to confirm the diagnosis (Stremmel 1991, Roberts 2003) [19,20]. In general, WD patients identified with liver disease are children and adolescents and are more youthful than the ones identified with neurological signs (Merle 2007) [21]. Many patients who have the most effective CNS signs and symptoms are in their 20s-40s. patients with WD may have a wide spectrum of liver sickness, ranging from asymptomatic elevations of serum aminotransferases to fulminant liver failure. Serum aminotransferase is increased in maximum patients with WD no matter age (Schilksky 1991) [22]. different sufferers with WD may additionally have findings and signs of autoimmune hepatitis, inclusive of autoimmune antibodies and improved IgG (Scott 1978, Milkiewicz, 2000) [23,24]. The scientific photograph may additionally resemble acute or continual viral hepatitis, without viral serum. markers. Even liver histology is not predictive or typical of WD until copper awareness is measured. Histological findings can range from fatty liver changes to excessive necro-inflammatory and fibrotic disorder and entire cirrhosis. especially, youngsters and teens with persistent energetic hepatitis of unknown etiology or autoimmune hepatitis and grownup sufferers with suspected autoimmune hepatitis or unresponsive to immuno suppressants need to be evaluated for WD (Roberts, 2003) [25]. WD ought to be dominated out in patients with fulminant liver failure of unknown etiology, mainly underneath the age of 35; WD sufferers with this presentation typically have some type of liver disease (Rector 1984, Ferlan Maroult 1999, Roberts 2003) [26,27]. related to Coombs-bad hemolysis. Anemia and markedly prolonged for thrombin time, unresponsive to vitamin ok, and innovative renal failure (Sallie 1992) [28]. some sufferers have bilirubin ranges of more than forty mg/dL, while serum alkaline phosphatase is regular or only mildly accelerated (Berman, 1991) [29]. In contrast to many kinds of toxic liver failure, liver failure in WD does now not commonly start with an excessive elevation of aminotransferase. In lots of sufferers with WD, AST degrees exceed ALT ranges (Emre 2001, Berman 1991) [30]. In most cohorts, the female-to-male ratio is about 2:1 for unexplained motives (Roberts, 2003). Serum ceruloplasmin can be reduced, even as serum copper and 24-hour urinary copper excretion are typically accelerated. it is very helpful if Kayser-Fleischer rings may be identified in this example; these patients must be tested with a slit lamp by an experienced ophthalmologist. patients with acute liver failure need diagnostic workup as soon as viable; if there is a robust suspicion or diagnosis of WD, the patient should be transferred to a transplant center on the same day. Neurological signs and symptoms in WD frequently resemble those visible in Parkinson's ailment, together with tremors and tension. Many sufferers record these symptoms. begin handwriting issues and dysarthria. Neurological signs may be related to moderate behavioral modifications, which can later progress to overt psychiatric contamination and melancholy, anxiety, and psychosis. As CNS involvement progresses, sufferers might also expand their WD
seizures and pseudobulbar palsy related to excessive dysphagia, aspiration, and pneumonia. despite the fact that many older WD patients present with neurological illness, the diagnostic workup frequently suggests huge liver involvement or maybe whole liver cirrhosis. Renal involvement of WD also can be gifted with aminoaciduria and nephrolithiasis (Aziza 1989, Nakada 1994, Chu 1996) [31,32,33]. There can be numerous non-neurological and. non-hepatic headaches of WD, including osteoporosis and arthritis cardiomyopathy, pancreatitis, hyperparathyroidism, and miscarriages. Kayser-Fleischer earrings are caused by corneal copper deposition (determine. 3). Once in a while, you may see the rings right now as a band of brown pigment near the limbus. In one-of-a-kind sufferers, the ring can pleasantly be identified by using a slit lamp. Very not often similar rings may be seen in non-WD sufferers.g., in a few patients with neonatal or chronic cholestasis (Tauber, 1993) [34]. Kayser-Fleischer earrings are detectable.in 50–60% of WD patients in most massive cohorts (Tauber, 1993, Roberts, 2003). Many young WD patients with liver sickness do not have such earrings (Giacchino, 1997) [35]. at the same time as nearly all patients within maximum cases neurologic signs do have them (Steindl 1997). WD patients may additionally produce other a whole lot much less specific eye modification, collectively. sunflower cataracts (Cairns 1963) [36]. Kayser-Fleischer earrings generally regress. chelation remedy or after LTX (Stremmel 1991, Schilksky 1994). diagnosis of Wilson's sickness may be hard. Consequently, a scoring gadget has. bean installed (Ferenci 2003) (desk 1), which is now encouraged with the aid of cutting-edge EASL suggestions (EASL 2012)
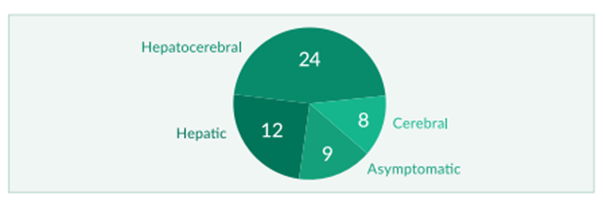
Figure 1: Clinical course of WD in 53 patients (modified from Stremmel 1991)
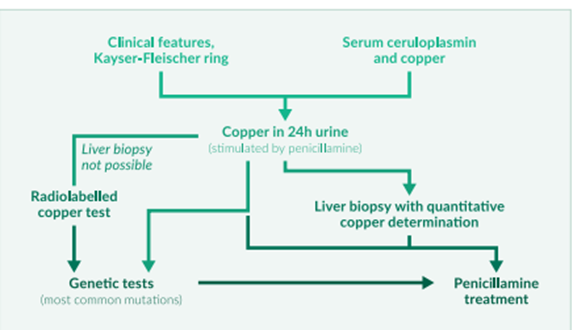
Figure 2: Diagnostic workup
Ceruloplasmin, the primary circulating copper transporter, is synthesized and secreted through hepatocytes. The 132 kg protein includes six copper atoms in line with. Molecule of Ceruloplasmin (holo ceruloplasmin) while the last part of the Protein does now not convey copper (ceruloplasmin). Ceruloplasmin acts as an acute. phase reactant and might therefore be increased through any inflammatory system it is able to additionally upward push in pregnancy and with the use of estrogens and oral contraceptives. One also desires to understand that the ordinary variety of serum ceruloplasmin is age-dependent: it is also low in toddlers until 6 months; in older kids, it can be better than in adults. As defined previously, serum degrees of. Ceruloplasmin is typically decreased in WD; however, this locating by myself is unreliable due to the fact low serum ceruloplasmin can be seen without WD and serum ceruloplasmin may also even be expanded in extreme WD and liver failure. Non-specific discounts of ceruloplasmin are commonly related. with protein deficiency or any end-level liver sickness. long-term parenteral vitamins may additionally lead to decreased levels of ceruloplasmin. Low serum ceruloplasmin is likewise an indicator of Menkes' ailment, a very rare X-linked inborn mistake of metabolism that results in an illness in copper transport due to mutations in ATP7A (Menkes 1999) [37]. Very not often, one can't measure serum. ceruloplasmin at all. This ceruloplasmin is a very rare genetic ailment. resulting from mutations inside the ceruloplasmin gene; but, patients with ceruloplasmin broaden iron and not copper overload (Harris 1998) [38]. maximum patients with WD have serum ceruloplasmin decrease than 20 μg/dl; this locating is diagnostic for WD, but, only when there are other findings, including a Kayser Fleischer corneal ring. in one potential screening examination, ceruloplasmin becomes. measured in 2867 patients presenting with liver disease, the best 17 of them had decreased ceruloplasmin tiers, and the most effective 1 of those subjects had WD (Cazuza 1997) [39]. As a consequence of deceased ceruloplasmin had a nice predictive fee of the best 6% inside the 2867 patients tested. In cohorts, approximately 20% of WD had everyday ceruloplasmin and no. Kayser-Fleischer rings (Steindl 1997, Gow 2000). most reports, but, show that more than 90% of WD patients have reduced serum ceruloplasmin (Walshe 1989, Lau 1990, Stremmel 1991) [40,41]. size of ceruloplasmin as an available marker can't reliably differentiate homo zygotes from heterozygote Serum copper corresponding to the lower serum ceruloplasmin, general serum copper is also normally decreased in WD. much like the diagnostic troubles in interpreting ceruloplasmin facts in WD patients with fulminant liver failure, serum copper can also, be every day in this case–even though serum ceruloplasmin is decreased. In acute liver failure, circulating copper can be accelerated due to the fact it's far hugely released from Injured hepatocytes. If ceruloplasmin is decreased, a regular or multiplied serum copper. typically suggests that there is a growth in free serum copper (not bound to ceruloplasmin). The free copper attention is calculated from the total copper. and ceruloplasmin values have also been proposed as a diagnostic check and for tracking WD. Its miles extended above 25 μg/dl in maximum untreated patients. (Everyday values are underneath 10–15 μg/dl). the amount of copper associated. With ceruloplasmin is three.15 μg of copper in keeping with mg of ceruloplasmin. thus, unfastened copper is the difference between the total serum copper in μg/dl and three times the ceruloplasmin concentration in mg/dl (Roberts 1998) [42]. increases in serum-unfastened but, copper isn't specific for WD and may be visible in all types of acute liver failure, in addition to marked cholestasis (Gross 1985, Martins 1992) [43, 44]. As a result, serum copper isn't endorsed as a primary device for the prognosis of Wilson's disorder (Ferenci 2003, EASL 2012) (desk 2) [45]. however, serum copper remains encouraged as a tool. reveal treatment (EASL 2012) (desk 3). Calculation of loose copper concentration relies upon seriously on the adequacy of the techniques used to degree total serum copper and ceruloplasmin; laboratories regularly honestly file that one of the checks is under a positive fee, making it not possible to calculate the quantity of unfastened copper. Urinary copper excretion Most sufferers with WD have multiplied urinary copper excretion above a hundred μg/24 hours, which is taken into consideration to be a boom in circulating unfastened copper (not sure to ceruloplasmin). some studies suggest that about 20% of sufferers with WD may additionally have 24-hour urinary copper excretion between 40 and one hundred μg/24 h (Steindl 1997, Giacchino 1997, Gow 2000, Roberts 2003). but, some increases in urinary copper excretion may be in severe cholestasis, continual active hepatitis, and autoimmune hepatitis (Frommer, 1981) [46]. It has. Bean suggested that penicillamine-inspired urinary copper excretion can be greater beneficial than a non-stimulation take a look at. In children, 500 mg of oral penicillamine is typically given to begin with after which 12 hours later all through a 24-hour urine series. all the WD children they looked at had higher tiers. 1600 μg copper/24 h and all sufferers with other liver diseases, such as autoimmune hepatitis and cholestatic liver ailment, had decreased values. It isn't. It is unclear whether or not this check has a similar discriminating strength in adults, where it has been utilized in numerous changes (Tu 1967, Frommer 1981) [47].
Hepatic copper awareness Liver copper content material above 250 μg/g liver dry weight remains the gold popular for diagnosing WD and is not seen in heterozygotes or different liver illnesses besides Indian formative years cirrhosis (Martins 1992) [48]. Biopsies (greater than 1 cm in period) for the size of liver copper determinations ought to be interested in a disposable Tru-reduce needle, positioned dry in a copper-free container, and shipped frozen (track 2000, Roberts 2003) [49]. Radiolabelled copper in WD, the incorporation of radiolabelled copper into ceruloplasmin is notably reduced. This takes a look at what is rarely used because of its problem. acquisition of the isotope and because of legal restrictions. Liver Biopsy Findings Histological findings in WD variety from some steatosis and hepatocellular necrosis to the image visible in intense autoimmune hepatitis with fibrosis and cirrhosis. patients identified at a younger age commonly have the extensive liver disorder; aged sufferers presenting with neurological signs and symptoms for the primary time often also have liver biopsy abnormalities (Stemmel 1991, Steindl 1997, Merle 2007) [50]. Detection of copper in hepatocytes, e.g., by way of staining. rhodamine does now not allow the prognosis of WD by recurring histochemistry (Geller 2000) (discern 4) [51]. Neurology and CNS MRI. Neurological signs in WD consist of Parkinsonian-like abnormalities with pressure, tremors, and dysarthria. In more significantly affected patients. Muscle spasms, contractures, dysphoria, and dysphagia may additionally occur. In patients with huge neurological signs, magnetic resonance imaging (MRI)often identifies abnormalities in basal ganglia, such as hyper intensity on T2-weighted imaging (Aisen 1995, van Wassenaer 1996) [52]. MRI of the CNS is superior to computed tomography to diagnose WD.

Figure Kayser-Fleischer ring in a patient with WD
Genetic studies. using mutation analysis in WD is limited because more than 200 ATP7B mutations have been described (www.medgen.med.ualberta. Ca/database.html). when the mutation is thought in a specific patient, gene. the evaluation may be useful for the circle of relatives screening or prenatal evaluation (Thomas 1995, Shah AB 1997, Illinois 1994) [53,54]. A few populations in Japanese Europe display a predominance of the H1069Q mutation. Recently, genetic evaluation is suggested as a primary tool for the analysis of Wilson's ailment (Ferenci 2003, EASL 2012) (desk2).
Treatment earlier than 1948, all patients with Wilson's ailment died rapidly after diagnosis. In 1948, intramuscular administration of the copper chelator BAL (dimercaprol) turned into introduced because the first treatment of WD (Cumming 1951, Denny-Brown 1951) [55, 56]. accompanied through the oral chelator penicillamine (1955), trientine (1969), and tetrathiomolybdate (1984). other treatment modalities include oral zinc salts (1961) and liver transplantation (1982). nowadays, maximum sufferers with WD continue to be on a lifelong pharmacologic remedy, commonly inclusive of a copper chelator and/or a zinc salt (parent 5). LTX is reserved for fulminant. liver failure and irreversible decompensation of liver cirrhosis. patients with a hit LTX no longer want WD remedy due to the fact LTX heals the biochemical defect. today, most physicians use oral chelators for the preliminary. remedy for symptomatic sufferers; many physicians start with Penicillamine, while some choose trientine. both drugs are in all likelihood equal. effective, with trientine having fewer aspect results. In sufferers with advanced neurological sickness, a few authors propose tetrathiomolybdate for primary therapy. mixture remedy
chelators and zinc salts can have additive consequences, affecting both urinary copper excretion and intestinal absorption. After removing a maximum of the gathered copper and regression of the most excessive clinical troubles, the chelator dose may be decreased and later replaced with zinc. Asymptomatic patients can be, to begin with, handled with a low-dose chelator or zinc salt. Compliance troubles had been shown to frequently cause recurrence of symptomatic WD and can result in fulminant liver failure, the need for LTX, or death. current EASL hints summarize the suggestions for the control of Wilson's ailment (EASL 2012) (table 3). Penicillamine. Penicillamine was the first oral copper chelator shown to be effective in WD (Walshe, 1955) [57]. the whole bioavailability of oral penicillamine stages among 40% and 70% (Bergstrom 1981) [58]. Many studies have proven this. penicillamine reduces copper accumulation and affords clinical benefit in WD (Walshe 1973, Grand 1975, Sternlieb 1980) [59,60, 61]. symptoms of the liver ailment are frequently remedied within the first 6 months of treatment. A non-compliance has happened. it's been proven to cause the development of liver ailment, liver failure, death, and LTX (Scheinberg 1987). but neurological signs may worsen. initiation of penicillamine treatment, it stays questionable how regularly this neurological deterioration takes place and whether or not it's far reversible; prices of neurological deterioration range from 10-50% in one-of-a-kind cohorts (Brewer 1987, Walshe 1993) [62]. A few authors even recommend now not the usage of penicillamine. In WD patients with neurological sickness (Brewer 2006). Penicillamine is related to many unfavorable results that result in its discontinuation in up to 30% of patients (for literature, see Roberts 2003). An early hypersensitivity response may also arise during the first three weeks, such as fever, skin rash, lymphadenopathy, neutropenia, thrombocytopenia, and proteinuria. Penicillamine has to be substituted for such early sensitivity. straight away using trientine. every other commonplace facet effect is nephrotoxicity. of penicillamine, which seems later and includes proteinuria and signs of tubular damage. In this situation, penicillamine ought to be instantaneous. Penicillamine can also cause a lupus-like syndrome with hematuria, proteinuria, superb antinuclear antibodies, and appropriate grazing syndrome. extra hardly ever, the drug can damage the conduction of the bone marrow. to thrombocytopenia or preferred aplasia. Dermatological aspect results include a desire for elastosis Serpiginous, pemphigoid lesions, lichen planus, and aphthous stomatitis. cases of myasthenia gravis, polymyositis, loss of taste, reduced IgA, and excessive retinitis have also been pronounced because of the management of penicillamine. To decrease the side effects of penicillamine, it must be started at a dose of 250 mg every day; the dose may be accelerated by way of 250 mg each week to a maximum each day quantity of one thousand to 1500 mg given in 2 to four divided doses in keeping with day (Roberts 2003). maintenance doses vary from 750 to one thousand mg/day, given in 2 divided doses. For children, the dose is 20 mg/kg/day divided into 2 or three parts. doses. Penicillamine must be administered 1 hour earlier than or 2 hours after a meal. because meals can hinder its absorption. After initiation of penicillamine remedy, serum ceruloplasmin may also initially decrease. The fulfillment of the remedy is monitored. a 24-hour urine copper dimension that should be between two hundred and 500 μg/day. In the long term, ceruloplasmin and loss of copper have to increase. regression to regular with penicillamine remedy (Roberts, 2003). Trientine (triene). The chemical structure of the copper-chelating trientine (triethylenetetramine dihydrochloride, AKA triene) differs from that of penicillamine. Trientine became normally used as an opportunity or opportunity for penicillamine, especially when the primary factor effects of penicillamine are not tolerable (Walshe, 1982) [63]. Trien rarely has any results. like penicillamine, the long-term trientine drug can also cause iron to accumulate in the liver in people with WD. Trientine is poorly absorbed from the gastrointestinal tract and a large 1% appears in the urine (Walshe 1982). Various doses from 750 to 1500 mg/day are given in 2 or 3 divided doses; For protective treatment, 750 or 1000 mg is given (Roberts 2003). A 20 mg/kg/day dose is usually recommended for domestic puppies. people. like penicillamine, trientine must be taken 1 hour before or 2 hours after a meal. The overall efficiency of copper chelation using the triene is measured as defined. penicillamine. Triene chelates several metals along with copper, zinc, and iron through urinary excretion and effectively removes accumulated copper from many organs in people with WD beyond moderate liver disease (Walshe 1979, Scheinberg 1987, Santos 1996, Saito 1991) [64, 65, 66, 67]. but it is miles, extremely debatable whether penicillamine is an extra effective copper chelator compared to triene; probably the difference in effectiveness is modest (Walshe 1973, Sarkar 1977) [68]. Worsening of neurologic disability may also be determined after initiation of triene therapy; worsening is much less common and much less suggested than after initiation of penicillamine therapy. Zinc. docs maximally update penicillamine or triene with zinc as a preservative while removing maximal copper accumulation. Zinc can also be transported as a pro drug in asymptomatic patients who were subsequently identified in accordance with their circle of relatives. but the current document lists the signs and symptoms of WD. it can occur regardless of zinc prophylaxis in asymptomatic patients (Mishra, 2008) [69] By the present review from India, there were 45 affected WD on each penicillamine and zinc sulfate. the maximum number of patients (84%) had Neuropsychiatric manifestations. the same old duration of correction with penicillamine and zinc before discontinuation of penicillamine changed to 107 months. All sufferers had to avoid penicillamine because of the financial burden. the afflicted then received zinc sulfate for 27 months and destroyed it for 44 months. Of the forty-five affected (90-8%), they remained robust. The simplest disabled person qualified for worsening dysarthria (Sinha 2008) {70}. Zinc now no longer acts as an iron chelator, but inhibits the gut. absorption of copper and is also thought to bind unfixed toxic copper (Brewer 1983, Schilksky 1989, Hill 1987). Zinc has almost no results. but it is uncertain whether zinc as monotherapy is an effective "interpreter" in symptomatic patients. there are many suggestions that copper may additionally accumulate in the liver regardless of zinc treatment, along with suggestions of fatal liver damage (Lang 1993, Walshe 1995). therefore, many authors use zinc mixed with a chelator. Neurological deterioration is uncommon with zinc (Brewer 1987, Czlonkowska 1996). approved doses of zinc vary in the literature: according to AASLD practice, the dosage is in milligrams of elemental zinc (Roberts, 2003). Older children and adults are given 100 and 50 mg/day in divided doses. Adherence to doses several times a day can be difficult; zinc wishes to be considered a minimum of two cases during the afternoon to be potent (Brewer 1998). various authors recommend using zinc sulfate 3 times in the afternoon as a loading dose of 10 mg and for maintenance treatment 10 mg 3 times in the afternoon. numerous boards approve the administration of 50 mg of zinc. acetate 3 times in the afternoon in adults. The form of zinc salt used was changed to match the difference in typical overall performance (Roberts, 2003). but zinc acetate modified to. In addition, it is recommended to motivate to the least possible pain in the digestive tract. while zinc binds with a chelator, the substances should be given at widely spaced intervals, potentially causing compliance problems. The effectiveness of the zinc treatment should be checked as described for penicillamine and zinc (Roberts 2003). Tetrathiomolybdate. Tetrathiomolybdate is an experimental copper Chelator not approved by FDA or EMA. It has been suggested as the initial. treatment of WD patients with neurological involvement. Early reports say. that tetrathiomolybdate stabilizes neurological disease and reduces circulating free copper in a matter of weeks (Brewer 1994, Brewer 1996). A more recent randomized study supports this view and suggests that zinc monotherapy is insufficient for treating neurological WD (Brewer2006). Vitamin E, other antioxidants, and diet. Since serum and hepatic concentrations of vitamin E levels may be reduced in WD (von Herbay 1994, Sokol 1994). It has been. Suggested to complement vitamin E intake. Some authors have also recommended it. taking other antioxidants; studies have not proven their effectiveness as yet. WD patients should avoid food with high copper content (nuts, chocolate, shellfish, mushrooms, organ meats, etc). Patients living in older buildings Should also check whether the water runs through copper pipes. Such dietary and lifestyle restrictions do not replace chelator or zinc therapy (Roberts 2003). Fulminant hepatic failure and LTX. Most WD patients with fulminant liver failure need LTX urgently to survive (Sokol 1985, Roberts 2003). However, in a long-term cohort study, only two patients died before LTX, being available (Stremmel 1991). It is a difficult clinical question whether WD patients are involved. Liver failure can survive without LTX. The prognostic score is used to help with this. The difficult decision includes bilirubin, AST, and INR (Nazer 1986). In any case, WD patients with signs of fulminant liver failure need to be transferred immediately (same day!) to a transplant center. WD patients with a chronic course of decompensated cirrhosis follow the usual rules for LTX. LTX cures metabolic defects and thus copper. metabolism returns to normal afterward (Groth 1973). The prognosis for WD after LTX is excellent, in particular when patients survive the first year (Eghtesad 1999). It is still unclear under which circumstances LTX may be. helpful for WD patients with neurological complications, which do not respond to drug therapy. In some patients, CNS symptoms regress after LTX. while other patients do not improve (for literature, see Brewer, 2000). Asymptomatic Patients. All asymptomatic WD subjects – usually identified by family screening–need to be treated by chelators or zinc to prevent life-threatening complications (Walshe, 1988, Brewer, 1989, Roberts 2003). It is unclear whether therapy should begin in children under. the age of 3 years. Maintenance Therapy. After the initial removal of excessive copper by chelators, some centers replace the chelators with zinc for maintenance therapy. It is unclear when such change is advisable and whether it might. be better to reduce the dose of chelators instead of replacing them with zinc. It is generally accepted that replacement of chelators with zinc should only be done in patients who are clinically stable for some years, have normal aminotransferase and liver function, have a normal free copper concentration, and a 24-hr urinary copper repeatedly in the range of 200–500 μg while on chelators (Roberts 2003). Long-term treatment with zinc may be associated. with fewer side effects than chelator treatment. Many patients are on trientine, however, have significant side effects, and this author believes one needs to replace trientine with zinc in such patients. In any case, therapy Either with a chelator or with zinc needs to be maintained indefinitely; any
interruption may lead to lethal liver failure (Walshe 1986, Scheinberg 1987). Pregnancy. Treatment must be maintained during pregnancy because an interruption has been shown to carry a high risk of fulminant liver failure (Shimono 1991). Maintenance therapy with chelators (penicillamine, trientine) or with zinc usually results in a good outcome for mother and child, although birth defects have (rarely) been documented (for literature See Sternlieb 2000). It is recommended that the doses of both chelators be. reduced, if possible, by about 50%, in particular during the last trimester to avoid potential problems in wound healing (Roberts, 2003). Zinc does not. need to be reduced. Monitoring of Treatment Monitoring should be done closely during initial treatment in all WD patients to look for efficacy (Table 4) and side effects. During the maintenance phase, patients should be checked at least twice a year. Clinical examinations include. neurological, ophthalmologic, and psychiatric consultations. Patients with liver involvement need to be checked carefully for signs of liver failure. Laboratory tests include measurements of serum copper and ceruloplasmin, calculation of free (non-ceruloplasmin-bound) copper, and 24-hr urinary copper excretion (Roberts 2003). While on chelating therapy 24-hr urinary copper excretion Should initially range between 200 and 500 μg; such a value can also suggest that the patient is adherent to the drug. After the removal of copper accumulation, Urinary copper excretion may be lower. The prognosis of WD is dependent on the. initial severity of the disease and then on adherence to the lifelong treatment. Patients treated before severe and potentially irreversible neurological and hepatic complications have a good prognosis approaching a normal life expectancy (Figure 6). Irreversible liver disease often can be treated. successfully by LTX while some patients with severe neurological disease do not get better despite optimal therapy Acknowledgment The completion of this research assignment could now not have been possible without the contributions and assistance of many individuals and groups. We're. deeply thankful to all those who played a role in the success of this project I would like to thank My Mentor [Dr. Naweed Imam Syed Prof branch of mobile Biology at the University of Calgary for their useful input and guidance for the duration of the research system. Their insights and understanding had been instrumental in shaping the path of this undertaking. Authors' Contribution would like to increase our sincere way to all the members of our take a look at, who generously shared their time, studies, and insights with us. Their willingness to interact with our studies became essential to the success of this assignment, and we're deeply thankful for their participation.
The authors received no financial support for this article's research, authorship, and/or publication. Conflict of Interest
The authors declare no conflict of interest
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.
