AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2640-1045/166
1 Assistant Lecturer of Endocrinology and Internal Medicine-School of Medicine-New Giza University.
2 Professor of Endocrinology and Internal Medicine, Head of Endocrinology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
3 Assistant Professor of Endocrinology and Internal Medicine, Endocrinology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
4 Assistant Professor of Histology and Cell Biology, Coordinator of Stem Cell Unit, Histology and Cell Biology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
5 Lecturer of Endocrinology and Internal Medicine, Endocrinology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
*Corresponding Author: Maged Hossameldin, Assistant Lecturer of Endocrinology and Internal Medicine-School of Medicine-New Giza University.
Citation: Maged Hossameldin, Mohamed Halawa, Laila Hindawy, Asmaa Abozeid, Dina Marawan, (2023), Study on Thyroid Function and Structure in Hypothyroid Rats after Umblical Cord Blood Derived Stem Cells Transplantation Versus their Conditioned Media, J, Endocrinology and disorders; 7(7); DOI:10.31579/2640-1045/166
Copyright: © 2023, Maged Hossameldin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 01 November 2023 | Accepted: 20 November 2023 | Published: 30 November 2023
Keywords: hypothyroidism; stem cells (SC); transplantation; conditioned media; hypothyroid rats; TFTs; UK
Background: Primary hypothyroidism is a chronic condition with significant morbidity. Providing physiological thyroid hormone replacement through pharmacological treatment remains controversial.
Aim of work: To evaluate the efficacy of human umbilical cord blood-derived mesenchymal stem cells (UM-MSCs) transplantation versus their conditioned media (CM) on thyroid function tests (TFTs) and thyroid structure in hypothyroid rats, also to compare intravenous versus its intranasal route.
Methods: This study was conducted on forty male Wistar rats divided equally into 4 groups. Group I served as a negative control group, group II received amiodarone (AMD, 100 mg/kg /day for one month) to induce hypothyroidism, group III (AMD+UM-MSCs) through intranasal and intravenous routes, and group IV (AMD+CM) through intranasal and intravenous routes. TFTs were measured at the beginning of the experiment, after 30 days of AMD administration, and then after ten days from the injection of stem cells and conditioned media (at day 40). Bodyweight and rectal temperature were measured weekly during the study. Rats were authentically sacrificed, the thyroid gland was extracted from each rat for histological studies (Hematoxylin and Eosin (H&E), electron microscopy (E/M), and Immunohistochemistry).
Results: Normalization of TFTs after ten days from transplantation in treated groups (p<0.05) showing no difference between intravenous and intranasal groups. Electron microscopy together with H&E showed restoration of thyroid follicles morphology. Furthermore, immune reactions for BCL2 (B Cell Lymphoma 2), iNOS (Inducible Nitric Oxide Synthase), and BAX (Bcl-2-associated X protein) in both stem cells and conditioned media groups were comparable to the control group.
Conclusion: Stem cells-derived conditioned media was as effective as umbilical cord-derived stem cells in reversing hypothyroid status in rats either by intravenous or intranasal routes.
Hypothyroidism is a worldwide problem that affects 5.3% of the population(Taylor, Albrecht et al. 2018). Under- and overtreatment are associated with increased somatic and psychiatric morbidity leading to earlier retirement and excess mortality. (Lillevang-Johansen, Abrahamsen et al. 2019). The current standard of care for the treatment of overt hypothyroidism is a replacement of thyroid hormone with levothyroxine (LT4), at doses that achieve a normal serum TSH level. (Nagy, Perros et al. 2020) However, several unresolved issues exist concerning patients who are biochemically well-controlled but unsatisfied with their treatment outcome. (Chaker, Bianco et al. 2017) Future studies should address whether alternative regimens could provide a solution for at least a proportion of patients with residual symptoms, e.g. combined formulas (Ettleson and Bianco 2020). So Regenerative medicine may give us a clue for better management.
Stem cells (SC) are unique, undifferentiated cells characterized by unlimited proliferation, multi-differentiation potency, and perpetual self-renewal. SCs also have unique proangiogenic, antifibrotic, and antiapoptotic features (Sun, Abelson et al. 2019) and are subdivided into four main categories: adult SC (ASC), embryonic SC (ESC), induced pluripotent SC, and amniotic fluid SC (Wu and Izpisua Belmonte 2016).
Compared with the counterparts of other origins like bone marrow and adipose tissue, UC-MSCs enjoy appealing benefits such as (a) collected in a noninvasive way; (b) a lower risk of infection; (c) a rare teratoma risk; (d) low immunogenicity with a good immunosuppressive ability; and (e) multipotency. (Nagamura-Inoue and He 2014).
Cell-free approaches present several advantages over cell therapy. Initially, the potential risks associated with the administration step are lower, as the injection of soluble factors and/or submicron particles decreases the occurrence of thrombosis and other cardiovascular events such as arrhythmia. Then, the potential risk of malignant transformation and tumorigenesis is almost excluded. Furthermore, cell-free products do not require the mandatory steps of isolation/thawing and in vitro expansion just before administration. (Bari, Perteghella et al. 2019).
Conditioned medium (CM) or secretome is media containing biologically active components obtained from previously cultured cells or tissues that have been released into the media substances affecting certain cell functions, composed of soluble proteins, extracellular vesicles (EV), lipids, microvesicles (MVs), or nucleic acids. (Wang, Zhang et al. 2020).
In the presented article we studied the efficacy of both umbilical cord blood-derived stem cells and their conditioned medium in the treatment of hypothyroidism in a rat model of amiodarone-induced hypothyroidism. Also, we aimed to compare the efficacy of the novel intranasal route of administration versus the conventional intravenous route.
Our study was conducted at the Stem Cell Unit at the Faculty of Medicine Ain Shams University, the period from 2018 to 2020, all animal handling, procedures, and sacrification were approved by Ain Shams University Ethical Committee.
Drugs
Amiodarone tablets were purchased from Sanofi-Aventis Egypt under the license from Sanofi-Aventis France (Patch No. D13130, certification code 19589/2010). The doses of amiodarone were prepared by soaking the 200 mg amiodarone tablet in 10 ml distilled water and calculated according to the weight of the rat (100mg/kg body weight/day) (Stoykov, van Beeren et al. 2007). After confirmation of hypothyroidism by measuring TFTs at the beginning of the study and after 30 days.
Experimental Design
Forty adult Wistar rats weighing 120 -180 grams were divided equally into four main groups (10 rats for each group): Group I: Negative Control Group, Group II: Positive Control group (rats receiving only Amiodarone for 30 days, AMD group), Group III: Stem Cell treated group (Single injection of 1 ml of 1× 106 cells/ml of UC-MSCs into tail vein, AMD+UC-MSCs group), which was further subdivided into Intravenous group (n=5) and Intranasal group (n=5). Group IV: CM treated group (Single injection of 0.5 ml of CM, AMD+CM group), which was further subdivided into Intravenous group (n=5) and Intranasal group (n=5).
TFTs were withdrawn at the beginning of the experiment, after 30 days of amiodarone administration and then after ten days from the injection of stem cells and conditioned media (at day 40) rats were sacrificed according to ethical rules. During the experiment periodic measurement of body weight and temperature (every two weeks).
At the end of the experiments, the thyroid gland of all experimental groups was extracted and processed for histological examination and morphological analysis (Hematoxylin & Eosin, electron microscope, and immunohistochemistry).
Patients and samples
Fetal umbilical cords were obtained after consenting the parents from three full-term infants delivered at 36–41 weeks of gestation. We have excluded all cases suffering from any genetic, structural anomalies or involving maternal diabetes, pre-eclampsia, eclampsia, IUGR, infectious diseases. This study was conducted following the approved guidelines of Ain Shams University Ethical Committee.
Informed Consent was signed by the mother late in pregnancy before delivery, explaining the study procedures, risks, and benefits from it. Also, these cells were not be used in another study and were not be used in genetic testing by any means, and any remaining cells were discarded properly. All data of the research including the donor and procedures remained confidential during and after the study.
Umbilical cord blood derived-Stem cells Culture
SCs were cultured from human fetal umbilical cord blood collected under aseptic conditions by cord blood bag collection set (manufactured by JMS, Singapore), under supervision of Gynecology and Obstetrics Hospital-Ain Shams University.
During the study, we planned to have umbilical cord samples from multiple donors (around 3 donors), an average of 20 ml of umbilical cord blood from each sample. We considered not mixing the samples from different donors together, but each study group received stem cells from different donors.
The umbilical cord blood sample was diluted by adding PBS at a ratio of 1:1. The blood mix was added slowly over the Lymphocyte Separation Medium, 100 ml (Manufactured by Lonza Verviers SPRL Belgium) in falcon tubes. For each 2 ml Lymphocyte Separation Medium, a 4 ml blood mix was added with care not to disturb the interface. The tubes were centrifuged at 2000 rpm (400 G) for 30 min. The mononuclear layer was aspirated slowly with a sterile Pasteur, washed with 3ml PBS, centrifuged at 1000 rpm (100 G) for 10 min. The supernatant was aspirated then resuspended in complete culture media containing Dulbecco’s modified Eagle’s medium-high glucose low glutamine (DMEM 4.5g/L Glucose w/ L-Glutamine 500ml, manufactured by Lonza Verviers SPRL Belgium) supplemented with 10 percentage FBS (fetal bovine serum Sterile Filtered, manufactured by Life Science) and 1 % penicillin/streptomycin). The cells were cultured at 37°C in a humidified 5% CO2 atmosphere, with a change of medium twice weekly.
Three days from initial seeding, nonadherent cells were removed by changing the medium. The adherent cells were fed twice a week and screened for confluency appearance after 14 days. MSCs at 90% confluence were harvested using 10 × Trypsin/EDTA and sub-cultured at 4000 cells/cm2 density. The medium was replaced two times per week. (Amati, Sella et al. 2017) The third passaged cells were used in this study.
Conditioned medium preparation
The third passaged cells, their complete medium was substituted with DMEM without antibiotics and FBS. Next, for 48 hours, the cells were cultured in a hypoxic condition in the incubator (1% O2, 5% CO2, and 94 % N2). To remove cell debris or detached cells, the medium was aspirated from hypoxic SCs and centrifuged at 1200 rpm for 10 min. Then the CM was filtered through a 0.22 μm filter to get rid of any contaminants and stored till the time of need at -80 °C before experiments. (Xing, Cui et al. 2014).
Fluorescence labeling of stem cells
Following the manufacturer’s instructions, the third passaged cells were labeled by PKH26 Red Fluorescent Cell Linker Kit for General Cell Membrane Labeling (Molecular probes, Sigma-Aldrich, St. Louis, Mo, USA). Briefly, for 2-5 min, the cells were incubated with PKH26 reagent at 25°C with gentle frequent shaking. Then an equal volume of serum was added and incubated for 1min to block the staining action. (Xue, Li J Fau - Liu et al.) After that, the cells were centrifuged at 1800 rpm for 10 min at 25°C. The supernatant was aspirated, the cell pellets were washed three times with PBS. Finally, the cell pellets were resuspended in PBS at a density of 1×106 cells/ml. Labeled cells were injected in rats of Group Ⅲ either intranasal or intravenous. (Shao-Fang, Hong-Tian et al. 2011).
Thyroid function tests
TSH, Free T4, and Free T3 were done using ELISA test kits were sold from (IBL International, Hamburg, Germany).
Histological study
Specimens of thyroid tissue were prepared for histopathological assessment. Fixation was done using 10% phosphate-buffered formalin, dried out in climbing grades of ethyl alcohol, cleared in xylol, and prepared to acquire paraffin blocks. Sections of 5µm thick were cut and stained with H&E stain. (Spencer and Bancroft 2013).
Electron microscope study
Small pieces of thyroid specimens were fixed in 2.5% glutaraldehyde for 2 h at room temperature (25° C) and then the specimens were post-fixed in osmium tetroxide. The specimens were dehydrated in ascending graded concentrations of ethanol and embedded in epoxy resin. Ultrathin sections (60-80 nm) cut with diamond knives were placed on copper grids, stained with uranyl acetate and lead citrate, and scoped. Copper grids were examined and photographed with Jeol, JEM- 1200 EX II Electron Microscope, Tokyo (Graham and Orenstein 2007).
Immunohistochemical study
Sections of thyroid tissue were deparaffinized in xylene for one hour, hydrated in descending grades of alcohol, and then rinsed in distilled water. Endogenous peroxidase was inactivated with a methanol solution containing H2O2 (1:50) for 10 min and washed with PBS. The tissue sections were blocked with 1.5% serum for 30 min, treated with anti iNOS polyclonal antibody (PA3-030A, Thermo Fisher Scientific, UK, 1:200), anti-BAX monoclonal antibody (ab32503, Abcam, UK,1:50), or anti-Bcl- 2 monoclonal antibody (14-6992-82, Thermo Fisher Scientific, UK, 1:100) overnight (Pacher, Beckman et al. 2007) Then, samples were incubated with AB enzymes for 30 min and rinsed in PBS. Positive signals were detected using peroxidase chromogenic substrates, diamino benzodiazepine (DAB) solution was applied to all sections for ten min. Sections were washed in distilled water and then counterstained with Mayer’s hematoxylin for 2 min. Negative controls included staining tissue sections with PBS without the primary antibody. (Jiang, Chen et al. 2016) (Koga, Hiromatsu et al. 1999).
The mean area percentage of iNOS, BAX, and BCL-2 expression was quantified by the image analyzer Leica Q win V.3 program installed on a computer connected to a Leica DM2500 microscope (Wetzlar, Germany). Measurements were done from five non-overlapping fields examined at objective lens X 40 from five slides from each group and the data were subjected to statistical analysis.
Statistical presentation and analysis of the present study were conducted, using the mean, SD, and Student t-test and ANOVA by SPSS version 18. Student’s t-test or one-way ANOVA was utilized for statistical comparisons. The results of the bioinformatics analysis were imagined utilizing SPSS software. The statistical significances were calculated as P values, and (P < 0> 0.05) was not statistically significant.
TSH
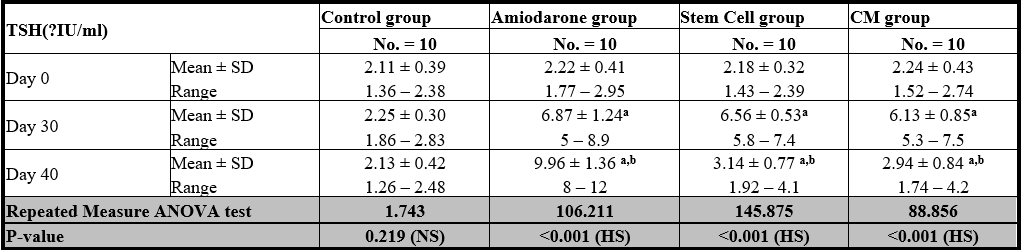
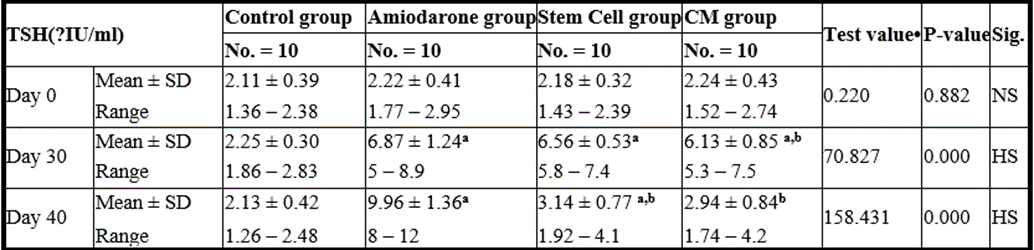
At the beginning of the study, TSH measurement was normal among four groups. On Day 30 after administration of AMD in Groups Ⅱ, Ⅲ, and Ⅳ there was a statistical difference (P-value lessthan 0.05) among Groups Ⅱ, Ⅲ, and Ⅳ. (Tables 1 and 2) (Figures 1 and 2).
P-value >0.05: Non significant (NS); P-value lessthan 0.05; Significant (S); P-value lessthan 0.01: highly significant (HS) •: Repeated measure ANOVA test
Table 1: Comparison between TSH at day 0, day 30, and day 40 in each group.
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS) •: One Way ANOVA test
Table 2: Comparison between the four studied groups regarding TSH at day 0, day 30, and day 40Comparison between the four studied groups regarding TSH at day 0, day 30, and day 40.
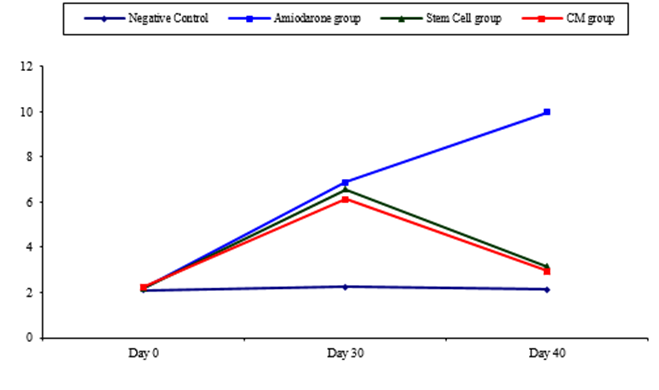
Figure 1: Comparison between TSH at day 0, day 30, and day 40 in each group.
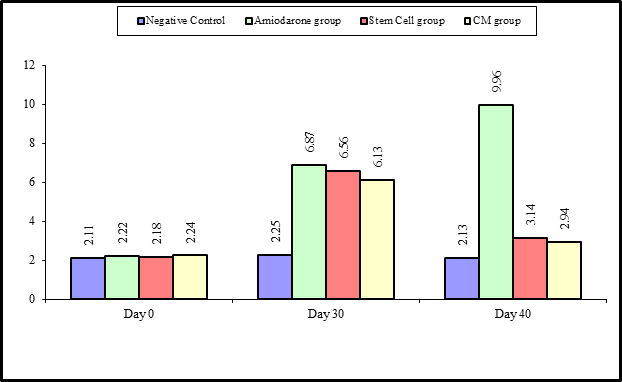
Figure 2: Comparison between the four studied groups regarding TSH at day 0, day 30, and day 40.
On Day 40, there was a highly statistical difference (P-value lessthan 0.01) between Groups Ⅲ and Ⅳ versus Group Ⅱ. Remarkably, no statistical difference between groups Ⅲ and Ⅳ. Notably, no statistical difference between Intravenous and Intranasal groups. (Table 11&12).
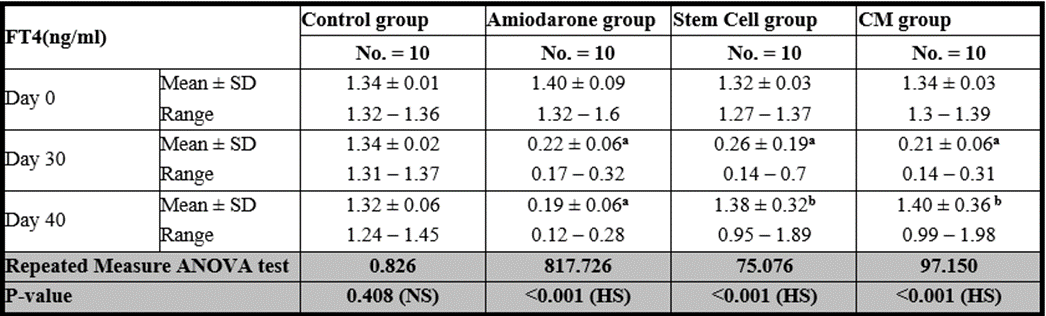
Free T4
At the beginning of the study, Free T4 measurement was normal among four groups. On Day 30 after administration of AMD in Groups Ⅱ, Ⅲ, and Ⅳ there was a statistical difference (P-value lessthan 0.05) among Groups Ⅱ, Ⅲ, and Ⅳ. (Tables 3 and 4) (Figures 3 and 4).
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS) •: Repeated measure ANOVA test
Table 3: Comparison between FT4 at day 0, day 30 and day 40 in each group.

P-value >0.05: Nonsignificant (NS); P-value is lessthan 0.05: Significant (S); P-value lessthan 0.01 •: One Way ANOVA test
Table 4: Comparison between FT4 at day 0, day 30 and day 40 in each group.
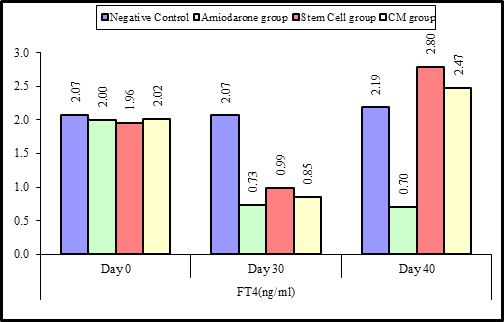
Figure 3: Comparison between FT4 at day 0, day 30, and day 40 in each group.

Figure 4: Comparison between the four studied groups regarding FT4 at day 0, day 30, and day 40.
On Day 40, there was a highly statistical difference (P-value lessthan 0.01) between Groups Ⅲ and Ⅳ versus Group Ⅱ. Remarkably, no statistical difference between groups Ⅲ and Ⅳ. Notably, no statistical difference between Intravenous and Intranasal groups. (Table 11&12).
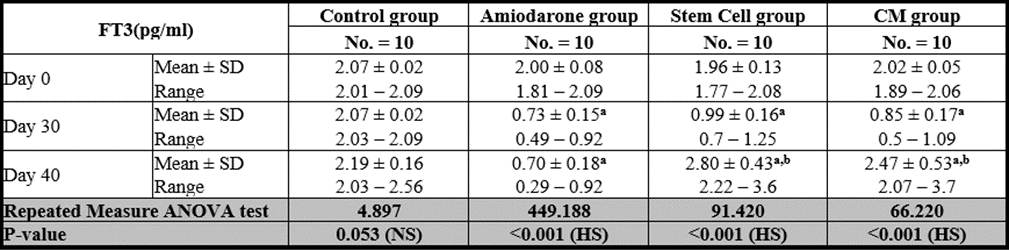
Free T3
At the beginning of the study, Free T4 measurement was normal among four groups. On Day 30 after administration of AMD in Groups Ⅱ, Ⅲ, and Ⅳ there was a statistical difference (P-value is lessthan 0.01) between Groups Ⅱ, Ⅲ, and Ⅳ and Group Ⅰ (Negative Control Group) and no statistical difference (P-value >0.05) among Groups Ⅱ, Ⅲ, and Ⅳ. (Tables 5 and 6) (Figures 5 and 6).
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS) •: Repeated measure ANOVA test
Table 5: Comparison between FT3 at day 0, day 30, and day 40 in each group.

P-value >0.05: Nonsignificant (NS); P-value is lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS) •: One Way ANOVA test
Table 6: Comparison between the four studied groups regarding FT3 at day 0, day 30, and day 40.
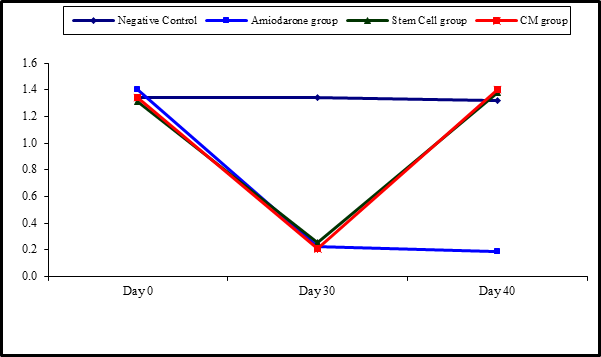
Figure 5: Comparison between FT3 at day 0, day 30, and day 40 in each group.
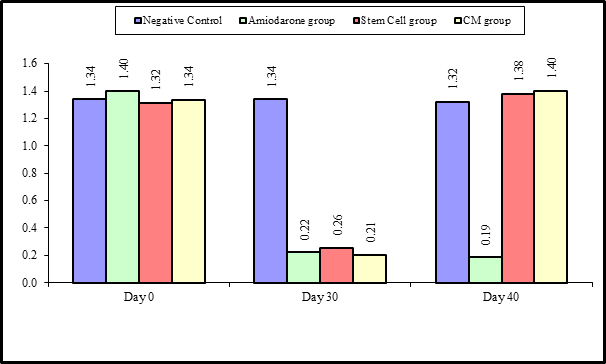
Figure 6: Comparison between the four studied groups regarding FT3 at day 0, day 30, and day 40.
On Day 40, there was a highly statistical difference (P-value lessthan 0.01) between Groups Ⅲ and Ⅳ versus Group Ⅱ. Remarkably, no statistical difference between groups Ⅲ and Ⅳ. Notably, no statistical difference between Intravenous and Intranasal groups. (Table 11 and 12)
Body weight
At the beginning of the study, weight measurement was normal among four groups. On Day 30 after administration of AMD in Groups Ⅱ, Ⅲ, and Ⅳ there was a statistical difference (P-value lessthan 0.05) between Groups Ⅱ, Ⅲ, and Ⅳ and Group Ⅰ (Negative Control Group) and no statistical difference (P-value >0.05) among Groups Ⅱ, Ⅲ, and Ⅳ. (Tables 7 and 8) (Figures 7 and 8).
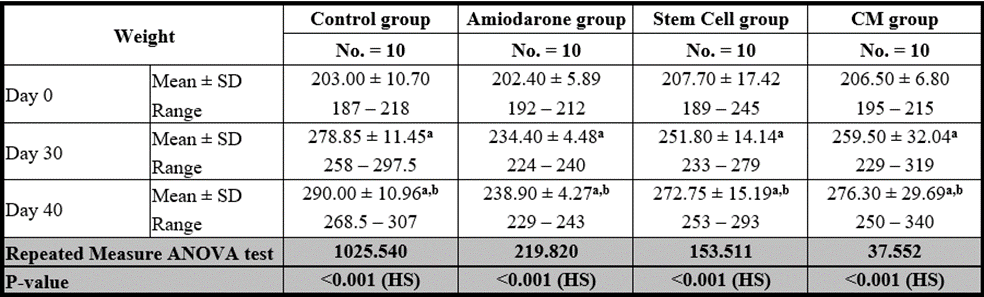
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS)
•: Repeated measure ANOVA test with post hoc analysis using Bonferroni test
Table 7: Comparison between the four studied groups regarding FT3 at day 0, day 30, and day 40.
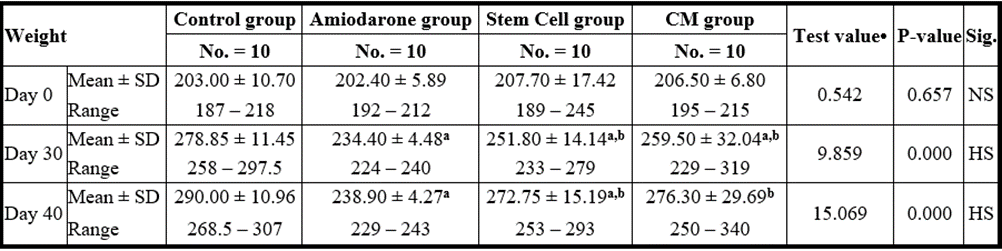
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS)
•: One Way ANOVA test a: Significant from WT group b: Significant from amiodarone group c: Significant from Stem cell group
Table 8: Comparison between the four studied groups regarding weight at day 0, day 30, and day 40.

Figure 7: Comparison between the four studied groups regarding weight at day 0, day 30, and day 40.
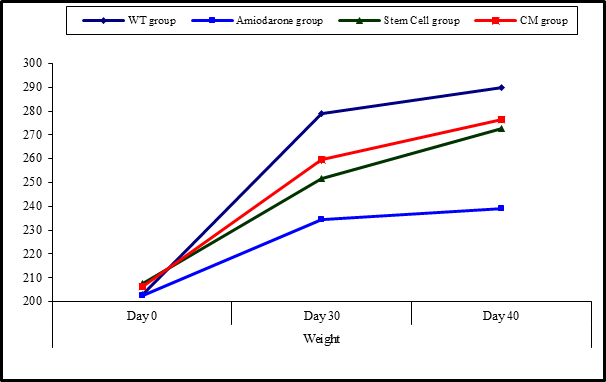
Figure 8: Comparison between weight at day 0, day 30, and day 40 in each group.
On Day 40, there was a statistical difference (P-value lessthan 0.05) between Groups Ⅲ and Ⅳ versus Group Ⅱ. Remarkably, no statistical difference between groups Ⅲ and Ⅳ. Notably, no statistical difference between Intravenous and Intranasal groups. (Table 11&12).
Temperature
At the beginning of the study, Temperature measurement was normal among four groups. On Day 30 after administration of AMD in Groups Ⅱ, Ⅲ, and Ⅳ there was a statistical difference (P-value lessthan 0.05) between Groups Ⅱ, Ⅲ, and Ⅳ and Group Ⅰ (Negative Control Group) and no statistical difference (P-value >0.05) among Groups Ⅱ, Ⅲ, and Ⅳ. (Tables 9 and 10) (Figures 9 and 10).
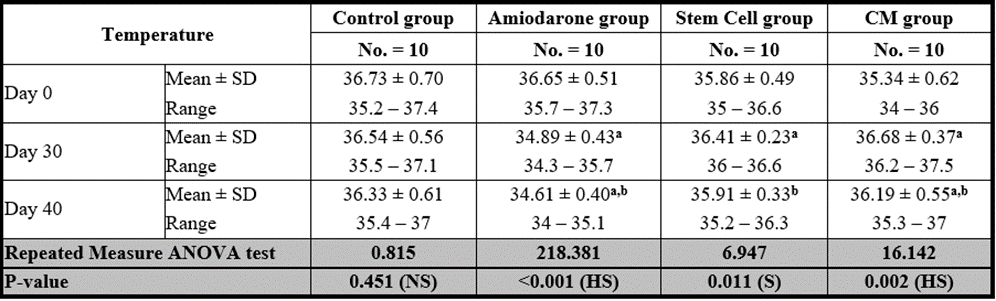
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS)
•: Repeated measure ANOVA test
Table 9: Comparison between the temperature at day 0, day 30, and day 40 in each group.
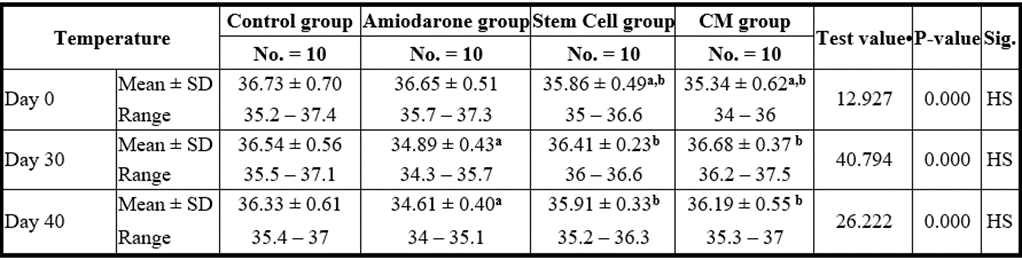
P-value >0.05: Nonsignificant (NS); P-value lessthan 0.05: Significant (S); P-value lessthan 0.01: highly significant (HS)
•: One Way ANOVA test
Table 10: Comparison between the four studied groups regarding the temperature at day 0, day 30, and day 40.

Figure 9: Comparison between the four studied groups regarding the temperature at day 0, day 30, and day 40.

Figure 10: Comparison between the temperature at day 0, day 30, and day 40 in each group.
On Day 40, there was a statistical difference (P-value lessthan 0.05) between Groups Ⅲ and Ⅳ versus Group Ⅱ. Remarkably, no statistical difference between groups Ⅲ and Ⅳ. Notably, no statistical difference between Intravenous and Intranasal groups. (Table 11 and 12).
Stem cell group
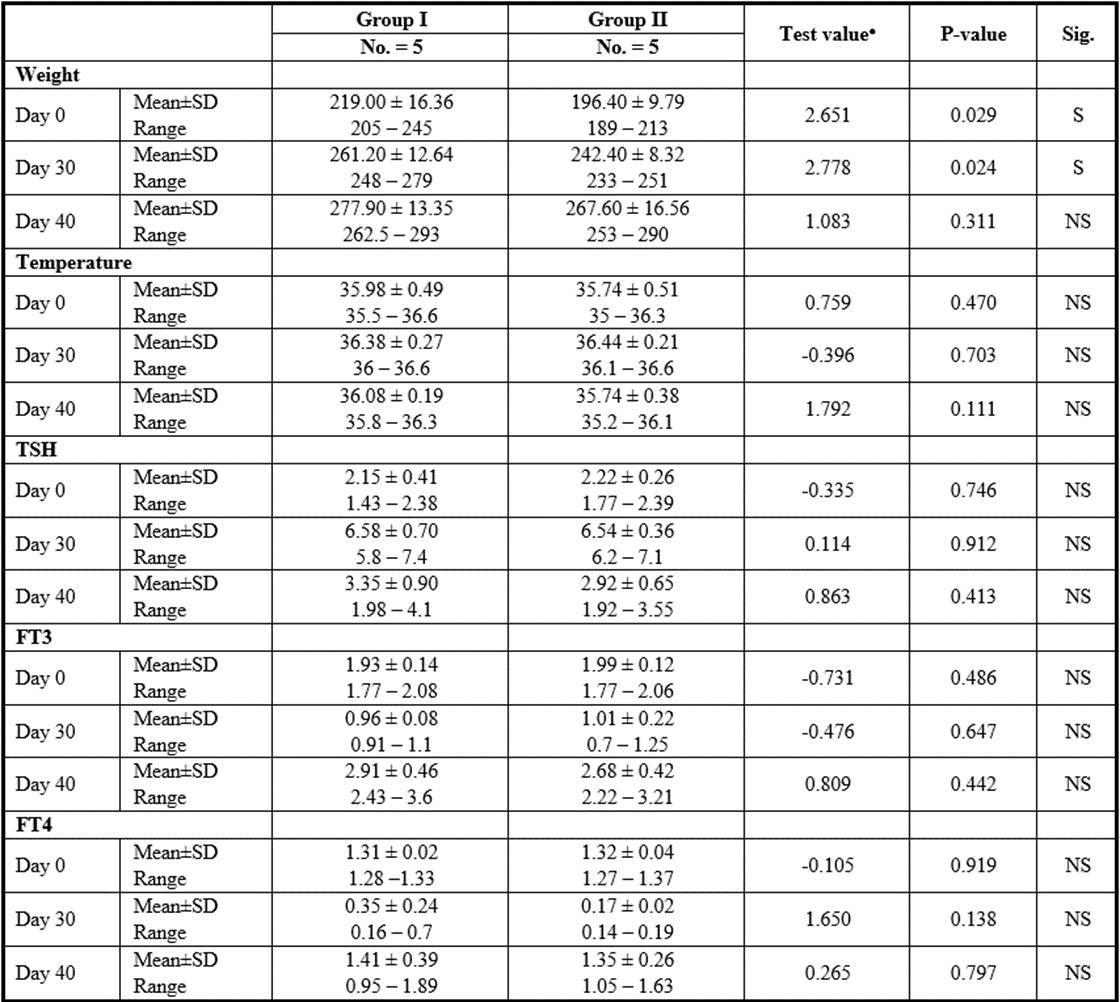
P>0.05: Nonsignificant; P lessthan 0.05: Significant; P lessthan 0.01: Highly significant
●: Independent t-test
Table 11: Comparison between SCs injection by intranasal vs intravenous routes regarding all parameters at day 0, day 30, and day 40.
CM Group
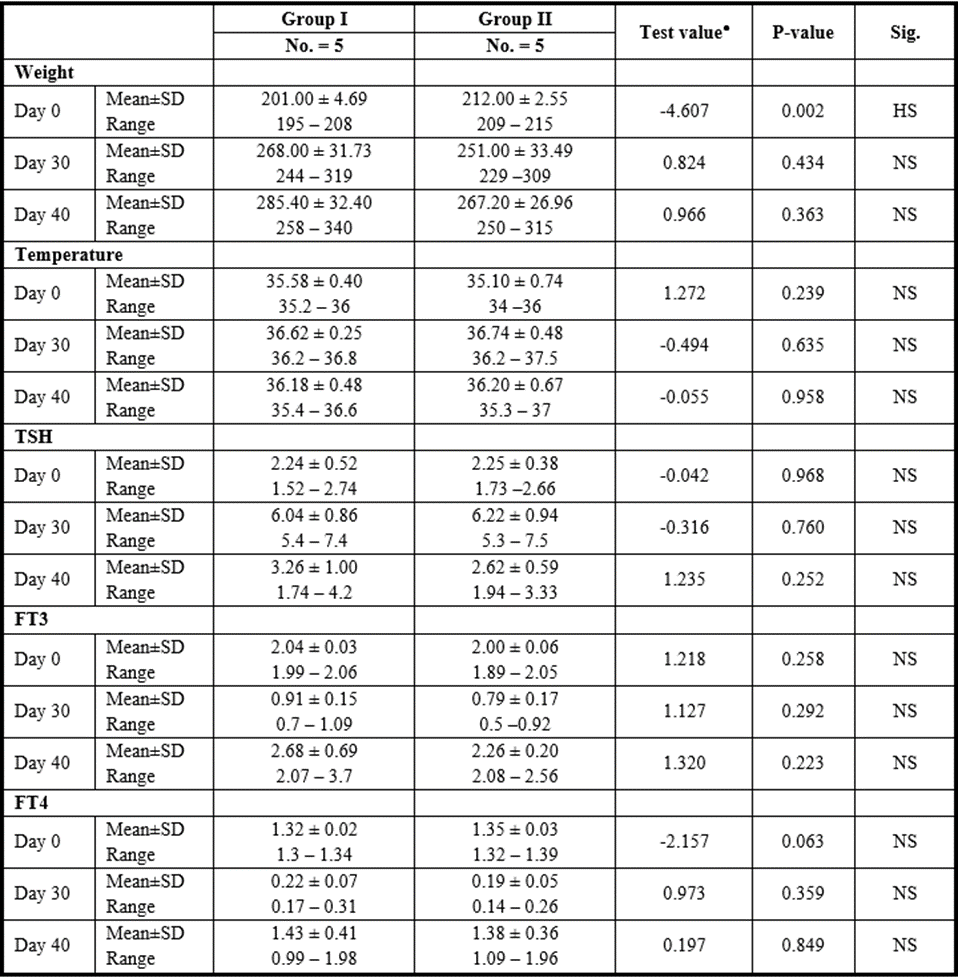
P>0.05: Nonsignificant; P lessthan 0.05: Significant; P lessthan 0.01: Highly significant
●: Independent t-test
Table 12: Comparison between CM injection by intranasal vs intravenous routes regarding all parameters at day 0, day 30, and day 40.
Light microscopic examination
H.&E. of the negative control group (group I) showed the thyroid gland was divided into lobules by connective tissue septa dipping from the capsule and consisted of thyroid follicles. Every single follicle was spherical and lined by a row of cuboidal follicular cells with central nuclei and few populations of small C cells (Parafollicular cells). Their lumina were filled with homogenous acidophilic colloid. (Figure 11 A).
On the other hand, the AMD group (group II) showed marked follicular disruption by epithelial atrophy, some follicular cells nuclei were apoptotic. Others were exfoliated and present as intraluminal aggregates of vacuolated cells. The lumen of some follicles appeared empty while others showed few colloids. The interfollicular tissue exhibited congested blood capillaries and foci of nonspecific lymphocyte infiltration. (Figure 11 B).
However, the transplantation of UC-MSCs (SC Group III) showed restored thyroid gland general architecture. The follicles were lined by simple cuboidal epithelium and contained colloid. Some cells with vacuolated cytoplasm were still noticed. No differences were noticed between intravenous or Intranasal groups. (Figures 11 C&D)
In addition, injection of CM (CM group IV) showed also nearly normal thyroid architecture represented by thyroid follicles lined with simple cuboidal epithelial cells. The follicles were filled with colloid which was homogenous and acidophilic. Blood capillaries were noticed in the interstitial tissue between follicles. Also, no significant differences were noticed between intravenous or Intranasal groups. (Figures 11 E&F).
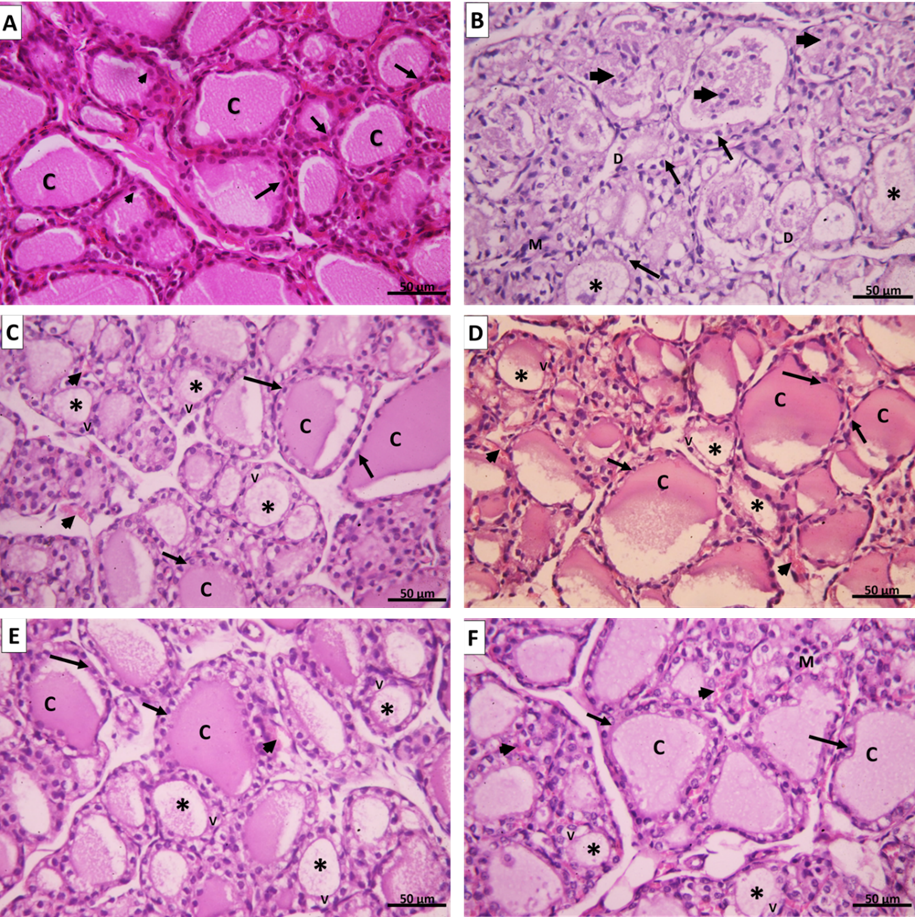
Figure 11: A. Group I control group showing thyroid follicles lined with cuboidal epithelium with round central nuclei (arrows) and few parafollicular cells with large nuclei (arrowheads). The lumen is filled with a homogenous eosinophilic colloid (C). B. Group II amiodarone group showing disorganized and distorted thyroid follicles. Some follicles appear empty (*); others have scanty colloid material mixed with exfoliated apoptotic cells (arrowheads). The thyroid follicular cells are ballooned with apoptotic nuclei (↑). The epithelium of some follicles exhibits focal disruption [D]. Mononuclear cell infiltration can be seen in the interfollicular tissue (M). C. Group III (SC intravenous), D. Group III (SC intranasal), E. Group VI CM intravenous, and F. Group VI (CM intranasal) showing thyroid follicles with variable sizes. Large follicles are lined with simple cuboidal follicular cells with vesicular nuclei (↑) and their lumen is filled with homogenous acidophilic colloid (C). Small thyroid follicles appeared with scanty colloid (*) and their lined epithelium still show vacuolations (V). Congested blood capillaries can be noticed in the interfollicular tissue (arrowheads). (H&E x 400).
Electron microscopic study
Assessment of ultrathin segments of the thyroid organ of Group I showed thyroid follicles fixed with cubical follicular cells lied on the slim basal lamina. Their cores were euchromatic with fringe chromatin. The cytoplasm showed very much created Golgi apparatus, rER, numerous lysosomes, and numerous secretory vesicles. The apical line of the follicular cells showed microvilli jutting in the lumen and the horizontal lines were conjoined with junctional complexes. (Figure 12 A).
Amiodarone treated group (group Ⅱ) showed a remarkable cellular change in the form of dilated rER, increased number of lysosomes and colloid vesicles, dilated Golgi bodies, mitochondrial distension. There was nuclear degeneration ranging from the irregular flattened nucleus with irregular nucleoli and dispersed chromatin to markedly shrunken ones. The free surface of the follicular cells showed partially lost microvilli. The lumen showed clusters of desquamated follicular cells. (Figure 12 B).
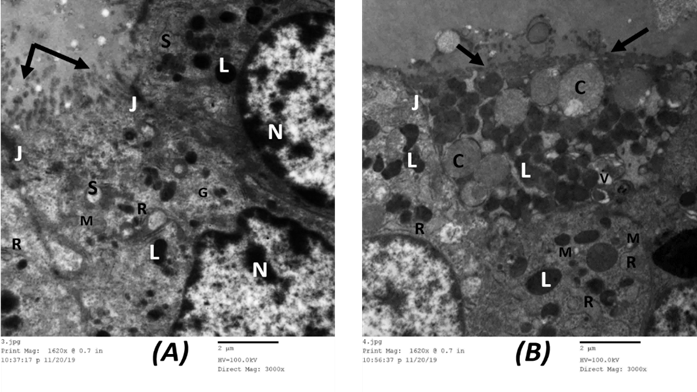
Figure 12: Electron microscopic picture of A. group I control rat showing follicular cells have euchromatic nuclei with peripheral and islet chromatin (N). The cytoplasm displays prominent rough endoplasmic reticulum (R), supranuclear Golgi apparatus (G), many lysosomes (L), mitochondria (M), and secretory vesicles (S). The apical surface shows many microvilli protruding into the lumen which is filled with colloid (↑). Junctional complexes could be observed between adjacent follicular cells (J). (x 3000). B. Group II showing the cytoplasm of follicular cells exhibit vacuolization (V) with marked dilatation of rough endoplasmic reticulum (R), numerous lysosomes (L), and large colloid droplets (C). Mitochondria appear dilated with disrupted cristae (M). The apical surface shows lost microvilli (↑). A junctional complex could be seen between follicular cells (J). (x 3000).
Group Ⅲ showed restoration of normal ultrastructure of the thyroid follicles comparable to the control group. Each follicle was lined by a single layer of cuboidal follicular cells with regular euchromatic nuclei. On the other hand, their cytoplasm revealed a well-organized rER, prominent Golgi complex, lysosomes, preserved mitochondria, and some secretory vesicles. The apical surface of the follicular epithelium revealed a moderate number of short microvilli projected into the lumen which was filled with moderately dense colloid. Typical junctional complexes were seen between the adjacent follicular cells (Figures 13 A&B).

Figure 13: Electron microscopic picture of A. Group III (SC intravenous) shows follicular epithelium with a euchromatic nucleus and peripheral chromatin (N). Cytoplasm shows mildly dilated rough endoplasmic reticulum (R), many lysosomes (L), and small secretory vesicles (S). Long microvilli can be seen protruding into the lumen (↑). Adjacent follicular cells are joined by a junctional complex (J). (x3000). B. Group III (SC intranasal) shows thyroid follicular cells with a euchromatic nucleus and peripheral chromatin (N). Cytoplasm still shows mildly dilated rough endoplasmic reticulum (R), few lysosomes (L). Few microvilli can be seen protruding into the lumen (↑). (x3000).
Similarly, group Ⅳ showed pictures like group Ⅲ, follicular epithelium appeared with irregular nuclei and prominent nucleoli. The cytoplasm showed mildly dilated basal rER, many lysosomes, colloid, and secretory vesicles. Intact microvilli were protruded in the follicular lumen (Figures 14 A&B).

Figure 14: Electron microscopic picture of A. Group VI (CM intravenous) and B. (CM intranasal) show follicular epithelium with a euchromatic nucleus and peripheral chromatin (N). Cytoplasm shows mildly dilated rough endoplasmic reticulum (R), few lysosomes (L), preserved mitochondria (M). Many microvilli can be seen protruding into the lumen (↑). (x3000).
Immunohistochemistry study
Group I (Negative Control) showed a negative immune reaction for the inflammatory marker iNOS (Figure 15 A), while group Ⅱ (AMD group) showed strong positive immune for iNOS (Figure 15 B). However, both group III (SC treated) and group IV (CM treated) showed mild iNOS immuno- histochemical reaction (Figures 15 C-F).

Figure 15: Representative immunohistochemical images for iNOS expression in the thyroid gland. A. Group I show a negative reaction for iNOS. B. Group II shows a positive cytoplasmic immune reaction for iNOS. C. Group III (intravenous), D. Group III (intranasal), E. Group IV (intravenous), and F. Group IV (intranasal) show mild immune reaction for iNOS. (iNOS immune staining x400).
Group Ⅰ showed a strong positive reaction for the antiapoptotic marker Bcl-2 (Figure 16 A), while the group II amiodarone group exhibit weak expression of Bcl-2 (Figure 16 B). Contrarily, group Ⅲ showed Bcl-2 immune reaction was moderate (Figures 16 C&D). Group Ⅳ showed also moderate Bcl-2 immune reaction similar to group III (Figures 16 E&F).

Figure 16: Representative immunohistochemical images for Bcl-2 expression in the thyroid gland. A. Group I show a strong positive reaction for Bcl-2. B. Group II shows mild cytoplasmic immune reaction for Bcl-2. C. Group III (intravenous), D. Group III (intranasal), E. Group IV (intravenous), and F. Group IV (intranasal) show positive cytoplasmic immune reaction for Bcl-2. (Bcl-2 immune staining x400)
Group Ⅰ showed no BAX expression; the apoptotic marker (Figure 17 A), while group Ⅱ (amiodarone treated group) showed a strong positive reaction for BAX [removed]Figure 17 B). Conversely, both group III and group IV showed mild BAX immune reaction (Figures 17 C-F).
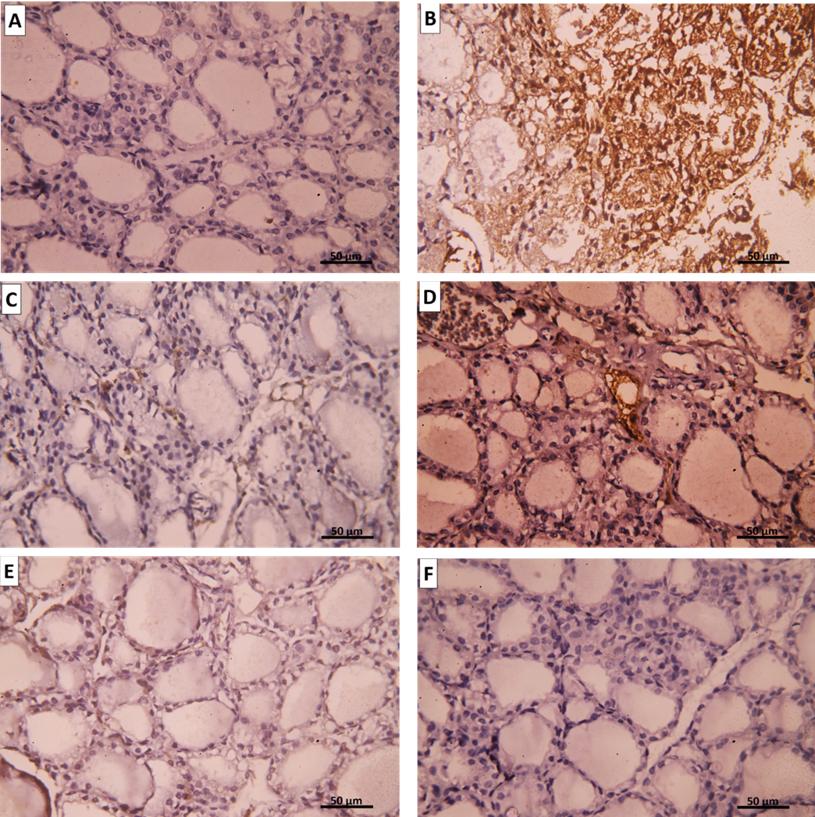
Figure 17: Representative immunohistochemical images for BAX expression in the thyroid gland. A. Group I show a negative reaction to BAX. B. Group II shows a positive cytoplasmic immune reaction for BAX. C. Group III (intravenous), D. Group III (intranasal), E. Group IV (intravenous), and F. Group IV (intranasal) show mild immune reaction for BAX. (BAX immune staining x400).
There was a significant increase (P lessthan 0.05) in the area percentage of iNOS positive cells in rats of amiodarone group II as compared to the rats of control group I. UC-MSCs treated group III as well as CM treated group IV showed a significant decrease (P lessthan 0.05) in the mean area percentage of iNOS immune positive cells compared to group II (Figure 19).

Figure 19: Histogram showing the mean area percentage of iNOS immune positive cells for the different study groups.
On the other hand, there was a significant decrease (P lessthan 0.05) in the area percentage of Bcl-2 positive cells in rats of group II as compared to the rats of control group I. Group III as well as group IV showed a significant increase (P lessthan 0.05) in the mean area percentage of Bcl-2 immune positive cells compared to group II (Figure 20).
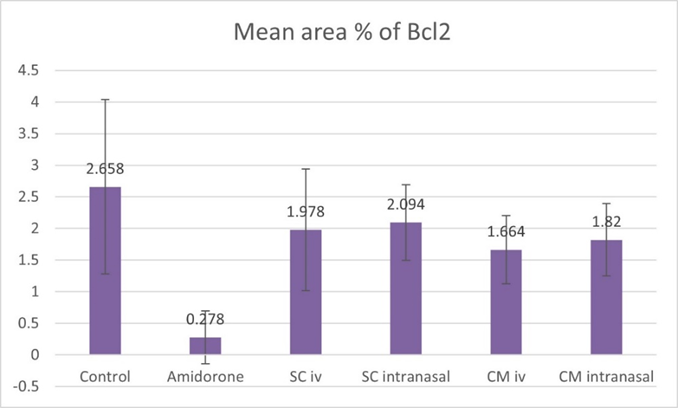
Figure 20: Histogram showing the mean area percentage of Bcl-2 immune positive cells for the different study groups.
There was a significant increase (P lessthan 0.05) in the area percentage of BAX positive cells in group II as compared to group I. UC-MSCs treated group III as well as CM treated group IV showed a significant decrease (P lessthan 0.05) in the mean area percentage of BAX immune positive cells compared to group II (Figure 21).

Figure 21: Histogram showing the mean area percentage of BAX immune positive cells for the different study groups.
Yet, there was no significant difference found among subgroups of intravenous and intranasal injected SC or CM.
Fluorescence Staining
To detect if the transplanted UC-MSCs traveled to thyroid tissue, we labeled the stem cells with PKH26 fluorescence material. Then we examined the unstained slides by fluorescent microscope for homing. SC group displayed many red fluorescence cells distributed all over the thyroid gland indicating homing of stem cells to thyroid tissue whether they were injected intravenously or intranasally (Figure 18).

Figure 18: A. Group III (SC intravenous). B. Group III (SC intranasal) shows the distribution of PKH26-labeled UC-MSCs in the thyroid gland, 40 days after transplantation (↑). (Fluorescence microscopy x400).
Regenerative medicine (RM) aims to repair and regenerate poorly functioning organs. RM is committed to supplanting and additionally fixing tissues and organs for practical reclamation and it holds guarantees in a wide cluster of fields (Mao and Mooney 2015), for example, advancing the recovery of local cell lines, development of new tissue or organs, demonstrating of illness states, and expanding the feasibility of existing ex vivo relocated organs. (Edgar, Pu et al. 2020)
Stem cell technologies address an advancement improvement in biomedical science. In the previous years, the interest for MSCs and their adapted CM exosomes has been expanding in regenerative medication. MSCs with the properties of differentiability, immunoregulatory capacity, and paracrine capacities make them good for the recovery of tissues and organs. There are some radiant advantages of MSCs-exosomes that assume a significant part in neurotic and physiological cycles, because of the impediments of MSCs in RM. MSCs-exosomes start quicker intracellular correspondence and copy the properties of the extracellular layer when contrasted with MSCs. (Maqsood, Kang et al. 2020)
In our study, we aimed to investigate the effectiveness of umbilical cord blood SCs and their CM in the regeneration of thyroid function, also to compare the conventional intravenous route of administration versus the novel intranasal route.
Amiodarone is a highly effective class III antiarrhythmic drug. It can be used to treat a wide range of arrhythmias. However, it is markedly concentrated in tissues leading to multiple adverse effects mainly on the thyroid gland. It usually leads to hypothyroidism and less commonly hyperthyroidism and relates to high iodine content within the molecule plus several unique intrinsic properties of amiodarone (Narayana et al., 2011).
In the present study, there was no mortality noticed all over the studied groups but showed lower body weight among group Ⅱ which received only AMD. This result agreed with another study that showed rats that administrated amiodarone had lower body weight than control rats (Stoykov, van Beeren et al. 2007). On the other hand, (Jiang, Chen et al. 2016) found that amiodarone-treated rats showed signs of hair and weight loss, and diarrhea compared with normal rats. Meanwhile, (Pantos, Mourouzis et al. 2002) also concluded that amiodarone administration had no effects on survival.
In the current study, administration of amiodarone for one month revealed many structural changes in the form of general disturbance of the gland architecture with ruptured, degenerated, and disturbed follicles accompanied by cellular exfoliation. These results match with the results of (Zickri and Embaby 2013) and (Jiang, Chen et al. 2016) who suggested that amiodarone exhibits direct toxicity on thyroid follicular cells, and thus induces thyroid changes in the form of cellular apoptosis and necrosis.
The present study showed that the follicular cellular lining in group II (AMD Group) exhibited vacuolation in the cytoplasm and the nuclei showed pyknosis and sometimes karyorrhexis. These findings were in agreement with the study of (Jiang, Chen et al. 2016) who postulated that these cellular changes were referred to as a sign of cellular apoptosis.
Also, this study showed that the amiodarone-treated group displayed histopathological findings in the form of congestion and hemorrhage in between the follicles. Some macrophages were spotted in the area surrounding the congested blood vessels. At the same time, some lymphocytic infiltrations were detected. (Jiang, Chen et al. 2016) explained that these findings may indicate an inflammatory response in the thyroid gland. Inflammation and oxidative stress are closely related processes, as well exemplified in obesity and cardiovascular diseases (Mancini, Di Segni et al. 2016).
Electron microscopic examination of the thyroid gland upon amiodarone administration revealed structural changes in cellular organelles of the thyroid follicle cells in the form of marked distension of rER and an increased number of lysosomes. (Pitsiavas, Smerdely et al. 1997) explained these findings by the accumulation of iodinated thyroglobulin which resists degradation by proteolytic enzymes. Also, disruption of sorting mechanisms leads to the accumulation of proteins inside rER resulting in its distension. In addition, amiodarone-induced mitochondrial swelling. (Rasheed and Arsanyos 2018) declared that mitochondrial distension occurred due to the accumulation of lipid droplets with subsequent inhibition of mitochondrial beta-oxidation by amiodarone therapy.
Inducible nitric oxide synthase (iNOS) in the present study revealed a marked expression in the amiodarone treated group compared to the control. To confirm oxidative stress and to detect the level of thyroid oxidant and antioxidant enzymes, further oxidative tissue markers were measured in thyroid tissue of different groups.
In our study Bcl-2 (anti-apoptotic factor) was significantly decreased in Group Ⅱ (AMD treated) rats compared to the control group (Group Ⅰ) which indicates an increased rate of apoptosis in affected tissue. Pernick (2018) referred that Bcl-2 prevents cells from undergoing apoptosis. Bcl-2 is an anti-apoptotic factor; the Bcl-2 protooncogene encodes an inner mitochondrial membrane protein that blocks programmed cell death. (Pernick 2018)
On the other hand, the amiodarone-treated group (Group Ⅱ) showed a significant increase in BAX expression; an apoptotic factor as compared to group I. In (Markovic, Todorović et al. 2010) study results show that BAX expression is significantly higher in the Wistar rat experimental model of thyroiditis than in the control group. These data propose that the role of apoptosis in the pathogenesis of experimental thyroiditis is due to the increased expression of BAX.
In the current study injection of either UC-MSCs or their CM in the rat model of hypothyroidism improved greatly the histopathological changes of the thyroid follicles. The thyroid follicles appeared well formed nearly like the normal appearance of normal thyroid, filled with a good amount of colloid formation. EM showed organized follicles with apical microvilli, well-developed Golgi apparatus, normal appearance of endoplasmic reticulum, mitochondria, and lysosomes. Furthermore, both groups showed Bcl-2 as an anti-apoptotic factor immuno-histochemical reaction was moderate, iNOS as oxidative stress marker showed a mild immune reaction. Also, both groups showed minimal immune reaction against apoptotic marker BAX.
These results go hand in hand with (da Silva, de Freitas et al. 2018) who found that the treatment of diabetic rats with mesenchymal stem cells was capable of a reduction of thyroid H2O2 generation reducing the oxidative stress produced by diabetes. Also, (Antonica, Kasprzyk et al. 2012) found that stem cells transplanted into mice generated functional thyroid tissue that was able to produce thyroid hormone in thyroid deficient animals as they showed a substantial increase in plasma T4 levels. This is in agreement with the study of (Gaide Chevronnay, Janssens et al. 2016) who transplanted stem cells into cystinosis mice. They unveiled that stem cells normalized thyroid function and protect follicular structures as half of them showed normal plasma T4 and TSH levels. Also, they detected overall improvement over non-grafted controls, proved by predominantly normal thyrocyte height and homogeneous colloid filling. This contradicts with (da Silva, de Freitas et al. 2018) who found that MSC treatment did not normalize thyroid function and TSH remained elevated.
The fluorescence microscopic examination to detect homing of stem cells to the thyroid gland revealed that the stem cells migrated from the bloodstream to the thyroid gland in the UC-MSCs group either stem cells administered intravenously or intranasally.
The exact mechanisms used by MSCs to migrate and home to tissues have not been fully clarified. It is generally assumed that these stem cells follow the same steps that were described for leukocyte homing. (Ullah, Liu et al. 2019) First of all, the cells encounter the endothelium by tethering and rolling, resulting in a deceleration of the cells in the blood flow. Secondly, the cells are activated by G-protein-coupled receptors, followed by an integrin-mediated, activation-dependent arrest in the third step. Finally, the cells transmigrate through the endothelium and the underlying basement membrane. (De Becker and Riet 2016)
In the current study, UC-MSCs and CM groups showed a significant increase in T3 and T4 levels when compared to the amiodarone group (Group Ⅱ) (highly significant difference P lessthan 0.05).
The foundation system of SCs is accepted to be interceded by the paracrine component, which is known as undeveloped cells adapted medium (CM) or secretome. (Shen, Lie et al. 2015) CM utilization could resolve security contemplations related to SCs transplantation, like tumorigenicity, the transmission of contaminations, immune incompatibility so will give some clinical advantage. CM (secretome) could be produced in large amounts and stored for a long time without loss of product potency and toxic cryo-preservative agents. It could decrease the expense and time for the creation and support of cell-based treatment and be changed for wanted impacts. (Vizoso, Eiro et al. 2017).
To claim therapeutic advantages in using MSC-CM and EVs over live whole cells, and to develop safe and effective cell-free therapies, there is a significant knowledge gap that needs to be addressed, including optimization of bioactive components, dosing regimens, and production methods. (Chen, Tsai et al. 2019) An enormous number of studies have discovered some of the key mediators in MSC-CM and EVs; however, there is variability in the content of the secretome due to several factors. (Vizoso, Eiro et al. 2017) These include donor’s health status (e.g. age, sex), MSCs origin (e.g. bone marrow, adipose, or UC), culture type (flask or bioreactor), the media and supplements types (e.g., fetal bovine serum, xeno-free, or chemically-defined media), and the microenvironment (e.g. oxygen tension and presence or absence of stress signals). (Willis, Kourembanas et al. 2017).
Exosome-based therapeutics address a generally encouraging cutting edge approach for treating a different number of diseases, especially sicknesses the pathogenesis of which includes an essential or major inflammatory provocative part. (Mohammadipoor, Antebi et al. 2018)The adequacy of MSC exosome medicines has been vigorously settled in various preclinical models, yet the improvement of huge scope exosome-based drugs and ensuing clinical preliminaries request the goal of a few mechanical and unthinking issues, mirroring the mindful route in obscure era for this generally modern field. (Kim, Nishida et al. 2016) Among the major issues to be resolved are the standardization of MSC culture conditions, and protocols for exosome harvest and storage. (Willis, Kourembanas et al. 2017)
The use of secretome-containing CM enjoys a few benefits contrasted with the use of stem cells, as CM can be manufactured, freeze-dried, packaged, and transported more easily. (Bari, Perteghella et al. 2019) Furthermore, as it is devoid of cells; there is no need to match the donor and the recipient to avoid rejection problems. Therefore, SC-derived CMs have a promising possibility to be produced as pharmaceuticals for RM. (Pawitan 2014)
Although MSC EVs lack the potential to directly form tumors, this does not imply that MSC-EV administration to human subjects is without any risk of promoting neoplasia. (Rani, Ryan et al. 2015)
Intravenous (IV) injection is the most widely recognized, least obtrusive, and most reproducible strategy, and accounting for almost half of all trials. Despite being the easiest way of transplantation, most of the cells become trapped in the lungs. (Lee, Kim et al. 2017).
According to the researches database, the least effective dose of the IV route that gives the highest response ranging from 70 to 190 million MSCs/patient/dose, and the median dose is 100 million MSCs/patient/dose. Only four trials showed that dose-dependent response gave improved outcomes. Otherwise, there is no difference or efficacy when using higher doses. (Kabat, Bobkov et al. 2020)
Our study results showed that after intranasal administration of SC in group Ⅲ and CM in group Ⅳ, showed effective restoration of TFTs. There was a significant statistical difference (P-value < 0>0.05)
There is an examination that goes against our outcomes showed, intranasal administration of thyroid hormones doesn't expand the substance of thyroid contents in the cerebrum and further raises the peripheral thyroid hormone levels. The data propose intranasal administration of thyroid hormone is certainly not a reasonable restorative methodology for MCT8 inadequacy, albeit elective details could be considered in the future to work on the nose-to-brain transport. (Grijota-Martínez, Bárez-López et al. 2020)
Detailed understanding of molecular mechanisms controlling stem cell self-renewal will assist us with creating novel strategies for in vitro development to conquer the restricted cell numbers and naivety of the cells. To beat these difficulties, further researches on the fundamental and molecular biology of UCSCs are required, novel clinical preliminaries ought to be created, and further developing UC public and private banking is of incredible interest. (Alatyyat, Alasmari et al. 2020).
In conclusion, we provide evidence the practicability and safety of UC stem cells and conditioned media therapy have been tested in clinical trials, but the optimal SC and their CM dose and delivery route for the treatment of Hypothyroidism should be studied. Although some problems remain for the use of stem cells to treat thyroid disorder, UCSC and their CM are still a promising form of cell therapy.
Regarding the route of cell administration, the IV route seems to be the best choice. Its advantages include bedside application, greater cell survival, and possible paracrine effect exertion. On the other hand, the Intranasal route appeared to be a promising route to administer SC.
Current research has generally centered around single treatment methodologies which have created a lot of information. Notwithstanding, further examinations are needed in both current and novel medicines. Further developed longitudinal examinations using blends of medicines joined with drug delivery approaches will probably deliver the most strong and advanced impacts while limiting incidental effects however will require broad endeavors from the field to exhaustively assess the impacts in unique cell types.
All researchers similarly contributed recorded as a hard copy research idea, writing search, information examination, composition arrangement, drafting the original copy, checking on, and altering the original copy; envisioning the information into a table.
No funding was received for this article.
The authors declare that they have no competing commercial or financial interests.
The authors wish to acknowledge Professor Dr. Samuel Refetoff, the University of Chicago for his assistance and guidance support.
| CM | Condition Media | EM | Electron Microscope |
| SCs | Stem Cells | IHC | Immunohistochemistry |
| TFTs | Thyroid Function Tests | BCL2 | B Cell Lymphoma 2 |
| AMD | Amiodarone | BAX | Bcl-2-associated X protein |
| UC | Umbilical Cord | iNOS | Inducible Nitric Oxide Synthase |
| Temp | Temperature | IUGR | intrauterine growth retardation |
| H. &E. | Hematoxylin and Eosin | ECM | extracellular membrane |
| LM | Light Microscope | MSCs | Mesenchymal Stem Cells |
| IV | Intravenous | UCSC | Umbilical cord stem cells |
Animals were grouped of five in animal cages with suitable ventilation, the temperature of 22-25° C, 12 hours light-dark cycle, and free access to food and water (containing Amiodarone) in the Animal House-MASRI-Faculty of Medicine- Ain Shams University. The animals were allowed to the new environment before for 7 days before experimental procedures to decrease the possible discomfort of animals. Animals were not exposed to unnecessary pain or stress and animal manipulation was performed with maximal care and hygiene.
At the end of the experiment, rats were sacrificed by overdose of anesthesia. Euthanasia was carried out quickly and painlessly in a location separate from animal rooms by the general anesthesia Diethyl Ether. All animal carcasses were packed in polyethylene bags before sending them for disposal. Animal remains disposal was done by incineration.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.

Dear Editorial Team, Journal-Clinical Cardiology and Cardiovascular Interventions, “Publishing my article with Clinical Cardiology and Cardiovascular Interventions has been a highly positive experience. The peer-review process was rigorous yet supportive, offering valuable feedback that strengthened my work. The editorial team demonstrated exceptional professionalism, prompt communication, and a genuine commitment to maintaining the highest scientific standards. I am very pleased with the publication quality and proud to be associated with such a reputable journal.” Warm regards, Dr. Mahmoud Kamal Moustafa Ahmed

Dear Maria Emerson, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews’, I appreciate the opportunity to publish my article with your journal. The editorial office provided clear communication during the submission and review process, and I found the overall experience professional and constructive. Best regards, Elena Salvatore.

Dear Mayra Duenas, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews Herewith I confirm an optimal peer review process and a great support of the editorial office of the present journal
Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. I am really grateful for the peers review; their feedback gave me the opportunity to reflect on the message and impact of my work and to ameliorate the article. The editors did a great job in addition by encouraging me to continue with the process of publishing.

Dear Cecilia Lilly, Editorial Coordinator, Endocrinology and Disorders, Thank you so much for your quick response regarding reviewing and all process till publishing our manuscript entitled: Prevalence of Pre-Diabetes and its Associated Risk Factors Among Nile College Students, Sudan. Best regards, Dr Mamoun Magzoub.

International Journal of Clinical Case Reports and Reviews is a high quality journal that has a clear and concise submission process. The peer review process was comprehensive and constructive. Support from the editorial office was excellent, since the administrative staff were responsive. The journal provides a fast and timely publication timeline.

Dear Maria Emerson, Editorial Coordinator of International Journal of Clinical Case Reports and Reviews, What distinguishes International Journal of Clinical Case Report and Review is not only the scientific rigor of its publications, but the intellectual climate in which research is evaluated. The submission process is refreshingly free of unnecessary formal barriers and bureaucratic rituals that often complicate academic publishing without adding real value. The peer-review system is demanding yet constructive, guided by genuine scientific dialogue rather than hierarchical or authoritarian attitudes. Reviewers act as collaborators in improving the manuscript, not as gatekeepers imposing arbitrary standards. This journal offers a rare balance: high methodological standards combined with a respectful, transparent, and supportive editorial approach. In an era where publishing can feel more burdensome than research itself, this platform restores the original purpose of peer review — to refine ideas, not to obstruct them Prof. Perlat Kapisyzi, FCCP PULMONOLOGIST AND THORACIC IMAGING.

Dear Grace Pierce, International Journal of Clinical Case Reports and Reviews I appreciate the opportunity to review for Auctore Journal, as the overall editorial process was smooth, transparent and professionally managed. This journal maintains high scientific standards and ensures timely communications with authors, which is truly commendable. I would like to express my special thanks to editor Grace Pierce for his constant guidance, promt responses, and supportive coordination throughout the review process. I am also greatful to Eleanor Bailey from the finance department for her clear communication and efficient handling of all administrative matters. Overall, my experience with Auctore Journal has been highly positive and rewarding. Best regards, Sabita sinha

Dear Mayra Duenas, Editorial Coordinator of the journal IJCCR, I write here a little on my experience as an author submitting to the International Journal of Clinical Case Reports and Reviews (IJCCR). This was my first submission to IJCCR and my manuscript was inherently an outsider’s effort. It attempted to broadly identify and then make some sense of life’s under-appreciated mysteries. I initially had responded to a request for possible submissions. I then contacted IJCCR with a tentative topic for a manuscript. They quickly got back with an approval for the submission, but with a particular requirement that it be medically relevant. I then put together a manuscript and submitted it. After the usual back-and-forth over forms and formality, the manuscript was sent off for reviews. Within 2 weeks I got back 4 reviews which were both helpful and also surprising. Surprising in that the topic was somewhat foreign to medical literature. My subsequent updates in response to the reviewer comments went smoothly and in short order I had a series of proofs to evaluate. All in all, the whole publication process seemed outstanding. It was both helpful in terms of the paper’s content and also in terms of its efficient and friendly communications. Thank you all very much. Sincerely, Ted Christopher, Rochester, NY.
