AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2640-1053/241
Senior Scientist, Department of Genetics & Robotics, NCMRR (National Center for Medical Rehabilitation Research), National Institutes of Health (NIH), Bethesda, Maryland, USA.
*Corresponding Author: A. Hameed Khan, Senior Scientist, Department of Genetics & Robotics, NCMRR (National Center for Medical Rehabilitation Research), National Institutes of Health (NIH), Bethesda, Maryland, USA. He can be reached at [email protected].
Citation: A. Hameed Khan, (2025), The Impact of Sequencing the Medical Genome for Specifically Identifying, Preventing and Treating Horrendous Diseases Such as Cancers, J. Cancer Research and Cellular Therapeutics. 9(4); DOI:10.31579/2640-1053/241
Copyright: © 2025, A. Hameed Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 14 May 2025 | Accepted: 22 May 2025 | Published: 29 May 2025
Keywords: sequencing genome; mutations; glioblastoma; genetic diversity; genome diseases; inbreeding; AZQ
Human Genome sequence (which serves as Reference Sequence) contains the sequence of the entire book of life, but the medical genome will sequence only the strip of DNA in the cancer cell genome which carry oncogene responsible for a specific disease. The Cancer Genome Atlas Project initially focused on identifying important deleterious genetic changes involved in Lung, Brain, Breast and Ovarian cancers and later investigation will include other cancers. When we sequence a single breast cancer cell and compare with the Reference Sequence, we found two deleterious mutations on Chromosore-17. Chrosome-17 is long chromosome it contains 92 million nucleotide bases and carries 1,394 genes. About 11 somatic mutations are discovered. Of the two deleterious mutations, the first is the mutation on BRCA1 gene responsible for causing breast cancer in both men and women. The second one is the BRCA2 gene responsible for causing prostate cancer in men. We first find a cure in animal then we translated the animal work in human. From the Structure Activity relationship, I have demonstrated that 1-Aziridine, 2,4-dinotrobenzamide (CB1954) showed the highest biological activity against a solid aggressive tumor like, Walker Carcinoma 256 in Rats. I translated this work in human by making AZQ. Of all the deadly cancers, the deadliest are Brain, Breast and Lungs. Brain cancer is the worst. Most patients die with in fourteen months of their diagnosis. By making AZQ (US Patent 4,233,215) to attack Glioblastoma, the Brain tumor, I used Quinone to transport two aziridines and two carbamates’ molecules attached to Quinone across the Blood Brain Barrier (BBB). AZQ is a pro-drug. It is activated in the presence of acid. As the glioblastoma grows, it uses glucose as a source of energy. Glucose is broken down to Lactic Acid. It is the acid which activate Aziridine and Carbamate molecules to generate the most powerful Carbonium ions which attack the growing Glioblastoma tumor which starts shrinking. Most untreated patients die within fourteen months. The treated patients are most likely to live 2-years longer. The residual cell could be treated by immune therapy. Literature search showed that AZQ is extensively studied.
To shut off a gene, two methods are available. One was developed by Professor Ross of London University by using Nitrogen Mustard which cross-link both strands of DNA. As his student, I used Aziridine, half as toxic, to bind to one strand of DNA shutting off the gene. Both methods are presented below.
The Impact of Sequencing Human Genomes are a series of lectures to be delivered to the scholars of the National Youth League Forum (NYLF) and in the International Science Conferences. NYLF scholars are the very best and brightest students selected from all over the USA and the world brought to Washington by Envision, an outstanding organization that provides future leaders of the world. I am reproducing here part of the lecture which was delivered at the International Science Conference that was PCS 6the Annual Global Cancer Conference held on November 15-16, 2019, in Athens, Greece.
I am describing below the use of highly toxic lethal chemical weapons (Nitrogen Mustard) which was used during WWI and its more toxic analogs developed as more toxic weapons during WWII. I described the use of Nitrogen Mustard as anti-cancer agents in a semi-autographical way to accept the responsibility of its use. When we publish research papers, we share the glory with colleagues and use the pronoun “We” but only when we share the glory not the misery. In this article by adding the names of my coworkers, the animal handers, I will share only misery. The Safety Committee is interested to know who generated the highly lethal Chemical Waste, how much was it generated and how was it disposed? I accept the responsibility. The article below sounds semi-autobiographical, it is, because I am alone responsible for making these compounds of Nitrogen Mustard, Aziridines and Carbamate. To get a five-gram sample for animal screening, I must start with 80 grams of initial chemicals for a four-step synthesis. To avoid generating too much toxic chemical waste, instead of using one experiment with 80 grams, I conducted 80 experiments with one gram sample, isolating one crystal of the final product at a time. The tiny amount of waste generated at each experiment was burned and buried at a safe place according to safety committee rules.
Objective: The purpose of the medical genome study is to specifically sequence all tumor cell genomes and identify the mutations responsible for causing the disease. The future generation of scientists (my students) will design drugs to shut off those cancerous genes. By making 1-Aziridine-2,4-dinitro Benzamide (CB1954), I have demonstrated that I could shut off gene in a solid aggressive Walker Carcinoma 256 in Rats. Later I translated this work in humans by making AZQ (US Patent 4,233,215) to transport DNA binding Aziridine and Carbamate Quinones across the Blood Brain Barrier to attack the rapidly growing Glioblastoma tumor cells in the brain. (most patients die with fourteen month of diagnosis). The Aziridine ring is stable in basic and neutral media but not in acidic medium. As the tumor in the brain grows rapidly, it uses glucose as a source of energy, Glucose is broken down to produce Lactic Acid. In the acidic media, the Carbamate and Aziridine ring opens generating Carbonium ions which attack the single strand of tumor DNA shutting off the genes reducing the tumor size. Treated patients are most likely to live 2 additional years.
Methods: Chloroquinones are treated with Aziridines in basic media to make Aziridine Quinone. Sodium salt of Carbamate is reacted with Chloroquinones. In depth synthetic procedures are described in the following publications [21-23].
Result: Aziridine Quinone is a novel class of drug that passes across the Blood Brain Barrier (BBB) and attack brain tumor DNA shutting off gene that causes Brain Cancer. Over the years, I made over 200 Aziridine compounds which were tested against experimental animal tumors. Forty-five of them are considered valuable enough to be patented by the U.S. Government (US Patent 4,146,622). Radio-labeled study showed that it is the Aziridine and Carbamate Moieties that bind to a single strand of DNA nucleotide Guanine shutting off genes. This discovery of AZQ opens path to treat a variety of cancers.
Conclusion: Dr. Khan is the discoverer of (AZQ). His work showed that chemicals that exhibit affinity for an organ could serve as a carrier for Aziridine groups to treat the cancer of that organ. There are about 220 different tissues in our body and they all could develop tumor if their DNA is mutated by exposure to radiations, chemical/environmental pollution, viral infection or genetic inheritance. Fortunately, there are several coloring dyes to color every tissue of our body. Dyes could be used as a carrier for the Aziridine groups to attack their tumor DNA. NIH considers Dr. Khan’s work is so valuable and innovative that he was honored with the “2004 NIH Scientific Achievement Award” one of the America’s highest awards in Medicine.
Our entire book of all life, our genome, is written in four genetic letters called nucleotides in a three-letter code called codon, and they are A (adenine), T (thymine), G (guanine) and C (cytosine). These four chemicals are called nucleotide. Out of four-letter text three-letter code for an amino acid called codon, The four-letter nucleotides A (adenine), T (thymine), G (guanine) and C (cytosine). These four chemicals are called nucleotide. The essence of life is information which is carried on these four nucleotides. These nucleotides are found in the nucleus of all living cells including humans, plants, and animals. Instruction in a single gene is written in thousands of AT/GC base pairs that are linked together in a straight line. A string of nucleotides is called DNA (Deoxyribose Nucleic Acid) –
Nobel prize was awarded to Crick, Watson & Morris Wilkins [1] for discovering the double helical nature of the DNA structure which is transcribed into a single stranded of RNA (in RNA the less water-soluble methyl group in Thiamine, T, is converted to more water-soluble Uracil, U, by replacing Methyl group with a Hydroxyl group) which leaves the nucleus and moves into Cytoplasm where it is translated in Ribosomes into Amino Acids leading to proteins). When thousands to millions of AT/GC base pairs contain information to make a single protein, we call that portion of AT/GC base pairs a gene (Nobel Prize was awarded to Khorana & Nauenberg for making a functional gene). The essence of life is information which is carried on these four nucleotides. These nucleotides are found in the nucleus of all living cells including humans, plants, and animals. Instruction in a single gene is written in thousands of AT/GC base pairs that are linked together in a straight line. A string of nucleotides is called DNA (Deoxyribose Nucleic Acid).
Nobel prize was awarded to Crick, Watson & Morris Wilkins [1] for discovering the double helical nature of the DNA structure which is transcribed into a single stranded of RNA (in RNA the less water-soluble methyl group in Thiamine, T, is converted to more water-soluble Uracil, U, by replacing Methyl group with a Hydroxyl group) which leaves the nucleus and moves into Cytoplasm where it is translated in Ribosomes into Amino Acids leading to proteins). When thousands to millions of AT/GC base pairs contain information to make a single protein, we call that portion of AT/GC base pairs a gene (Nobel Prize was awarded to Khorana & Nauenberg for making a functional gene).
A normal cell becomes abnormal when exposed to radiations, chemical/environmental pollution, viral infection or genetic inheritance.
To design a drug to shut off an abnormal gene, we need to know the exact location of the deleterious gene. By sequencing mutating cell genome and comparing with the Reference Sequence, the disease-causing mutations could be identified by any of the following methods: By comparing with Reference Sequence. SNIP map or Hap Map or Microarray.
In microarray thousands of flagrance tag are placed on a single chip. When patients DNA extracts are passed on RNA chips, it hybridized with the chip complimentary nucleotides forming DNA. Single stranded DNA or RNA have affinity to hybridize. Genes occur on a single stranded mRNA. It has a start codon AUG which codes for amino acid Methionine and several hundred codons later one of the three stop codons (UAG, UGA, of UGG) appears and DNA synthesis stops. The colored hybridized DNA identifies the specific mutation responsible for causing the disease. specific DNA could be obtained from the patient from SNP data.
SNIP map (Single Nucleotide Poly Morphism is a form of genotyping, which is the measurement of more general genetic variation. SNPs are one of the most common types of genetic variation. An SNP is a single base pair mutation at a specific locus at each thousand base pairs of the genome, usually consisting of two alleles (where the rare allele frequency is > 1%).) or from RFLP data (Restriction fragment length polymorphism (RFLP) which is a technique that exploits variations in DNA sequences. DNA from differing sources will have variations or polymorphisms throughout the sequence. Using Restriction Enzymes, these differences in sequences may be teased out.) In case of rapidly dividing cancer cells, most mutations occurred either due to deletion or insertion of DNA base pairs or when the nucleotide structure damage occurred. Such mutation by radiation found in laborers working in radioactive mine fields or living close to radioactive power plants exposed to radiation. Most mutation in cancer patients occurred due to rapid replication of cells. Insertion and deletion of a piece of DNA occurs frequently. Imagine A child is conceived on day one has a single cell by the time the child is grown up to age 21, that single cell has replicated over one hundred trillion times. Mistakes occurred during fast replication.
There are 220 different tissues in our body. Any of those tissues could become cancerous if exposed to radiation, chemical environmental pollution, viral infection or genetic inheritance. Accumulation of mutations are responsible for causing cancer. Mutated genes carry wrong instruction to make wrong proteins. These wrong proteins create Havoc in our body. Mutated genes disrupt biological pathway, disrupt DNA expression processes, invade neighborly tissues, disrupt normal gene function, spread site to site, disrupt the DNA repair processes, produce uncontrol cell growth, produce new tumors far from the site of origin. Accumulation of these mutation over the ears permanently transfer the normal function of the cell to become cancer cell. Accumulation of wrong protein over lifetime shut off the normal function of organs. By treating with various medicine. the Physician try to revive the function of one organ while the other organs fail. One by one when several organs fail, the body switches off all other organs and the patient dies.
In medical genome, every cancer gene will be sequenced and will be aligned with the Reference sequence (the sequencing of the entire human genome including good and bad genes will serve as a Reference Sequence). By comparing the cancer genome with the Reference Sequence, we could identify which chromosome on which nucleotide in the cancer genome base has been altered, how it is altered and how can it be fixed? The Sequence of every cancer gene will not only help us identify the mutated gene but also help us design drugs to shut off that gene.
We cannot design novel drugs unless we find the abnormal mutations responsible for causing that disease. The reading of the total genetic information that makes us human is called the Human Genome. The reading the entire book of our life is authorized by the US Congress under The Human Genome Project. It will answer the most fundamental question we have asked ourselves since the dawn of human civilization. What does it mean to be human? What is the nature of memory and our consciousness? Our development from a single cell to a complete human being? The biochemical nature of our senses, the process of our aging. The scientific basis of our similarity and dissimilarity, Similarity that all living creatures from a tiny blade of grass to the mighty Elephant including Man, Mouse, Monkey, Mosquitos and Microbes are all made of the same chemical building blocks and yet we are so diverse that no two individuals are alike even identical twins are not identical, they grow up to become two separate individuals.
In 1990, US Congress authorized three billion dollars to NIH to decipher the entire Human Genome under the title, “The Human Genome Project.” We found that our genome contains six billion four hundred million nucleotides bases, half comes from our father and another half comes from our mother. Less than two percent of our Genome contains genes which code for proteins. The other 98 percent of our non-coding region of our genome contains switches, promoters, terminators etc. The 46 Chromosomes present in each cell of our body are the greatest library of the Human Book of Life on planet Earth. Chromosomes carry genes which are written in nucleotides. Before sequencing (determining the number and the order of the four nucleotides bases on a Chromosomes), it is essential to know how many genes are present on each Chromosome in our Genome. The Human Genome Project has identified not only the number of nucleotides on each Chromosome, but also the number of genes on each chromosome.
The Reference Sequence:
The following list provides the detailed composition of each Chromosome including the number of nucleotides and the number of genes on each Chromosome:
We found that the Chromosome-1 is the largest Chromosome carrying 263 million A, T, G and C nucleotides bases and it has only 2,610 genes. The Chromosome-2 contains 255 million nucleotides bases and has only 1,748 genes. The Chromosome-3 contains 214 million nucleotide bases and carries 1,381 genes. The Chromosome-4 contains 203 million nucleotide bases and carries 1,024 genes. The Chromosome-5 contains 194 million nucleotide bases and carries 1,190 genes. The Chromosome-6 contains 183 million nucleotide bases and carries 1,394 genes. The Chromosome-7 contains 171 million nucleotide bases and carries 1,378 genes. Chromosome-8 contains 155 million nucleotide bases and carries 927 genes. The Chromosome-9 contains 145 million nucleotide bases and carries 1,076 genes. The Chromosome-10 contains 144 million nucleotide bases and carries 983 genes. The Chromosome-11 contains 144 million nucleotide bases and carries 1,692 genes. The Chromosome-12 contains 143 million nucleotide bases and carries 1,268 genes. The Chromosome-13 contains 114 million nucleotide bases and carries 496 genes. The Chromosome-14 contains 109 million nucleotide bases and carries 1,173 genes. The Chromosome-15 contains 106 million nucleotide bases and carries 906 genes. The Chromosome-16 contains 98 million nucleotide bases and carries 1,032 genes. The Chromosome-17 contains 92 million nucleotide bases and carries 1,394 genes. The Chromosome-18 contains 85 million nucleotide bases and carries 400 genes. The Chromosome-19 contains 67 million nucleotide bases and carries 1,592 genes. The Chromosome-20 contains 72 million nucleotide bases and carries 710 genes. The Chromosome-21 contains 50 million nucleotide bases and carries 337 genes. Chromosome-22 contains 56 million nucleotides and carries 701 genes Finally; the sex chromosome of all females called the (X) contains 164 million nucleotide bases and carries 1,141 genes. The male sperm chromosome contains 59 million nucleotide bases and carries 255 genes. [2-6]
If you add up all genes in the 23 pairs of Chromosomes, they come up to 26,808 genes and yet we keep on mentioning 24,000 genes needed to keep us function normally. As I said above, a gene codes for a protein, not all 24,000 genes code for proteins. It is estimated that less than 19,000 genes code for protein. Because of the alternative splicing, each gene codes for more than one protein. All functional genes in our body make less than 50,000 protein which interact in millions of different ways to give a single cell. Millions of cells interact to give a tissue, and 220 tissues give make an organ and several organs make a man, mouse, or a monkey. Out of 24,000 genes, 21,000 genes code for good proteins of commercial importance. Pharmaceutical companies sell those proteins as food supplements. The remaining 3,000 genes are responsible for causing diseases. Sequencing Human Genome provided two important pieces of information: First, sequence provides the identification of the mutation responsible for causing diseases. Second, it helps us design new small molecules to shut off those genes.
Next important steps are identification of mutated genes, prevention of disuses, and treatment either by gene therapy or drug therapy.
Fitz Haber, a German Army officer, worked on the development of Chemicals as a Weapon of War. He was responsible for making deadly Nerve gases sch as Nitrogen Mustards. Before WWI, he was honored with a Nobel Prize for capturing Nitrogen directly from the atmosphere for making Nitrate fertilizers by burning the element Magnesium in the air forming its Nitride. Upon hydrolysis, Nitride is converted to its Nitrate. Using this method, we could make unlimited amount fertilizer. Nitrate is also used for making explosives. Soon after the WWI, Haber was charged with a crime against humanity for releasing hundreds of cylinders of Chlorine gas on the Western front killing thousands of soldiers in the trenches. When Germany lost the war and Allied forces were looking for Haber. When they reached his residence, his son shot himself and his wife committed suicide. Haber went in hiding in Swiss Alps. After the War, German Government got his release as a part of the peace negotiations. Haber returned home to the hero’s welcome. Although he promised never to work on the chemical weapons again, secretly he continued to develop more lethal analogs of highly toxic chemicals like Nitrogen Mustards. It was Haber who first made the notorious Bis-dichloro-ethyl Methyl Amine. Because it smells like Mustard seeds, it is called Nitrogen Mustard. During the next 20 years, before the beginning of WWII, hundreds of more toxic analogs of Nitrogen Mustard were developed. The bad news is that they are highly toxic, and the good news is that they shut off genes.
Fossil bone extracts of soldiers who died of the chemical poisoning found in the trenches showed that the soldiers died slowly and were freeze to death. It was not clear if the effect was due to cold winter or due to chemical poisoning. Later, deaths of soldiers were due to chemical poisoning was confirmed.
One of the most disastrous bombing attacks against allied ships during the entire war took place. on Bari was an air attack by German bombers on Allied forces and shipping in Bari, Italy, on 2 December 1943, during World War II. US Liberty ship John Harvey carrying one hundred ton of Nitrogen mustard gas was hit by a German bombing raid, on Bari harbor releasing gas and mustard containing oil slick into harbor. Survivors who were exposed to the fumes had developed severe burns to the eyes, skin and respiratory track. Lt. Col Steward Alexander, a US army Physician determined the nature of the injuries but was too late to help victims, Clinical examination found that those who had initially survived the blast and who had been immersed in the water covered in burning oil were dying from late mustard gas poisoning that had profound depression of WBC, Alexander sent tissue samples of the victim to America where R.F. Clark later recorded. Alexanders Clincal observations were immediately recognized to have significant about therapy of neoplastic disorder of the leucopoietic system. SS John Harvey was a U.S. World War II Liberty ship. This ship is best known for carrying a secret cargo of mustard gas and whose sinking by German aircraft in December 1943 at the port of Bari in south Italy caused an unintentional release of chemical weapons.
Several American [merchant] ships with [U.S. Navy] Armed Guards aboard were at Bari on that fateful day [when a German air raid occurred]. When the last bomb had fallen, and the last ship exploded, and the large fires had run their course, 17 ships had been sunk and six damaged
About 90 percent of the gas casualties were American, the bulk of them merchant seamen. Since no U.S. hospital facilities were yet available in Bari - equipment for all but one of the U.S. hospitals scheduled for the area were destroyed in the bombing - casualties were hospitalized in British installation. Scientists observed that most patients were freeze to death due to the massive destruction of WBC. This information helps us design drugs to control the destruction of WBC.
Professor WCJ Ross of London University was the first person who used Nitrogen Mustard, a chemical weapon, to attack DNA for Cancer Treatment. Radiolabeled study showed that Nitrogen Mustard shut off a gene by cross-linking both strands of DNA that we inherit one strand from each parent. It was the same Cross-linking agents such as Nitrogen mustard made by Haber. Ross rationale was that cancer cells divide faster than the normal cell; by using Nitrogen Mustard he could use cross linking DNA and prevent cell division. Once he demonstrated that he could shut off a gene by cross-linking DNA; he could shut off any mutated gene including the genes of all 220 tissues present in a human by finding a dye that could specifically color that tissue. He could attach the Nitrogen Mustard group to the dye and attack the cancer genes in any one those 220 tissues.
Ross was the first person to use war chemicals successfully to treat cancer. Although such drugs are highly toxic, more cancer cell will be destroyed than the normal cells. Over decades, Ross made several hundred derivatives of Nitrogen Mustard as cross-linking agents. Some of the Nitrogen Mustards are useful for treating cancers such as Chlorambucil for treating childhood leukemia (Children suffering from Childhood Leukemia have a very high WBC count over 90,000 cells/CC. In sick children, most of the WBCs are premature, defected and unable to defend the body from microbial infections. Ross brought the WBC level from 90,000 down to 5,000/CC) and Melphalan and Myrophine for treating Pharyngeal Carcinomas. Because of the high toxicity of Nitrogen Mustard, new drugs could not be developed to treat other types of Oral or Lung Cancers.
The supreme intellect for Drug Design is Ross, an Englishman, who is a Professor of Chemistry at the London University. Professor WCJ Ross is also the Head of Chemistry Department at the Royal Cancer Hospital, a post-graduate medical center of London University. Ross was the first person who designed drugs for treating Cancers. He designed drugs to cross-link both strands of DNA that we inherit one strand from each parent. Cross-linking agents such as Nitrogen mustard are extremely toxic and were used as chemical weapons during the First World War. More toxic derivatives were developed during the Second World War. Using the Data for the toxic effect of Nitrogen Mustard used during the First World War, Ross observed that Soldiers exposed to Nitrogen Mustard showed a sharp decline of White Blood Cells (WBC) that is from 5,000 cell/CC to 500 cells/CC. Ross rationale was that cancer cells divide faster than the normal cell, by using Nitrogen Mustard to cross linking both strands of DNA, one can control and stop the abnormal WBC cell division in Leukemia patients. It was indeed found to be true. Professor Ross was the first person to synthesize many derivatives of Nitrogen Mustard. By using an analog of Nitrogen Mustard, called Chlorambucil, he was successful in treating Childhood Leukemia. In America, two Physicians named Goodman and Gilman from the Yale University were the first to use Nitrogen Mustard to treat cancer in humans. Nitrogen Mustards and its analogs are highly toxic. Ross was a Chemist, over the years, he synthesized several hundred derivatives of Nitrogen Mustard molecules to modify toxicity of Nitrogen Mustard. [7-12]
Although analogs of Nitrogen Mustard are highly toxic, they are more toxic to cancer cells and more cancer cells are destroyed than the normal cells. Toxicity is measured as the Chemotherapeutic Index (CI) which is a ratio between toxicity to Cancer cells versus the toxicity to Normal cells. Higher CI means that the drugs are more toxic to cancer cell. Most cross-linking Nitrogen Mustard have a CI of 10 that is they are ten times more toxic to cancer cells. Some of the Nitrogen Mustard analogs Ross made over the years several useful drugs for treating cancers such as Chlorambucil for treating childhood leukemia (which brought down the WBC level down to 5,000/CC). Children with Childhood Leukemia treated with Professor Ross Chlorambucil showed no sign of Leukemia even after 20 to 25 years. Chlorambucil made Ross one of the leaders of the scientific world. He also made Melphalan and Myrophine for treating Pharyngeal Carcinomas. [14].
At London University, I was trained as an Organic Chemist in the Laboratory of Professor WCJ Ross of the Royal Cancer Hospital, a post-graduate medical center of the London University. After working for about ten years at the London University, I moved to America when I was honored by the Fogarty International Fellowship Award by the National Institutes of Health, NIH, and the National Cancer Institute, NCI, of the USA. NIH has been my home for over a quarter of a century, I designed anti-cancer drugs to shut off mutated genes. All three Common Allele diseases have genetic origin. The rationale I used to synthesize anti-cancer drugs could be used to treat the other two old age diseases like Alzheimer or cardiovascular diseases. In the following sections, I will describe in detail how anti-cancer drug like AZQ was designed to shut off Glioblastoma genes which cause Brain Cancer in humans. Using the same rational, we will consider how each of the other two diseases namely cardiovascular disease and Alzheimer could be treated by shutting off their genes to save human life: The order of these diseases is arranged based on the level of funding provided by NIH specifically by the NCI (National Cancer Institute).
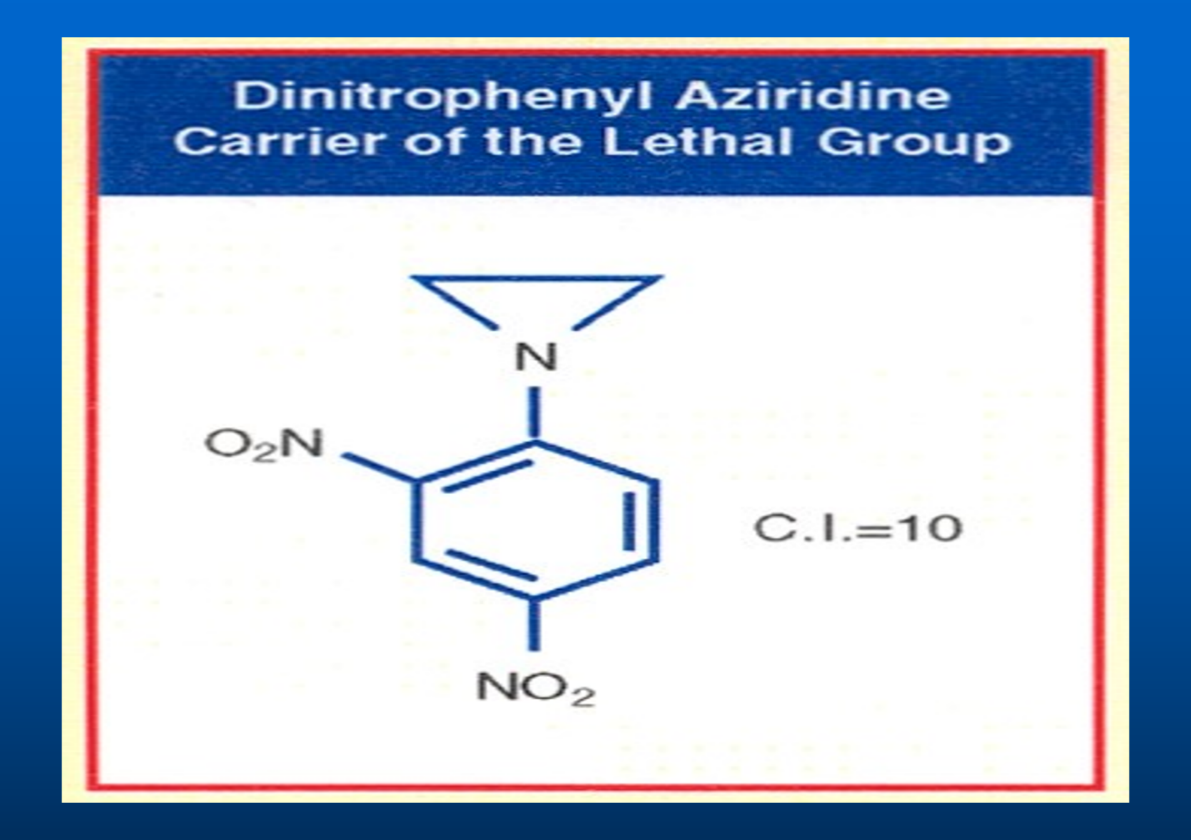
As I said above, Professor Ross was designing drugs to attack both strands of DNA simultaneously by cross-linking using Nitrogen Mustard analogs, which are extremely toxic. As a part of my doctoral thesis, I was assigned a different path. Instead of cross-linking DNA, I am to design drugs to attack only one strand of DNA. This class of drugs is called Aziridines. (The maker of Aziridine, James Warwick, died by exposure to Aziridine. When absorbs through the skin, Aziridine kills WBC. Because of the lack of WBC, JW freeze to death.) Over the years, I made over 100 Dinitrophenyl Aziridines derivatives. One of them is Dinitro benzamide (CB1954) which gives a CI of 70 highest ever recorded. CB1954 wipes out a solid tumor by attacking the DNA of Walker Carcinoma 256, a solid aggressive tumor in Rat.
Nitrogen Mustards are highly toxic because they have neither specificity nor selectivity. They attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates remain inactive in the basic and neutral media. They become activated only in the presence of Acidic media.
I used a simple rationale, the Aziridine attacks DNA in acidic medium, particularly the N-7 Guanine. The dye Dinitro benzamide has great affinity for Walker Tumor [19, 20]. The Aziridine dinitro benzamide (CB1954) stains the tumor. As the tumor grows, it uses Glucose as a source of energy. Glucose is broken down to Pyruvic Acid. It is the acid which attacks the Aziridine ring. The ring opens to generate a Carbonium ion which attacks the most negatively charged N-7 Guanine of DNA shutting off the Walker Carcinoma gene in Rat. To continue my work, I was honored with the Institute of Cancer Research Post-Doctoral Fellowship Award of the Royal Cancer Hospital of London University. To increase the toxicity of CB1954 to Walker Carcinoma, I made additional 20 analogs as a postdoctoral fellow. When I attached one more Carbonium generating moiety, the Carbamate moiety to the Aziridine Dinitrobenzene, the compound Aziridine Dinitro benzamide Carbamate was so toxic that its Therapeutic Index could not be measured. We stop the work at the London University for safety concerns.
I continued my work on the highly toxic Aziridine/Carbamate combination in America when I was offered the Fogarty International Fellowship Award to continue my work at the National Cancer Institute (NCI) of the National Institutes of Health (NIH). I brought the idea from London University of attacking one strand of DNA using not only Aziridine, but also Carbamate without using the same dye Dinitro benzamide.
My greatest challenge at NCI is to translate the animal work which I did in London University to humans. One day, I came across a paper which described that radio labeled Methylated Quinone cross the Blood Brain Barrier in mice. When injected in mice, the X-ray photograph showed that the entire radioactivity was concentrated in the Mice’s Brain within 24 hours. I immediately realized that Glioblastoma multiforme, the brain tumor in humans, is a solid aggressive tumor like Walker Carcinoma in Rats. I decided to use Quinone moiety as a carrier for Aziridine rings to attack Glioblastomas. By introducing an additional Carbamate moiety, I could increase its toxicity several folds. I planned to use this rational to translate animal work to human by introducing multiple Aziridine and Carbamate moieties to the Quinone to test against Glioblastomas in humans. Attaching two Aziridines and two Carbamate moieties to Quinone, I named this novel compound AZQ. By treating brain cancer with AZQ, we observed that Glioblastoma tumor not only stop growing, but also start shrinking. I could take care of at least one form of deadliest old age cancers that is Glioblastomas. Literature search showed that AZQ is extensively studied.
As I said above, Glioblastomas, the brain cancer, is a solid and aggressive tumor and is caused by mutations on several Chromosomal DNA. Mutations on DNA is the result of damaging DNA nucleotides by exposure to radiation, chemical and environmental pollution, viral infections or genetic inheritance. The other factors responsible for causing DNA mutations are due to the fast rate of replication of DNA. For example, the bacteria E-coli grows so rapidly that within 24 hours, a single cell on a petri dish forms an entire colony of millions when incubated on the Agar Gel. Mistakes occur in DNA during rapid replication such as Insertion of a piece of DNA, Deletion, Inversion, Multiple Copying, Homologous Recombination etc.
When an additional piece of nucleotide is attached to a DNA string, it is called Insertion, or a piece of DNA is removed from the DNA string; it is called Deletion or structural Inversion of DNA is responsible for mutations. Since the gene in DNA codes for Proteins, Insertion and Deletion on DNA have catastrophic effects on protein synthesis. Glioblastomas represent such an example. In Glioblastomas, three major changes occur on Chromosomes (C-7, C-9 & C-10) and two minor changes occur on Chromosomes (C-1 & C-19). These mutations are responsible for causing brain cancers in humans. In a normal human cell, Chromosome-7 which is made of 171 million nucleotide base pairs and it carries 1,378 genes. When Insertion occurs on Chromosome-7. Ninety-seven percent of Glioblastoma patients are affected by this mutation. On the other hand, a different mutation occurs on Chromosome-9 which is made of 145 million nucleotide base pairs, and it carries 1,076 genes. A major Deletion of a piece of DNA occurs on Chromosome-9 which results in eighty- three percent patients who are affected by this mutation. A minor Deletion of DNA also occurs on Chromosome-10 which is made of 144 million base pairs, and it carries 923 genes. Although it is a minor deletion of a piece of DNA and yet it contributes to ninety-one percent patients with Glioblastoma. To a lesser extent, small mutation occurs on Chromosome-1 (the largest Chromosome in our Genome). It is made of 263 million nucleotide base pairs and carries 2,610 genes) and Chromosome-19 (it is made of 67 million base pairs and carries 1,592 genes) is also implicated in some forms of Glioblastomas.
All known Glioblastomas causing genes are located on five different Chromosomes and carries a total of 9,579 genes. It appears impossible to design drugs to treat Glioblastomas since we don’t know which nucleotide on which gene and on which Chromosome is responsible for causing the disease. With the completion of the 1,000 Human Genome Project, it becomes easier. By simply comparing the patient’s Chromosomes with the one thousand genomes, letter by letter, word by word and sentence by sentence, we could identify the difference called the variants with precision and accuracy, the exact variants or mutations responsible for causing the disease. Once the diagnosis is confirmed, the next step is how to treat the disease.
With the Quinone ring, I could introduce different combinations of Aziridine rings and Carbamate moieties and could create havoc for Glioblastomas. My major concern was how toxic this compound would be to the human brain cells. Fortunately, brain cells do not divide, only cancer cells divide.
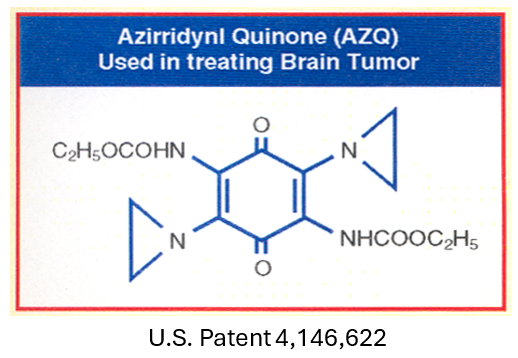
Our Rational Drug Design work began in the University of London, England, and completed in the Laboratory of the National Cancer Institute (NCI), of the National Institutes of Health (NIH), in Bethesda, Maryland, USA. Over this period, we conducted over 500 experiments which resulted in 200 novel drugs. They were all tested against experimental animal tumors. Forty-five of them were considered valuable enough to be patented by the US Government (US Patent 4,146,622). One of them is AZQ. Radiolabeled studies showed that AZQ can cross organ after organ, cross the Blood Brain Barrier, cross the nuclear membrane and attack the nuclear DNA shutting off the gene. X-ray studies showed that radioactivity is concentrated in the tumor region. Glioblastoma stops growing and start shrinking. For the discovery of AZQ, I was honored with the “2004 NIH Scientific Achievement Award” one of America’s highest awards in medicine and I was also honored with the India’s National Medal of Honor, “Vidya Ratna” a Gold Medal. (see Exhibits 1,2,3,4)
As a part of my doctoral thesis, I was assigned a different path. Instead of cross-linking both strands of DNA by Nitrogen Mustard, I am to design drugs to attack only one strand of DNA by making Aziridine analogues. We decided to use Aziridine moiety (as an intermediate of Nitrogen Mustard) that would be an excellent active component to shut off a gene by binding to a single strand of DNA. To deliver Aziridine to the target site which is the N-7 Guanine of DNA, we decided to use Dinitrophenyl (DNP) moiety as a drug delivery agent. DNP is a dye which colors the tissues of the experimental animal tumor such as Walker Carcinoma 256 in Rats.
It is well known that analogs of DNP such as Dinitrophenol disrupts the Oxidative Phosphorylation of the ATP (Adenosine Triphosphate) which provides energy to perform all our body functions. To provide energy to our body function, the high energy phosphate bond in ATP is broken down to ADP (Adenosine Diphosphate) which is further broken down to AMP (Adenosine Mono Phosphate), the enzyme Phosphokinase put the inorganic phosphate group back on the AMP giving back the ATP. This cyclic process of Oxidative Phosphorylation is prevented by Dinitrophenol. As a part of my doctoral thesis, I decided to use Dinitrophenol as a drug delivery method for the active ingredient aziridine. The analog of DNP such as Aziridine Dinitrophenol could also serves as a dye which stains Walker Carcinoma 256, a solid and most aggressive tumor in Rat. The first compound I made by attaching the C-14 radiolabeled Aziridine to the DNP dye. The Dinitrophenyl Aziridine was synthesized using Dinitrochlorobenzene with C-14 radiolabeled Aziridine in the presence of Triethyl amine which removes the Hydrochloric Acid produced during the reaction. When the compound Dinitrophenyl Aziridine was tested against the implanted experimental animal tumor, the Walker Carcinoma 256 in Rats, it showed a TI (Therapeutic Index) of ten. The TI of ten was like most of the analogs of Nitrogen Mustard. Since this Aziridine analog was not superior to Nitrogen Mustard, it was dismissed as unimportant.
On further reexamination of the X-ray photographs of Dinitrophenyl Aziridine, it appeared that most of the radioactivity was concentrated at the injection site. Very little radioactivity was observed at the tumor site. It was obvious that we need to make derivatives of Dinitrophenyl Aziridine to move the drug from the injection site to the tumor site. Because of the lack of fat/water solubility to be effective drug delivery method, Dinitrophenyl Aziridine stays at the injection site, a very small amount of radioactivity was found on the tumor site.
I immediately realized that by altering structure, I could enhance biological activity by making water and fat-soluble analogs of Dinitrophenyl Aziridine. By attaching water soluble groups, I should be able to move the drug from the injection site to the tumor site. To deliver 2,4-Dinitrophenylaziridine form the injection site to tumor site, I could alter the structure of 2,4-Dinitrophenylaziridine by introducing the most water-soluble group such as ethyl ester to the least water-soluble group such as Cyano- group or to introduce an intermediate fat/water soluble such as Amido group.
An additional substituent in the Dinitrophenyl Aziridine could give three isomers, Ortho, Meta, and Para substituent. Here confirmational chemistry plays an important role in drug delivery method. Ortho substituent always give inactive drug. Model building showed that because of the steric hinderance, Aziridine could not bind to DNA shutting off the genes. On the other hand, Meta and Para substituents offer no steric hindrance and drug could be delivered to DNA. When injected in Rat, because of the high solubility, most of the drugs was pass down through urine and extracted the drug from Rat urine by chloroform, The following chart showed that I synthesized all nine C-14 radiolabeled analogs of 2,4-Dinitrophenyl aziridines and tested them against implanted Walker Carcinoma 256 in Rats
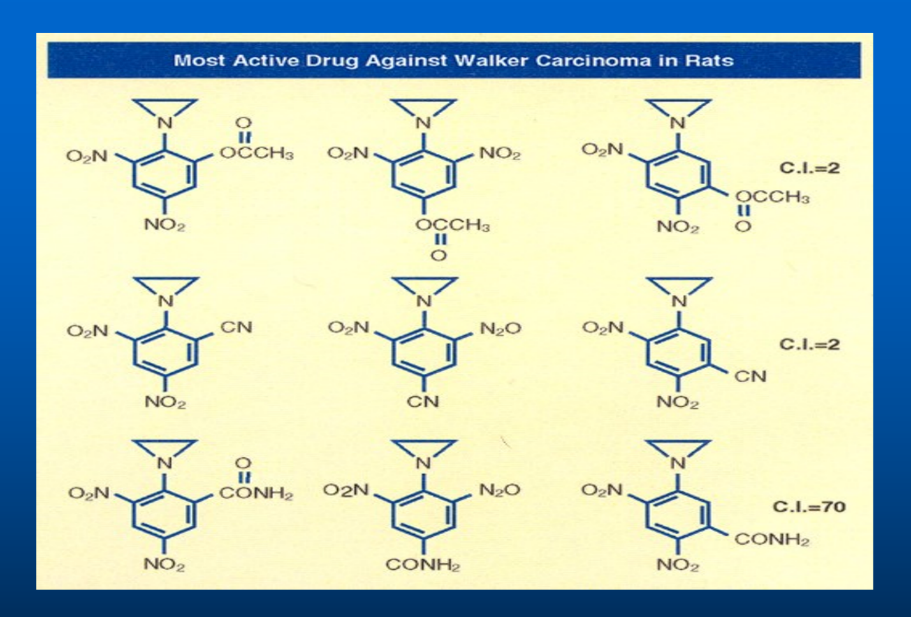
Derivatization of Dinitro phenyl Benzamide based on Partition Coefficient
The most water-soluble substituent:
The first three compounds on top line of the above chart carry all three isomer of the most water-soluble Ethyl Ester group attached to 2,4-Dinitropehny aziridine. The compound in vivo is hydrolyzed Ethyl Ester to produce most water-soluble carboxylic group. Since it is the most water-soluble substituent, within 24 hours of injection in Rats, the entire radioactive compound was passed down from in the Rat urine and it can be extracted by Chloroform. Since the Ortho position was not available for DNA binding, it showed no biological activity, but the third compound in which Ortho position was free to bind to DNA showed some anti-tumor activity in Rats.
The least water-soluble substituent:
On the other hand, when the least water-soluble Cyano-group was attached to all three isomers of the 2,4-Dinitrophenyl aziridine compound as shown in the second line of the above chart, most of the compound stayed at the injection site. Only the last Cyano-derivative attached to DNA showed some anti-tumor activity.
The moderately soluble Amido-substituent:
The last line of the above chart showed that the first two Amido groups were sterically hindered and did not bind to DNA and showed no biological activity, but the last compound presents the perfect drug delivery method. The entire drug was delivered from the injection site to the tumor site. The drug 1-Aziridine, 2,4-dinitro, 5-benzamide (CB1954) showed the highest anti-tumor activity. It has a CI of seventy; it is seventy times more toxic to cancer cells, the highest toxicity ever recorded against Walker Carcinoma 256 in Rats. [14-17]
As I said above, Nitrogen Mustards are highly toxic because they have neither specificity nor selectivity. They attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates serve as pro-drug and remain inactive in the basic and neutral media. They become activated only in the presence of acid produced by growing cancer cells. Aziridine attacks DNA in acidic medium, particularly the N-7 Guanine. The dye Dinitro benzamide has great affinity for Walker Tumor. The Aziridine Dinitro benzamide (CB1954) has the highest toxicity to Walker Tumor cells ever recorded. As the tumor grows, it uses Glucose as a source of energy. Glucose is broken down to Lactic Acid. It is the acid which activates the Aziridine ring. The ring opens to generate a carbonium ion which attacks the most negatively charged N-7 Guanine of DNA (as shown below) shutting off the Walker Carcinoma gene in Rat. The following conjugate structure shows how CB1954 binds to a single stranded of DNA shutting off the gene.
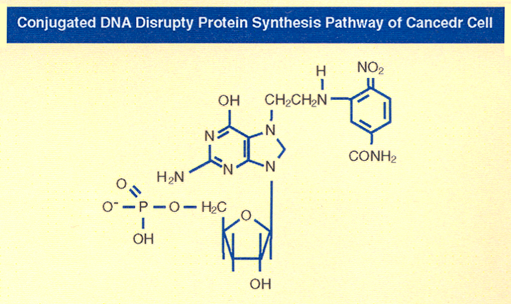
Conjugated DNA Disrupting Protein Synthesis Pathway of Cancer Cell
For the discovery of CB1954, The University of London, honored with the Institute of Cancer Research (ICR) post-doctoral fellowship award to synthesize more analogs of CB1954. Over the years, I made over a hundred additional analogs of Dinitro phenyl aziridines. To increase the toxicity of CB1954 to Walker Carcinoma, I made additional 20 analogs as a postdoctoral fellow. When I attached one more Carbonium ion generating moiety, the Carbamate moiety to the Aziridine Dinitrobenzene, the compound Aziridine Dinitro benzamide Carbamate was so toxic that its Therapeutic Index could not be measured. We stopped work. Further work at London University was discontinued for safety reasons.

The Best and the Worst Dinitro phenyl Aziridine Analogs
Although Aziridine Carbamate is extremely toxic, it is also very useful in testing the sensitivity of tumors in Tumor Bank. Over the years, some tumors in the tumor bank could become resistant. If a tumor culture survives in a petri dish by adding a solution of Aziridine Dinitrobenzene Carbamate, it means that this tumor has become resistant over the years and must be replaced by new sensitive tumor cells.
As a part of the inter-government agreement between UK and USA, all novel drugs developed in England were sent to the National Cancer Institute (NCI) in America for further screening. To translate animal work to human, I was invited to continue my work on the highly toxic Aziridine/Carbamate combination in America when I was offered the Fogarty International Fellowship Award to continue my work at the National Cancer Institute (NCI) of the National Institutes of Health (NIH), USA. For making more Aziridine/Carbamates, I brought the idea from London University of attacking one strand of DNA using not only Aziridine, but also Carbamate without using the same dye Dinitro benzamide. My greatest challenge at NCI is to translate animal work to humans.
In developing drugs for treatments, we poison bad DNA selectively. All poisons are a class of chemicals that attack all DNA good and bad alike. Chemicals that cause cancer, at a safe level, can also cure cancer. Science teaches us to selectively attack bad sets of DNAs without harming the good sets of DNAs. Poisons are injurious to living creatures. There is a small class of chemical, when exposed to humans, disrupt the function of DNAs, and make normal cells abnormal and they are called cancer causing chemicals or carcinogens. I must confess, we still use surgery to cut off a cancerous breast; we still burn cancer cells by radiation; and we still poison cancer cells by chemicals. The largest killer of women is breast cancer. After all the treatment, the remaining cancer cells return as metastatic cells and kill breast cancer patients in three years. A decade from now, these methods could be considered brutal and savage, but today that is all we have. We hope to develop new treatment for Breast Cancer. Hope means never ever to give up.
Glioblastoma (GBM) is a primary type of brain cancer which originates in the brain, rather than traveling to the brain from other parts of the body, such as the lungs or breasts. GBM is also called glioblastoma multiforme which is the most common type of primary brain cancer in adult humans. Attaching Nitrogen Mustard group to a carrier dye will produce highly toxic compound which will have neither specificity nor selectivity. Such a compound will attack all dividing cells whether they are normal or abnormal. On the other hand, the analogs of Aziridines and Carbamates serves as prodrugs that is they remain inactive in the basic and neutral media. They become activated only in the presence of acid produced by cancer cells.
Designing drugs to treat Glioblastoma, the human brain cancers:
One day, I heard an afternoon lecture at the NIH in which the speaker described that radio labeled Methylated Quinone crosses the Blood Brain Barrier (BBB) in mice. When injected in mice, the X-ray photograph showed that the entire radioactivity was concentrated in the Mice’s brain within 24 hours. I immediately realized that Glioblastoma multiforme, the brain tumor in humans, is a solid aggressive tumor like Walker Carcinoma in Rats. I decided to use Quinone moiety as a novel drug delivery molecule to cross BBB (Blood Brain Barrier) delivering Aziridine rings to attack Glioblastomas. By introducing an additional Carbamate moiety, I could increase its toxicity several folds. I planned to use this rationale to translate animal work to human by introducing multiple Aziridine and Carbamate moieties to the Quinone molecule to test against Glioblastomas in humans.

The Structure of a non-toxic and non-addictive Quinone used for crossing the Blood Brain Barrier (BBB)
With the Quinone ring, I could introduce two Aziridine rings and two Carbamate moieties and could create havoc for Glioblastoma. Within three years, I made 45 analogs of Quinone. One of the Quinone carries two aziridines and two carbamate moieties which was highly toxic to Glioblastoma. The tumor stopped growing and started shrinking. I named the Di-aziridine Dicarbamate Quinone, AZQ. My major concern was how toxic this compound would be to normal brain cells. Fortunately, brain cells do not divide, only cancer cells divide. AZQ acts as a Prodrug. A Prodrug is a compound carrying a chemical by masking group that renders it inactive and non-toxic. Once the prodrug reaches a treatment site in the body, removing the mask frees the active drug to go only where it is needed, which helps avoid systemic side effects. Aziridine and Carbamate show selectivity. As I said above, to grow rapidly, cancer cells use Glucose as a source of energy. Glucose is broken down to produce Lactic acid. It is the acid which activates the pro-drug aziridine and carbamate moieties generating Carbonium ions attacking Glioblastoma which stop growing and starts shrinking.
My drug AZQ is successful in treating experimental brain tumors because I rationally designed to attacks dividing DNA. Radio labeled studies showed that AZQ bind to the cancer cells DNA and destroy brain tumor and normal brain cells are not affected at all. AZQ is a new generation of drugs. Not so long ago, brain cancers mean death. Now, we have changed it from certain death to certain survival. The immunologists in our laboratories are developing new treatment techniques by making radio labeled antigens to attack remaining cancer cells without harming normal cells.
We have cured many forms of cancer. We have eliminated childhood leukemia, Hodgkin disease, testicular cancer and now AZQ type compounds which are being developed rationally. While most anti-cancer drugs such as Adriamycin, Mitomycin C, Bleomycin etc, in the market are selected after a random trial of thousands of chemicals by NCI, AZQ is rationally designed for attacking the DNA of cancer cells in the brain without harming the normal cells. We are testing combinations of these drugs to treat a variety of experimental cancers in animals.
DNA Binding Aziridines:
I decided to use Quinone moiety as a carrier for Aziridine rings to attack Glioblastomas. By introducing an additional Carbamate moiety, I could increase its toxicity several folds. I planned to use this rational to translate animal work to human by introducing multiple Aziridine and Carbamate moieties to the Quinone to test against Glioblastomas in humans. Over the years, I made dozens of analogs of Aziridine Quinone. By attaching two Aziridines and two Carbamate moieties to Quinone, I synthesized the most useful compound, Diaziridine Dicarbamate Quinone, I named this novel compound AZQ. Over a three-year period, I made 45 analogs of AZQ. They were all considered valuable enough to be patented by the US Government (US Patent 4,233,215). By treating brain cancer with AZQ, we observed that Glioblastoma tumor not only stops growing, but it also starts shrinking. I could take care of at least one form of deadliest old age cancer, Glioblastomas. Literature search showed that AZQ is extensively studied as a pure drug and in combination with other anti-cancer drugs.
Single Strand DNA Binding Aziridine and Carbamate
As I said above, Glioblastomas, the brain cancer, is a solid and aggressive tumor and is caused by mutations on several sites in chromosomal DNA. Deleterious genetic mutations are the result of damaging DNA nucleotides by exposure to radiation, chemical and environmental pollution, viral infections, or genetic inheritance. The other factors responsible for causing DNA mutations are due to the fast rate of replication of DNA. For example, the bacteria E-coli grows so rapidly that within 24 hours, a single cell on a petri dish containing nutrients forms an entire colony of millions when incubated on the Agar Gel. Mistakes occur in DNA during rapidly replication such as Insertion of a piece of DNA, Deletion, Inversion, Trans location, Multiple Copying, Homologous Recombination etc. When an additional piece of nucleotide is attached to a DNA string, it is called Insertion, or a piece of DNA is removed from the DNA string; it is called Deletion or structural Inversion of DNA is also responsible for mutations. Since the gene in DNA codes for Proteins, Insertion and Deletion on DNA have catastrophic effects on protein synthesis. With the Quinone ring as a carrier across BBB, I could introduce different combinations of Aziridine rings and Carbamate moieties to Quinine and could create havoc for Glioblastomas. My major concern was how toxic this compound would be to the human brain cells. Fortunately, brain cells do not divide, only cancer cells divide.
Attempting to find the site of mutations on Glioblastomas represents the greatest challenge. In Glioblastomas, three major changes occur on Chromosomes (C-7, C-9 & C-10) and two minor changes occur on Chromosomes (C-1 & C-19). These mutations are responsible for causing brain cancers in humans. Let us examine the effect on each chromosome. In a normal human cell, Chromosome-7 which is made of 171 million nucleotide base pairs, and it carries 1,378 genes. When Insertion occurs on Chromosome-7. Ninety-seven percent of Glioblastoma patients are affected by this mutation. On the other hand, a different mutation occurs on Chromosome-9 which is made of 145 million nucleotide base pairs, and it carries 1,076 genes. A major Deletion of a piece of DNA occurs on Chromosome-9 which results in eighty- three percent patients who are affected by this mutation. A minor Deletion of DNA also occurs on Chromosome-10 which is made of 144 million base pairs, and it carries 923 genes. Although it is a minor deletion of a piece of DNA and yet it contributes to ninety-one percent patients with Glioblastoma. To a lesser extent, small mutation occurs on Chromosome-1 (the largest Chromosome in our Genome). It is made of 263 million nucleotide base pairs and carries 2,610 genes) and Chromosome-19 (it is made of 67 million base pairs and carries 1,592 genes) is also implicated in some forms of Glioblastomas.
All known Glioblastomas causing genes are located on five different chromosomes and carries a total of 9,579 genes. It appears impossible to design drugs to treat Glioblastomas since we do not know which nucleotide on which gene and on which chromosome is responsible for causing the disease. It becomes possible by using C-14 radiolabeled Aziridines, we can confirm the binding site of a nucleotide on a specific gene and on a specific chromosome. By comparing with the mega sequencing genome project, we can further confirm the sites of mutations.
With the completion of the 1,000 Human Genome Project, it becomes easier. By simply comparing the patient’s genome with the sequencing of 1000-genomes, letter by letter, word by word and sentence by sentence, we could identify the differences called the variants with precision and accuracy, the exact variants, or mutations responsible for causing the disease. Once the diagnosis is confirmed, the next step is how to treat the disease. As I explained above, by making CB 1954 to treat solid Walker Carcinoma in Rats, I established the structure activity relationship, and by making AZQ to treat human Glioblastoma, we have demonstrated that all bad genes can be shut off using Aziridine or Carbamate or both as attacking agents to shut off a gene. If you plan to develop drugs to treat other cancers, all we need to do is to identify carriers such as coloring dyes which stains a specific tumor. By attaching Aziridines and Carbamate moiety to carriers to the dyes, we could attack other tumors. [18-20]
One of the greatest challenges of nanotechnology is to seek out the very first abnormal cell in the presence of billions of normal cells of our brain and shut off the genes before it spread. I worked on this assignment for about a quarter of a century; conducted over 500 experiments which resulted in 200 novel drugs. They were all tested against experimental animal tumors. Forty-five of them were considered valuable enough to be patented by the US Government (US Patent 4, 146, 622 & 4,233,215). One of them is AZQ which not only stops the growth of Glioblastoma, but also the tumor starts shrinking. For the discovery of AZQ, I was honored with, “The 2004 NIH Scientific Achievement Award.” One of America’s highest Award in Medicine. I was also honored with the India’s National Medal of Honor, “Vidya Ratna” a Gold Medal. (see Exhibits 1,2,3,4)
2004 NIH Scientific Achievement Award
Presented to
Dr. Hameed Khan
By
Dr. Elias Zerhouni,
The Director of NIH
During the NIH/APAO Award Ceremony held on December 3, 2004.
Dr. Khan is the Discoverer of AZQ (US Patent 4,146,622 & 4,233,215), a Novel Experimental Drug Specifically Designed to shut off a Gene that causes Brain Cancer for which he receives a 17-year Royalty for his invention (License Number L-0I9-0I/0). To this date, more than 300 research papers have been published on AZQ. The award ceremony was broadcast live worldwide by the Voice of America (VOA). Dr. Khan is the first Indian to receive one of America’s highest awards in Medicine.

NIH Scientific Achievement Award
His Excellency, Dr. A.P.J. Abdul Kalam,The President of India
Greetings
Dr. A. Hameed Khan,

Discoverer of anti-cancer AZQ, after receiving 2004, Vaidya Ratna,
The Gold Medal, One of India’s Highest Awards in Medicine
At The Rashtrapathi Bhavan (Presidential Palace), in Delhi, India, During a Reception held on April 2, 2004.

Gold Medal for Dr. Khan

Dr. A. Hameed Khan, a Scientist at the National Institutes of Health (NIH) USA, an American Scientist of Indian Origin was awarded on April 2, 2004. Vaidya Ratna; The gold Medal, one of India’s Highest Awards in Medicine for his Discovery of AZQ (US Patent 4,146,622) which is now undergoing Clinical Trials for Treating Bran Cancer.
After surgically removing the breast tumor from the breast cancer patient, the residual remaining tumor cell becomes resistant and returned in three years as metastatic cancer kill the patients. We need to design drugs to kill every cancer cell. Only drugs can remove the remaining residual tumor cell. Male and female hormones can act as carriers for Aziridine and Carbamate molecules. They act as pro-drugs. They are activated only in the presence of acid. As breast cancer cell grow, they use glucose as a source of energy. In the absence of Oxygen, Glucose is broken down to Lactic Acid. It is the acid which activate Aziridines and Carbamate molecules producing the post powerful Carbonium ions which attack the tumor cell shutting off the gene.
The next generation of scientists, my students, will face the great challenge to design novel drugs to treat breast cancer the largest killer of women.
In the early history for developing drugs for treatments of cancers, we poison bad DNA selectively. All poisons are a class of chemicals that attacks DNA molecules good and bad alike. Chemicals that cause cancer, at a safe level, can also cure cancer. Science teaches us to selectively attack bad sets of DNAs without harming the good sets of DNAs. Poisons are injurious to living creatures. There is a small class of chemical, when exposed to humans, disrupt the function of DNAs, and make normal cells abnormal and they are called cancer causing chemicals or carcinogens.
In the absence of any rational drug, I must confess, we still use surgery to cut off a cancerous breast; we still burn cancer cells by radiations; and we still poison cancer cells by chemicals. After all the current treatments, the remaining cancer cells return within three years as metastatic cells and kill breast cancer patients. A decade from now, these methods could be considered as brutal and savage, but today that is all we have. Based on rational design, we hope to develop new treatment for Breast Cancer. Hopes means never ever to give up.
To design drug rationally to treat Breast Cancer, and to shut off a gene of a specific cancer by using Aziridine or Carbamate, we need a carrier for these groups. For example, to treat Breast and Prostate cancers in humans, may I suggest that we try using hormones which could serve as carriers for Aziridine and Carbamate moiety. Could I use the same rationale for treating Breast tumor as I used for making AZQ for treating Brain cancer? Although BRCA1 gene located on Chromosome-17 (which is made of 92 million nucleotide base pairs carrying 1,394 genes) has been identified years ago, we wonder why it has been so difficult to treat Breast Cancer. By the time the Breast Cancer diagnosis is confirmed in a patient, the BRCA1 gene has accumulated more than three thousand mutations. Genotyping of the blood would also show that composition of many cells carrying mutated cell for creating secondary deposits. It is also believed that by the time Breast Cancer is confirmed, metastatic cancer cells have already been spread from liver lung on its way to brain. Since all other organs including breast could be removed and replaced by breast implant except brain, I thought that protecting brain is utmost important treatment. Once AZQ (US Patent 4,233,215) is developed to protect the brain, I could focus on the Breast and Prostate Cancers.
Radiolabeled studies showed that male hormone Testosterone has great affinity for female Breast, Ovary, and Fallopian tube cells. On the other hand, Estrogen, the female hormone, has great affinity for male prostate gland. By using male and female hormones as carriers, I could attach multiple Aziridine rings and Carbamate ions to both Hormones to attack the Breast and the Prostate cancer.
In a Breast tumor, within the start and stop codon, BRCA1 gene has captured over two hundred thousand nucleotide base pairs. The BRCA1 genes carries about three thousand mutations. These mutations are caused by radiations, chemical or environmental pollutants, viral infection, or genetic inheritance. To attack the mutated nucleotides among the three thousand cells in BRCA1 gene, I could use male hormone, Testosterone, and bind multiple radio labeled Aziridine and Carbamate ions to attack BRCA1 mutations. By using MRI, I could show how many radio-labeled nucleotides were bound to which mutations. Out of seventeen positions on Testosterone, only three positions that is 1,3 and 17 are available for substitution on Testosterone ring system.

Carl Djerassi (C. Djerassi et al. J. Amer. Chem. Soc. 72. 4534 (1950) [21] had demonstrated that we could activate additional positions for substitutions on hormone ring system such as the position 9 and 10 by reacting with Bromo-acetamide which introduce a Bromo ion on position 10 which could be de-brominated by Collidine to introduce a 9,10 double bond which we could further brominate to produce 9,10 dibromo compound. These bromo ion could be replaced by additional Aziridines or Carbamate ions. We could increase or decrease the number of Aziridine and Carbamate ions to get maximum benefit by further brominating position such as 15 and 16 to introduce additional Aziridine and Carbamate moieties.
Similarly, we could use the female hormone Estrogen and by attaching multiple Aziridine and Carbamate ions to attack Prostate tumor in Men. Since out of seventeen positions, only three positions are available on Estrogen ring as well; again, we could increase or decrease the number of Aziridine and Carbamate ions to get the maximum benefit by using Djerassi’ method as we did with Testosterone. The above methods are novel approach to designing drugs to treat Breast and Prostate cancers using genetic make-up of a patient to treat metastatic cancers. The next generation scientists, my students, will have the opportunity to translate this dream into reality.
We always wonder what is right and what is wrong. What is right for one person is it right for others. Could we limit the behavior of other because it affects us all?
If the impact of sequencing the human genome on the medical genomic is to be successful, we must sequence the first cell from the 8-cell embryo. Families with a long history of serious illnesses should be asked to donate a single cell from the fertilized ovum. By sequencing the embryo genome and comparing with the Reference Sequence, we could identify mutations responsible for causing the illnesses. For example, you find mutations on Chromosome-17 (Chromosome-17 is made of 92 million bases and carries 1,294 genes). we found two deleterious mutations on Chromosome-17. One BRCA1 responsible for causing Breast Cancer in both men and women. The second is the BRCA2 gene responsible for causing prostate cancer in men. Once mutated genes are identified, we can design drugs to shut off those genes. Medical Genome promises precision medicine. I know two methods to design drugs to shut off those genes. First method was used by Professor Ross of London University. He used deadly war chemicals like Nitrogen Mustard to cross-link the DNA and shut off that gene. His drug Chlorambucil is used to treat childhood leukemia. As his student, I used Aziridine to make AZQ to shut off Glioblastoma gene. Aziridines are pro-drug, they have to be activated by acid to open the ring. There are 220 tissues in our body, they could all become mutated if exposed to radiations, chemical environmental pollution, viral infection or genetic inheritance. If the single tumor cell from the 8-cell embryo shows deleterious mutation, we could discard this cell and use a new cell free from all genetic defects and still have a healthy baby. The 8-cell embryo is not alive, it could be frozen at minus seventy degree in liquid Nitrogen for years. The fertility clinics and cryo-preservation labs are full of frozen embryos.
Of all three cancers, three cancers are deadly: Brain cancer, Breast cancer and lung cancer. Most of us believe that people have a right to live and also, they have the right to die. Why we should risk our lives to save smokers. We are convinced that we can treat the illness, but we cannot treat addiction. Once they recover, the smokers go back to smoking. In breast cancer, tumor cells could be removed by surgery, unfortunately, the remaining residual cells returns within three years as metastatic cancer and kill the patient. 450,000 women die of breast cancer each year. My drug AZQ reduce the glioblastoma and prolong the patient’s life for at least two additional years.
Who should have this information besides the parents. The following examples will explain that we need new ethical principle based on modern science:
Recently in a woman, we found a mutation on Chromosome-5 which is responsible for causing Thrombosis leading to sudden heart attack. It is a dominant mutation. If she were to have three more brothers or sisters, 75% will come down with this disease. Fortunately, she has only one younger brother who refuses to take the test. Since it is a dominant gene, he has a 50% chance of coming down with this disease. He is now 16-years old. He is eligible to apply for the driver’s licenses. He has a right to a driver’s licenses. Should we allow him to drive a car. What if he gets a hears attack while driving the car in a crowded street or high way. He could take away the life of several innocent people. Where his rights end and others right begin. Should we allow him to get the driver’s licenses without taking the genetic test?
If a woman carrying a mutated fetus or seriously handicapped fetus, should we allow religious leaders to deny abortion. I also oppose abortion on scientific ground because when terminating the pregnancy, all life lines to the fetus are cut off. It could have disasterous effect on mother’s health. Instead, I suggest your sequence any of one of the 8-cell embryo, sequence it and comparing with it the Reference Sequence. If we detect a deleterious mutation, we could discard the cell and use another on free from any genetic defects. As I said above these cells are not alive, they could be frozen below seventy degrees in liquid Nitrogen for years.
The major cause of lung is smoking. Smokers have a right to smoke. When their rights ends where my nose begins. People have a right to live and they have right to die. Should lung cancer smokers be treated. The answer is Yes. Most senior scientists would not like to develop drugs to treat lung cancer. As I said above most highly toxic war chemicals are used to turn off those genes. Scientists wonder should they risk their life by making such drugs to save the life of smokers. Even if develop such drugs to treat lung cancer. What use once they recover, they will go back to smoking, we can treat illness, but we cannot treat addiction. The major culprit of tobacco is Nicotine. Scientists know that an enzyme mono-amino oxidase add oxygen to Nicotine to make Nicotine-N- Oxide. All N-oxides are carcinogens. How should we control smoker’s behavior?
Suppose by sequencing the whole genome and comparing with the Reference Sequence, we identify a mutation in the retinoblastoma gene which could result in blindness. Is there a reason to have incurably blind children in our highly competitive society?
Nanopore Sequencer will provide cheaper and faster sequencer. If the price of per genome comes down to $100 per genome. In your annual checkup with your family physician, he will send you to the sequencing lab to have your genome sequenced and send it to him. He will compare your genome with the Reference Sequence and identify the mutations responsible for your current condition. Once identified the mutations, he will provide the correct medicine. This is called the precision medicine. No more guess work or trail and error work. The problem is who should see your genome besides your and your doctor. Your life insurance or medical Insurance agent. Should your employer see your genome sequence. The 8-cell embryo will help us identify all deleterious mutations specially in families that have long history of serious illnesses. If such families want to have children, they should be recommended to have conception of in vitro fertilization using one of the cell from their 8-cell embryo free from all genetic defects.
We need new ethical principles based on modern science. The old ethical principle also came from people’s head. But they were based on social and religiously acceptable principles or in case of Devenport purely on racial basis. They were not based on scientific facts. We need debates and discussions from all members of our society including religious leaders to draw guidelines for scientists working in the Labs. One person cannot provide answers to all the ethical concerns. What I want to do is to raise these concerns your mind. My aim will be fulfilled if I have made you think along these lines.
Opinion expressed in this lecture is mine and does not represent NIH policy.
Dr. Hameed Khan was born in (Hyderabad Deccan) India, educated in England and received his doctorate degree in Organic Chemistry from the University of London. He is a recipient of the Institute of the Cancer Research postdoctoral fellowship award of the Royal Cancer Hospital, University of London and a recipient of the Fogarty International postdoctoral fellowship award of the National Institutes of Health (NIH), and the National Cancer Institute of USA. He is a discoverer of AZQ (US Patent 4, 233-215) for which he was honored with the “2004 NIH Scientific Achievement Award” one of Americas’ highest awards in medicine for discovering AZQ for treating brain cancer. He was also honored with a Gold Medal by the Government of India. He is a Fellow of the American Institute of Chemistry and was elected to the American Science Advisory Board. After working for 30 years, Dr. Khan retired from the Lab and Administrative duties (conducted 250 Study Sections). After retirement, he volunteered to continue to teach NYLF Scholars on the mission of the NIH Speaker’s Bureau. . As a part of NIH Speaker’s Bureau’s mission, Dr. Khan served as an Adjunct Professor teaching the Scholars of the NYLF (National League Youth Forum) on the Impact of Science on Society. Currently, he is delivering a series of lectures on-line on the Impact of Sequencing Human Genome on the designing novel drugs to diagnose, prevent and treat cancer. All his lectures are published in scientific journals and can be found on Guggle and Facebook.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti