AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2693-7247/077
1. Department of Pathology, Centro Universitário FMABC – FMABC, Santo Andre, São Paulo, Brazil.
2. Department of Health, Biomedical Sciences, Ibirapuera University – UNIB, Santo Andre, São Paulo, Brazil.
3. Biomedicine Course, Centro Universitário Italo Brasileiro, São Paulo, São Paulo, Brazil.
*Corresponding Author: Emerson Barbosa da Silva, Biomedicine Course, Centro Universitário Italo Brasileiro, São Paulo,SãoPaulo,Brazil
Citation: Carolina S Matheus, Camila dos Santos Chagas, Daniela Rodrigues Pereira, Anita de Carvalho Garcias, Patrick Gabriel dos Santos Pessini, Emerson Barbosa da Silva (2022) Use of Dolutegravir in Hiv Treatment. J. Pharmaceutics and Pharmacology Research 5(6); DOI: 10.31579/2693-7247/077
Copyright: © 2022 Emerson Barbosa da Silva, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 09 March 2022 | Accepted: 18 March 2022 | Published: 26 April 2022
Keywords: dolutegravir; coping with HIV/AIDS; antiretroviral drugs.
The present work deals with the theme of the use of the retroviral Dolutegravir in the fight against the HIV virus, aiming to present the pharmacological aspects of the drug. The thematic focus was guided by two major interconnected questions: a) how has the evolution of the disease occurred in Brazil and which policies have been adopted in the fight against HIV/AIDS; b) how is Dolutegravir being used to face this epidemic? The methodological procedures adopted were the research of some normative documents and materials considered of reference in the area. As a result, it was evidenced that because it is a drug that had its use approved in August/2013 by the FDA in the USA and by the European Commission in January/2014, Dolutegravir presents itself as a new efficient pharmacological option for the treatment/coping of HIV/AIDS in the country, incorporated in 2017 to the ministry of health to minimize infection in patients with cross-resistance to other drugs used.
Infection with the HIV virus began to be observed in the mid-20th century. Initial reports say that the disease emerged in central Africa found in the immune system of chimpanzees and the African green monkey, undergoing a mutation. Some experiments prove that the missing link in the passage from the first primates to man seems to be related to the issue of handling the meat of infected chimpanzees in Africa. The disease, then taken to small communities in the central region, spread around the world with globalization. (Pinto; Pinheiro; Vieira, et al., 2007).
Human immunodeficiency syndrome (AIDS) is a pandemic disease whose etiological agent is the HIV virus and a retrovirus (virus that contains the enzyme reverse transcriptase) that belongs to the lentivirinae family. (Antunes, 2012)
The main target of the HIV virus during infection is TCD4 and TCD8 lymphocytes, for this reason, the clinical picture of the disease is characterized as a function of the blood count of lymphocytes and viral load in the infected individual and the clinical conditions of the patient. (Rodrigues; Minari; Almeida; Jorge; Machado; Junior; Furine et al., 2014).
AIDS (acquired immunodeficiency syndrome), in Portuguese Acquired Immunodeficiency Syndrome (AIDS) is an infectious disease that attacks the immune system, causing its deficiency and allowing some opportunistic infections to settle in the patient's body such as: tuberculosis, intestinal diseases, pneumonia, thrush, herpes simplex, tumors such as Kaposi's sarcoma, by which the weakened organism allows the development of opportunistic diseases. (Souza and Almeida, 2003)
Drugs have been developed over the years, since the discovery of AIDS in the 1980s. These are: Reverse Transcriptase Nucleoside inhibitors, Abacavir, Didanosine , Lamivudine, Zidovudine and Tenofovir; Non-Nucleoside Reverse Transcriptase Inhibitors, Efavirenz, Nevirapine, Etravirine; fusion inhibitor, Efurvitide ; entry inhibitor, Maraviroc; protease inhibitors (enzyme that matures viral proteins), Fosamprenavir, Atazanavir, Darunavir, Lopinavir, Nelfinavir, Ritonavir, Saquinavir, Tipranavir; and inhibitors of Enzyme Integrase (enzyme that integrates viral DNA into the genetic material of the host cell) Raltegravir and Dolutegravir. (Brito, 2011).
Dolutegravir was a drug developed by the company GSK (GlaxoSmithKline), it is a potent inhibitor of the integrase enzyme, responsible for the insertion of viral DNA into Human DNA (the cell's genetic code). Thus, it inhibits the replication of the virus and its ability to infect new cells. (Min S., Sloan L., Dejesus E., et al., 2011)
In Brazil, the drug was incorporated into the Ministry of Health in 2017 in the STD/AIDS program by the Unified Health System (SUS) (RENAME, 2017), used in combination with other antiretroviral drugs, is a drug of first choice in the treatment of the epidemic. . (Castellino S, Moss L., Wagner D, et al., 2013).
Brazil is one of the pioneer countries in the control of the disease and 80% of the population has an undetectable viral load, the medication is distributed free of charge to the Brazilian population that needs treatment. Today, the carrier lives well with HIV due to the commitment of the Ministry of Health in caring for HIV-positive patients, through the free distribution of antiretrovirals. However, due to lack of education of the young population, new cases among this group have been growing. (Freitas O., Pereira L., 2008).
In the midst of this awareness policies are being carried out in schools, with the aim of instructing and informing young people about the virus, highlighting the importance of preventing the disease. (www.aids.gov.br).
The present work aims to present the pharmacological aspects of the drug Dolutegravir, as well as its effectiveness in the fight/treatment of Aids/HIV.
To carry out the work, a literature review was carried out, through the Virtual Health Library (BVS), SciELO (www.scielo.org) and LILACS (http://lilacs.bvsalud.org/) databases, in articles published in English, Portuguese and Spanish. To carry out the research, the described ones used were: Dolutegravir, Antiretroviral Drugs, Integrase Inhibitors and HIV/Aids. In addition to some normative documents and reference materials in the area (books and websites).

Source: NIH’s Office of AIDS Research
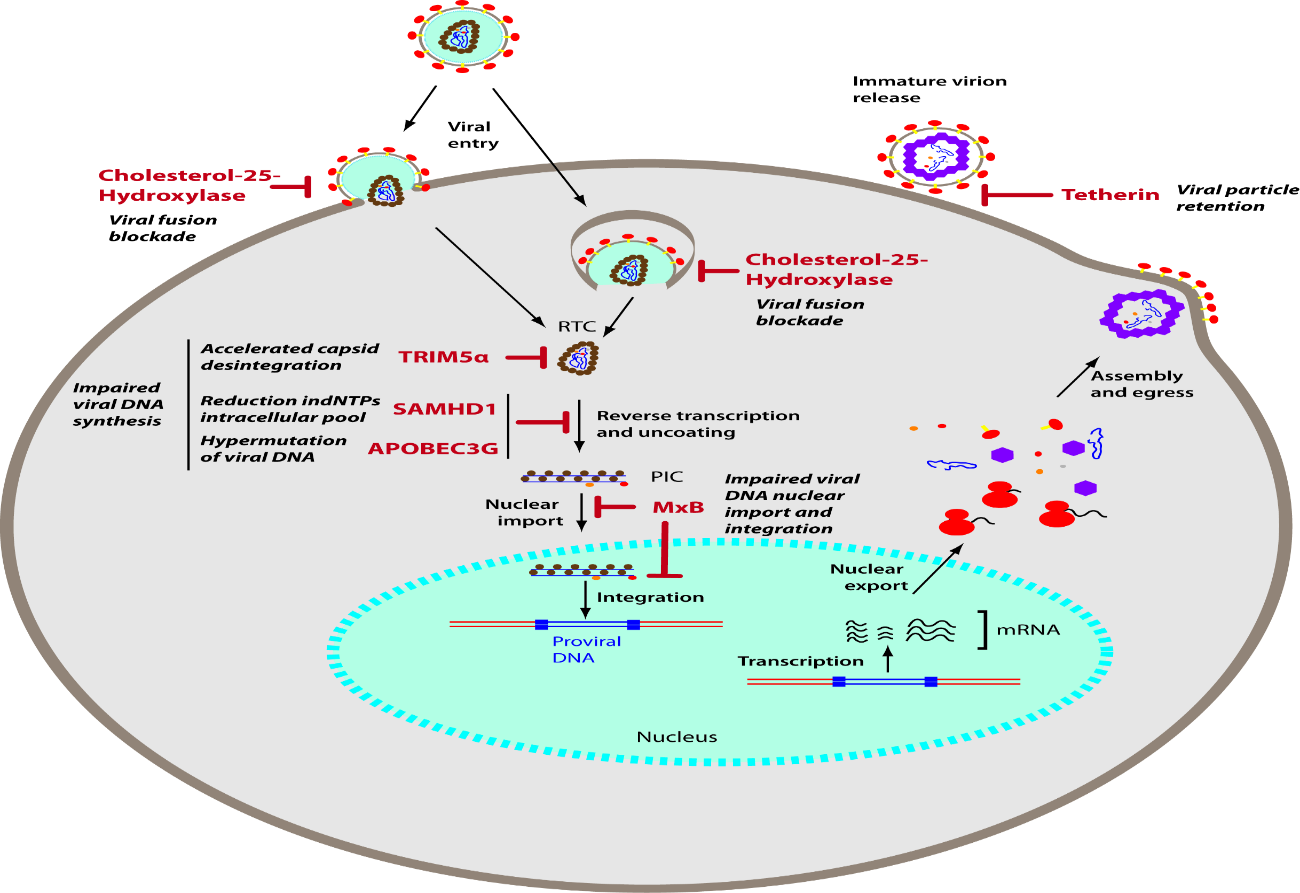
The GP120 and PG41 portion are responsible for the recognition of CCR5 and CXCR4 receptors present in the lymphocytes target cells of the virus. Integrase: Integrates viral DNA into human DNA, protease breaks long proteins into smaller proteins for the formation of a new virus and reverse transcriptase transforms viral RNA into Viral DNA. RNA is the Genetic Material of HIV. (Rodrigues; Minari; Almeida; Braz; Machado; Junior; Furine et al., 2014).
Source: Trends in Basic and Therapeutic Options in HIV Infection
Patients become infected with HIV through contact with blood, sharing syringes, blood transfusions and sexual contact without using a condom. The virus falls into the bloodstream and the virus particles reach their target cell through specific receptors present on the membrane. The receptors used by the virus are CCR5 or CXCR4 (HIV2). The protein responsible for the recognition of HIV is the GP120 portion, this portion binds to specific receptors on the cell membrane, allowing the fusion of the virus into the cytoplasm. Inside the cytoplasm, 3 enzymes responsible for its replication are released, namely: reverse transcriptase, protease and entregrase. (Lima; Cunha et al., 2008).
Reverse transcriptase is responsible for reverse transcription (conversion of RNA into viral DNA), when transcription occurs, the enzyme entegrase (enzyme responsible for integrating the viral genetic material into human DNA) comes into action, the protease is responsible for the maturation of the capsule virus to form a new virus that is released by budding and can infect another cell. (Brito; 2011).
The Human Immunodeficiency Virus (HIV) initiates the process through an interaction of the glycoprotein (GP120) with the cell's CD4 receptor and consequently induces the V3 loop of GP120 to bind to the CCR5 or CXCR4 receptor. By activating the GP41 glycoprotein and allowing the viral capsule to attach to the target cell, then depositing the capsid in the cell's cytoplasm, then the capsid breaks down releasing RNA, this viral genetic material undergoes the action of the reverse transcriptase enzyme that synthesizes the viral DNA from this RNA, with the viral DNA synthesized, the integrase enzyme binds this DNA to the DNA of the host cell, then the cell activates the gene transcription process, the viral DNA will also be transcribed and new viral particles will appear. The protease enzyme synthesizes the proteins necessary for the development of the viral particle. (Paker; Junior, et al., 2000).
Antiretroviral drugs act by interrupting or hindering the viral cycle, preventing the action of the integrase enzyme from binding the viral DNA to the cell's DNA; or inhibiting the function of the protease enzyme to synthesize the important proteins for the development of the viral particle and the antiretroviral fusion inhibitors have the function of acting on the transmembrane glycoproteins of the virus, not allowing or hindering the connection with the cellular receptors, preventing the infection from occurring. (Pinto; Pinheiro; Viera et al., 2007).
Integrase inhibitors (INI) are the newest class of antiretrovirals proven by the Food Drug Administration (FDA) for the treatment/combat of HIV, based on their efficacy and safety profiles (WALMSLEY et al., 2013).
INI act on the HIV integrase enzyme – one of the three essential enzymes for the replication of the virus, preventing the process of integrating the viral DNA into the host cell genome (HAZUDA et al., 2009). More specifically, the INI's are responsible for blocking the transfer process of the viral DNA strand, whose 3' hydroxyl groups formed by the pre-integration complex should be covalently linked to the 5' phosphate groups of the host cell's DNA exposed by the action of the host cell itself. viral enzyme in question (GANDHI; GANDHI, 2014).
The interruption of the binding of the viral DNA to the host DNA is made possible by the chelation of the two divalent metal cations present in the active site of the integrase (HAZuDA et al., 2009; MIN et al., 2010). Thus, the active site is occupied and the enzyme disconnects from the 3' ends of viral DNA, paralyzing the integration process (KATLAMA; MURPHY, 2012; SHAH et al., 2014). Furthermore, integrase inhibitors are effective against cases of resistance to other drugs such as: reverse transcriptase inhibitors; non-nucleoside reverse transcriptase; of protease and fusion (KATLAMA; MURPHY, 2012; SHAH et al., 2014).
Raltegravir and elvitegravir were the first drugs used in the treatment of HIV patients, more recently Dolutegravir (DTG) (KANDEL; WALMSLEY, 2015; CID-SILVA et al., 2017). DTG is an evolution of integrase inhibitors, due to its superiority over previous generations in terms of antiretroviral potency; dosage, side effects and genetic barrier to resistance (KATLAMA; MURPHY, 2012).
Dolutegravir, approved by the FDA on August 13, 2013, is a second-generation integrase enzyme inhibitor with an antiviral potency and tolerability similar to first-generation integrase inhibitors (Raltegravir and Elvitegravir). Administration of dolutegravir is indicated for the treatment of HIV-infected adults and children over 12 years of age in combination with other drugs. Dolutegravir, whose structure is shown in Figure 3, binds to the active site of the integrase enzyme, blocking the integration of viral DNA. This being an essential step in the replication cycle, HIV stops producing new viral particles.

Source: Der Pharma Chemica
Dolutegravir is designed to have: 1) high antiretroviral potency; 2) low dose of ligament; 3) given once daily without drug booster; 4) improved resistance profile with greater genetic barrier to resistance (KATLAMA; MURPHY, 2012). Regarding the effectiveness of DTG, it is possible to mention its high antiviral potency for wild-type and antiretroviral-resistant viruses, which is important due to the increasing tendency of resistance to treatments. Initiation of antiretroviral therapy with a DTG-containing regimen is recommended for initial preferential treatment and therapeutic rescue in cases of ARV therapy failure, as well as post-exposure therapy. This drug has very rapid inhibition of viral replication as well as a superior genetic barrier to other regimens containing first-generation integrase inhibitors (KATLAMA; MURPHY, 2012; KANDEL; WALMSLEY, 2015).
In 2017, dolutegravir became part of the National List of Essential Medicines (Rename) of the Unified Health System (SUS). Currently, in Brazil, 76,713 people use DTG as a first line and another 45,645 have switched to DTG. Altogether, there are more than 122,000 Brazilians living with HIV using dolutegravir in their antiretroviral treatment regimens, a number that represents 19% of the total of 572,000 Brazilians receiving antiretroviral treatment free of charge through the SUS. It is also noteworthy that 87% of people who started treatment in 2018 started with DTG. (aids.gov.br).
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the retroviral deoxyribonucleic acid (DNA) integration strand transfer step, a step essential to the HIV replication cycle.
The pharmacokinetics of dolutegravir are similar in healthy subjects and those living with HIV. The pharmacokinetic variability of dolutegravir is low to moderate.
Tmax two to three hours after administration in tablet form. The linearity of dolutegravir pharmacokinetics is dose and formulation dependent. In general, following oral administration of tablet formulations, dolutegravir sodium exhibited non-linear pharmacokinetics with less than dose-proportional increases in plasma exposure between 2 and 100 mg; however, the increase in dolutegravir exposure appears dose-proportional in the range of 25 to 50 mg. Dolutegravir sodium can be administered with or without food. Food increased the extent and reduced the rate of absorption of dolutegravir. The bioavailability of dolutegravir depends on the content of the meal: low, moderate and high fat meals increase absorption. The absolute bioavailability of dolutegravir has not been determined.
According to in vitro data, dolutegravir is highly bound (approximately 99.3%) to human plasma proteins. The apparent volume of distribution (following oral administration of the suspension formulation, Vd/F) is estimated to be 12.5 L. Plasma protein binding of dolutegravir was concentration independent. The concentration ratios of drug-related radioactivity in whole blood and plasma ranged, on average, between 0.441 and 0.535, indicating minimal association of radioactivity with cellular components of blood. The free fraction of DTG in plasma is estimated to be about 0.2% to 1.1% in healthy subjects, about 0.4% to 0.5% in subjects with moderate hepatic dysfunction, 0.8% to 1.0% in individuals with severe renal dysfunction and 0.5% in patients living with HIV-1. Dolutegravir is found in cerebrospinal fluid (CSF). In 12 dolutegravir plus abacavir/lamivudine (3TC) naive subjects for 16 weeks, the mean CSF concentration of dolutegravir was 15.4 ng/mL at week 2 and 12.6 ng/mL at week 16, ranging from 3.7 to 23.2 ng/mL (comparable to plasma concentration of unbound drug). The CSF dolutegravir concentration ratio: plasma ranged from 0.11% to 2.04%. CSF concentrations of dolutegravir exceeded the IC50, which confirms the median CSF HIV-1 RNA reduction of 2.2 log from baseline after 2 weeks and 3.4 log after 16 weeks of treatment (see Pharmacodynamics, in Pharmacological Characteristics). Dolutegravir is present in both female and male genitalia. The AUC in cervicovaginal fluid, cervical tissue and vaginal tissue ranged from 6% to 10% of the corresponding plasma AUC at steady state. In semen the AUC was 7% and in rectal tissue it was 17% of the corresponding plasma AUC at steady state.
component (9.7% of the total dose administered in a human mass balance study). Dolutegravir is the predominant circulating substance in plasma; renal elimination of unchanged drug is low (< 1>
Dolutegravir has a terminal half-life of approximately 14 hours and an apparent clearance (CL/F) of 0.56 L/h.
Since its emergence in the early 1980s, the HIV/AIDS epidemic has redesigned its profile of infected people over the years.
As a result, there was a rapid spread of AIDS cases in Brazil. To contain the advances, the country started to develop health actions that could respond to the demands placed in daily life. The consolidation of the SUS and the creation of the National STD/AIDS Policy represented great advances in the development of prevention programs and policies, and treatments available for free and universal access, with the State being the main responsible for ensuring health for all People. Living with HIV/AIDS (PLWHA).
According to the HIV Clinical Monitoring Report, the increase in diagnosis among people living with HIV and the increase in the number of people undergoing treatment are highlights in Brazil. The country has been advancing more and more in access to antiretroviral drugs, optimizing and reducing the number of pills for patients undergoing treatment, thus ensuring free access to all.
Dolutegravir, one of the most modern antiretrovirals in the world, is shown to be quite efficient in the treatment/combating AIDS/HIV, the introduction of Dolutegravir in the SUS only contributed more to achieving the established goals.
In the midst of this scenario, pharmaceutical care becomes important, as it promotes the rational use of the drug, maintaining the effectiveness and safety of treatment for PLWHA.
Therefore, Dolutegravir is a very promising drug, where, if used with the prescription/indication and the proper monitoring of a specialized doctor, it becomes a great alternative for the improvement in the clinical picture of HIV carriers, in addition to guaranteeing a longer and healthier life for carriers.