AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2693-7247/086
University of California, Irvine, Orange, CA
*Corresponding Author: Helmy M. Guirgis Department Pharmacy University of California, Irvine, Orange, CA
Citation: Helmy M. Guirgis, (2022) The Impact of The Immune Check Point Duration of use on Cost in Lung Cancer. J. Pharmaceutics and Pharmacology Research. 5(6); DOI: 10.31579/2693-7247/086
Copyright: © 2022, Helmy M. Guirgis, This is an open access article distributed under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cite
Received: 05 April 2022 | Accepted: 18 April 2022 | Published: 30 May 2022
Keywords: lung cancer; monotherapy; pembrolizumab (pembro); atezolizumab (atezo); cemiplimab (cemi)
Background: Monotherapy and combinations of Pembrolizumab (Pembro), Atezolizumab (Atezo) and Cemiplimab (Cemi), prolonged overall survival (OS) in advanced/metastatic non-small cell lung cancer (a/m NSCLC). Pembro demonstrated 5-year OS gain. The duration of therapy of the immune check point inhibitors (ICI) has not been defined. One-year adjuvant Durvalumab (Durv) and Atezo significantly prolonged OS. Neoadjuvant few cycles resulted in positive outcomes. Costs are relatively expensive, multiplying with prolonged use. The estimated 2019 CAR-T cost was $450,000. The Affordable Insulin bill 6833 capping insulin monthly cost at $35 was approved by the U.S. House of Representative. There are unmet needs for coherent drug cost policies and containment. We aimed 1- Explore the factors which impact ICI costs in lung cancer stages 2- Navigate cost-saving strategy based on generics, therapy duration and monotherapy utilization thresholds at $450,000 and $550,000 for combinations Methods: Clinical studies outcomes were quoted. Annual drug prices were calculated. Results: Estimated annual Pemetrexed (Peme) costs were $113,793, generic chemicals < $1,000 and Bevacizumab (Bev) $150,126 vs $148,000, mean 6 ICI. Pembro 2-year costs were $334,652. The 3- $501,978 and 5- $836,630 were above the $450,000. Atezo + Bev+ Peme combination had the highest 2-year $722,977 costs, above $550,000. Atezo + Peme costs were $422,725, Pembro + Peme $448,445 and Cemi + Peme $425,385, not significantly different. Costs decreased by 25% using generics. Extending ICI use by 6-12 months increased combination costs by 25-50%. Adjuvant 1-year Durv costs were $148,013 and Atezo $154,446, half the 2-year. Using response rates, cost of neoadjuvant Nivolumab (Nivo) 2-4 cycles were $25,000 - $50,000. Conclusion: Generics, short ICI duration use, neo-adjuvants, and utilization thresholds reduced costs.
Advanced/metastatic non-small cell lung cancer (a/m-NSCLC), Adverse events (AEs), Atezolizumab (Atezo), Bevacizumab (Bev), Biosimilar (Bio), Cemiplimab (Cemi), Confidence Interval (CI), Cytotoxic T-lymphocyte -associated antigen 4 (CTLA), Dorvolumab (Dorvo), Hazard Ratio HR), Nivolumab (Nivo), Immune check point inhibitors (ICI), Ipilimumab (Ipi), Pembrolizumab (Pembro), Pemetrexed (Peme), Programmed death receptor-1 (PD-1). Programmed death receptor-ligand-1 (PD-L1), Squamous (sq).
The 1 st immune check point inhibitors (ICI) Pembrolizumab (Pembro) was introduced in 2016. It significantly prolonged the overall survival (OS) in 1st line advanced/metastatic non-small cell lung cancer (a/m NSCLC) with high programmed death receptor-1 (PD-L1), lacking epidermal growth factors (EGFR) and anaplastic lymphoma kinase (ALK) genomic aberrations (1). Survival and 5-year OS were further confirmed (2-5). Duration of therapy after 2-years has not been clearly defined. Atezolizumab (Atezo) (6) and Cemiplimab (Cemi) (7) later demonstrated OS. Chemo-drugs in combinations with Pembro (8), Atezo (9-11), and Cemi (12) showed effectiveness regardless of PD-L1. Nivolumab/Ipilimumab (Nivo/Ipi), with and without chemo, have also shown OS gain (13,14). One-year adjuvant Durvalumab (Durv) (15) and Atezo significantly prolonged OS (16). Value (18-21) and cost effectiveness (22-23) were extensively studied. However, drug costs have rarely been scrutinized except by the press, media, and few scattered reports (24). ICI costs are rather expensive, multiplying with further therapy. CAR-T cell therapy 2019 cost was estimated at $450,000 (17). The H.R. Affordable Insulin bill 6833 to cap the cost of insulin prices at $35 per month was approved by the U.S. House of Representative. There are unmet needs for coherent drug cost policies and containment. We Open Access Research Article Journal of Pharmaceutics and Pharmacology Research Helmy M. Guirgis * AUCTORES Globalize your Research J Pharmaceutics and Pharmacology Research Copy rights@ Helmy M. Guirgis et.al. Auctores Publishing LLC – Volume 5(6)-086 www.auctoresonline.org ISSN: 2693-7247 Page 2 of 5 aimed 1- Explore the factors which impact ICI costs in various lung cancer stages 2-Navigate cost-saving strategy based on generics, shorter ICI duration, use of neoadjuvant therapy and $450,000 utilization thresholds for monotherapy use and $550,000 for combination
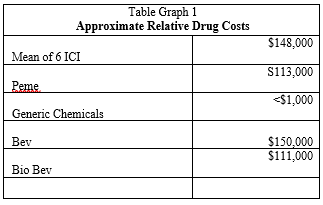
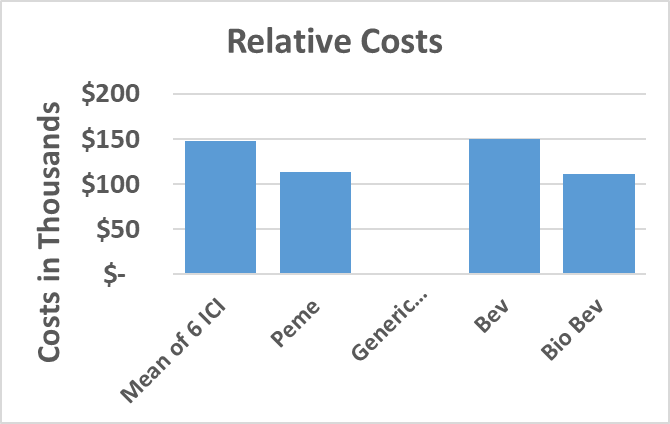
A-Monotherapy: The estimated annual costs of the 3 approved ICI in 1st-line a/mNSCLC in PD-L1 >50% were Pembro $167,326, Atezo $154,446 and Cemi $154,896. The mean 6 ICI was $148,431. Pemetrexed (Peme) was $113,793 and generic chemical drugs < $1,000. Bev cost was $150,126 and Bio-similar Bev $111,566, 0.74 cost of Bev (Graph 1).
The 2-year Pembro costs were $334,652, not significantly different from the 35 cycles. One added year increased costs to $501,978, $51,078 above the $450,000 threshold by $51,978. Pembro has demonstrated 5-year survival. If used for 5 years, cost would be $836,630 (Table (1).
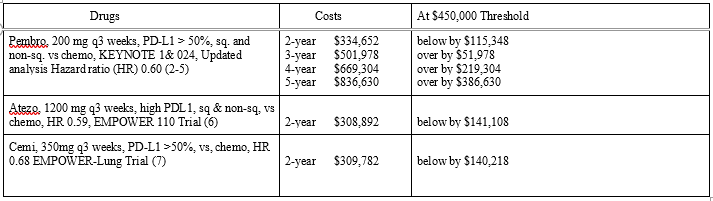
Atezo 2-year costs (6) were $308,892 and Cemi, approved early 2021 (7), was $309,782. There was no significant cost difference between the 3 ICI. All were below $450,000 (Table 1).
Both 1-year adjuvant Durvalumab (Durv) trial (15) after chemo-radiation of unresectable stage III and Atezo following chemotherapy in resected IB-IIIA (16) reported significant OS. Durv cost was $148,013 and Atezo $154,446, essentially half the 2-year costs.
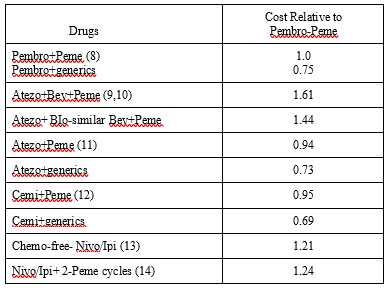
Combinations: The $550,000 threshold was set to cover the $100,000 cost of patent chemo-drugs. Peme annual price was $113,793, 0.68 that of
Pembro. The 2-year combination costs of Pembro-Peme (8), Atezo+Bev+Peme, Atezo+Bio-Bev+Peme, Atezo+chemo (9-11), Cemi-chemo (12), Nivo/Ipi and Nivo/Ipi+Peme (13,14) were shown in Table 2. Atezo+Bev+Peme demonstrated the highest costs at $722,977, with Bio-similar lower at $645,857. Nivo/Ipi and Nivo/Ipi + 2-Peme cycles hovered around $550,000. Lower costs were demonstrated by Pembro+ Peme at $448, 445, Atezo+Peme $442,725 and Cemi+Peme $423,585. Using generics instead of Peme, costs decreased to the 2-year ICI monotherapy baseline. Extending combination use beyond 2-years by 6-12 months increased costs by 25-50%.

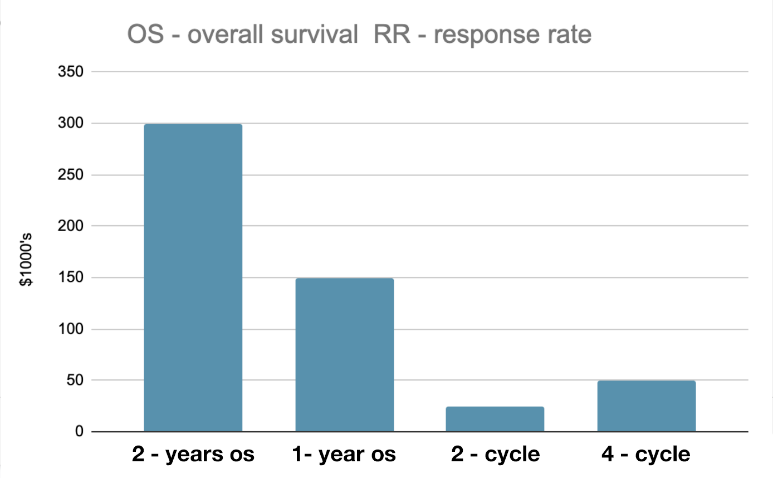
In graph 2, ICI costs were depicted in various stage of lung cancer. Costs were the highest in a/mNSCLC, twice the adjuvant therapy. Neoadjuvant therapy using 2-4 cycles resulted in positive responses in early lung cancer stages (26-28) at $25,000 - $50,000 costs.
Sales are constrained by high costs, disproportionately targeting the financially- disadvantaged patients and nations. Cost is a sensitive and complicated subject to tackle. ICI costs are relatively expensive. Synthesis is technically complicated, time consuming and costly. With no guarantee of success, it is fair and imperative that the pharmaceutical companies retrieve their investments in such highly competitive business.
In the present work, posted drug prices constituted the sole basis of drug comparison. Value and cost effectiveness of the ICI have been extensively studied by the parent drug companies. The HR of the monotherapy and combination therapies were overlapping in the cited clinical studies with no clear difference. With exception of neoadjuvant trials, OS and the HR were well documented. The observations that 20% of Pembro-treated patients in 1st-line a/mNSCLC with PDL1 > 50% survived 5 years seemed to justify the 2-year costs. Pembro, the first ICI synthesized, has, so far, the distinctive advantage of long-term OS benefit. The 3-year costs were $501,978, above $450,000 and multiplied with further use. Treatment of 1,000 patients, a small subset of a/mNSCLC, would be a heavy economic burden to bear. There was no significant cost difference between monotherapy Pembro, Atezo and Cemi. Costs could play a differentiating factor should one ICI have a significant cost reduction.
Peme, an inhibitor of the folate-dependent enzyme first reported in 2013 (25), is expected to lose its patency in the ensuing few years. Peme annual price was $113,793, with cost doubling if used for 2-years. On turning generic, there would be a steep drop in cost and a sharp rise in use. The ICI class, with its longer duration of action, has essentially replaced Peme in 1st-line a/mNSCLC in most of the affluent nations.
The most expensive 2-year Atezo+Bev+Peme combination was $722,977, far above the 550,00. Its Bio-similar regime was $645,857, lower by 11%. It would be self-inflicted wound to incur high costs considering the availability of cheaper combinations. Costs of Nivo/Ipi+2-Peme cycles were more expensive than Nivo/Ipi with $13,130, at approximate $550,000 costs. Pempro+Peme and Atezo+Peme and Cemi-Peme costs were less expensive. Combination costs would drop by about 25% using generics. The role of generics is presently being threatened by shortage and supply route disruptions. At present, there is no head-to-head outcome and safety comparison between one ICI and another. It is doubtful that such study would be undertaken in the future.
Cost saving was demonstrated using bio-similar, generics and adjuvant therapy. However, the clearest cost-saving evidence was using neoadjuvant Nivo. At cost fraction, few 2-4 cycles, with or without chemo (26,27) showed positive outcome. Based on the results of the CheckMate 816 study (NCT02998528), demonstrating statistically significant improvement in event-free survival (EFS), the FDA approved nivolumab plus chemotherapy vs chemotherapy alone among patients with early NSCLC (28). Circulating DNA biomarker is presently being explored to signal tumor clearance (29).
Cost divergence in drug prices between US and Germany was previously noted (30,31) with prices tending generally to be higher in the US where some drugs first originated. At present, cost reforms (32,33) have not been widely accepted. Application of utilization thresholds would lower costs and help consumers. Drug companies would also benefit through wider global distributions and sales. Cost containment needs to be a shared responsibility between dug companies, medical scientists and practicing physicians.
In summary, Pembro, Atezo and Cemi 2-year costs seemed fair and reasonable in 1st-line a/m NSCLC with PDL1 > 50%. Pembro 3-year costs multiplied with further use, supporting the adoption of utilization threshold strategy. At 2 years, Atezo+Bev+Peme combination demonstrated the highest cost.
Pempro+Peme and Atezo+Peme and Cemi-Peme were lower and dropped further using generics. Adjuvant therapy was 50% the 2-year costs. In the neo-adjuvant space, few cycles ICI resulted in EFS at minimal costs. Duration use, generics, utilization thresholds and neo-adjuvants resulted in cost containment.
Sales are constrained by high costs, disproportionately targeting the financially- disadvantaged patients and nations. Cost is a sensitive and complicated subject to tackle. ICI costs are relatively expensive. Synthesis is technically complicated, time consuming and costly. With no guarantee of success, it is fair and imperative that the pharmaceutical companies retrieve their investments in such highly competitive business.
In the present work, posted drug prices constituted the sole basis of drug comparison. Value and cost effectiveness of the ICI have been extensively studied by the parent drug companies. The HR of the monotherapy and combination therapies were overlapping in the cited clinical studies with no clear difference. With exception of neoadjuvant trials, OS and the HR were well documented. The observations that 20% of Pembro-treated patients in 1st-line a/mNSCLC with PDL1 > 50% survived 5 years seemed to justify the 2-year costs. Pembro, the first ICI synthesized, has, so far, the distinctive advantage of long-term OS benefit. The 3-year costs were $501,978, above $450,000 and multiplied with further use. Treatment of 1,000 patients, a small subset of a/mNSCLC, would be a heavy economic burden to bear. There was no significant cost difference between monotherapy Pembro, Atezo and Cemi. Costs could play a differentiating factor should one ICI have a significant cost reduction.
Peme, an inhibitor of the folate-dependent enzyme first reported in 2013 (25), is expected to lose its patency in the ensuing few years. Peme annual price was $113,793, with cost doubling if used for 2-years. On turning generic, there would be a steep drop in cost and a sharp rise in use. The ICI class, with its longer duration of action, has essentially replaced Peme in 1st-line a/mNSCLC in most of the affluent nations.
The most expensive 2-year Atezo+Bev+Peme combination was $722,977, far above the 550,00. Its Bio-similar regime was $645,857, lower by 11%. It would be self-inflicted wound to incur high costs considering the availability of cheaper combinations. Costs of Nivo/Ipi+2-Peme cycles were more expensive than Nivo/Ipi with $13,130, at approximate $550,000 costs. Pempro+Peme and Atezo+Peme and Cemi-Peme costs were less expensive. Combination costs would drop by about 25% using generics. The role of generics is presently being threatened by shortage and supply route disruptions. At present, there is no head-to-head outcome and safety comparison between one ICI and another. It is doubtful that such study would be undertaken in the future.
Cost saving was demonstrated using bio-similar, generics and adjuvant therapy. However, the clearest cost-saving evidence was using neoadjuvant Nivo. At cost fraction, few 2-4 cycles, with or without chemo (26,27) showed positive outcome. Based on the results of the CheckMate 816 study (NCT02998528), demonstrating statistically significant improvement in event-free survival (EFS), the FDA approved nivolumab plus chemotherapy vs chemotherapy alone among patients with early NSCLC (28). Circulating DNA biomarker is presently being explored to signal tumor clearance (29).
Cost divergence in drug prices between US and Germany was previously noted (30,31) with prices tending generally to be higher in the US where some drugs first originated. At present, cost reforms (32,33) have not been widely accepted. Application of utilization thresholds would lower costs and help consumers. Drug companies would also benefit through wider global distributions and sales. Cost containment needs to be a shared responsibility between dug companies, medical scientists and practicing physicians.
In summary, Pembro, Atezo and Cemi 2-year costs seemed fair and reasonable in 1st-line a/m NSCLC with PDL1 > 50%. Pembro 3-year costs multiplied with further use, supporting the adoption of utilization threshold strategy. At 2 years, Atezo+Bev+Peme combination demonstrated the highest cost.
Pempro+Peme and Atezo+Peme and Cemi-Peme were lower and dropped further using generics. Adjuvant therapy was 50% the 2-year costs. In the neo-adjuvant space, few cycles ICI resulted in EFS at minimal costs. Duration use, generics, utilization thresholds and neo-adjuvants resulted in cost containment.
Sales are constrained by high costs, disproportionately targeting the financially- disadvantaged patients and nations. Cost is a sensitive and complicated subject to tackle. ICI costs are relatively expensive. Synthesis is technically complicated, time consuming and costly. With no guarantee of success, it is fair and imperative that the pharmaceutical companies retrieve their investments in such highly competitive business.
In the present work, posted drug prices constituted the sole basis of drug comparison. Value and cost effectiveness of the ICI have been extensively studied by the parent drug companies. The HR of the monotherapy and combination therapies were overlapping in the cited clinical studies with no clear difference. With exception of neoadjuvant trials, OS and the HR were well documented. The observations that 20% of Pembro-treated patients in 1st-line a/mNSCLC with PDL1 > 50% survived 5 years seemed to justify the 2-year costs. Pembro, the first ICI synthesized, has, so far, the distinctive advantage of long-term OS benefit. The 3-year costs were $501,978, above $450,000 and multiplied with further use. Treatment of 1,000 patients, a small subset of a/mNSCLC, would be a heavy economic burden to bear. There was no significant cost difference between monotherapy Pembro, Atezo and Cemi. Costs could play a differentiating factor should one ICI have a significant cost reduction.
Peme, an inhibitor of the folate-dependent enzyme first reported in 2013 (25), is expected to lose its patency in the ensuing few years. Peme annual price was $113,793, with cost doubling if used for 2-years. On turning generic, there would be a steep drop in cost and a sharp rise in use. The ICI class, with its longer duration of action, has essentially replaced Peme in 1st-line a/mNSCLC in most of the affluent nations.
The most expensive 2-year Atezo+Bev+Peme combination was $722,977, far above the 550,00. Its Bio-similar regime was $645,857, lower by 11%. It would be self-inflicted wound to incur high costs considering the availability of cheaper combinations. Costs of Nivo/Ipi+2-Peme cycles were more expensive than Nivo/Ipi with $13,130, at approximate $550,000 costs. Pempro+Peme and Atezo+Peme and Cemi-Peme costs were less expensive. Combination costs would drop by about 25% using generics. The role of generics is presently being threatened by shortage and supply route disruptions. At present, there is no head-to-head outcome and safety comparison between one ICI and another. It is doubtful that such study would be undertaken in the future.
Cost saving was demonstrated using bio-similar, generics and adjuvant therapy. However, the clearest cost-saving evidence was using neoadjuvant Nivo. At cost fraction, few 2-4 cycles, with or without chemo (26,27) showed positive outcome. Based on the results of the CheckMate 816 study (NCT02998528), demonstrating statistically significant improvement in event-free survival (EFS), the FDA approved nivolumab plus chemotherapy vs chemotherapy alone among patients with early NSCLC (28). Circulating DNA biomarker is presently being explored to signal tumor clearance (29).
Cost divergence in drug prices between US and Germany was previously noted (30,31) with prices tending generally to be higher in the US where some drugs first originated. At present, cost reforms (32,33) have not been widely accepted. Application of utilization thresholds would lower costs and help consumers. Drug companies would also benefit through wider global distributions and sales. Cost containment needs to be a shared responsibility between dug companies, medical scientists and practicing physicians.
In summary, Pembro, Atezo and Cemi 2-year costs seemed fair and reasonable in 1st-line a/m NSCLC with PDL1 > 50%. Pembro 3-year costs multiplied with further use, supporting the adoption of utilization threshold strategy. At 2 years, Atezo+Bev+Peme combination demonstrated the highest cost.
Pempro+Peme and Atezo+Peme and Cemi-Peme were lower and dropped further using generics. Adjuvant therapy was 50% the 2-year costs. In the neo-adjuvant space, few cycles ICI resulted in EFS at minimal costs. Duration use, generics, utilization thresholds and neo-adjuvants resulted in cost containment.