AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2692-9406/005
*Corresponding Author: Mohamed Hisham Aref, Military Technical College, Biomedical Engineering Department, El-Fangary Street, Cairo, EGYPT.
Citation: Mohamed Hisham. Aref., Ibrahim H. Aboughaleb., Yasser H. El-Sharkawy. (2020) Spatiotemporal Thermal Contours mapping of ex- vivo Bovine Liver Radiofrequency Thermal Ablation utilizing Hyperspectral Image and its Associated K-Mean Clustering Algorithm. Biomedical Research and Clinical Reviews, 1(1): Doi:10.31579/2692-9406/005
Copyright: © 2020 Mohamed Hisham Aref. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 14 April 2020 | Accepted: 30 April 2020 | Published: 06 May 2020
Keywords: liver tumor; hyperspectral imaging; rf thermal ablation; k-mean clustering algorithm, tissue thermal depth
Significance: Hepatocellular carcinoma (HCC) is considered as worldwide health problem with a poor diagnosis due to limited detection techniques. Thermal ablation is the dominant modality to treat liver tumors for discriminating patients who are not allowed to have surgical intervention. Knowing that, observing or foreseeing the size of the subsequent tissue putrefaction during the Thermal Ablation techniques is a difficult undertaking.
Aim: To examine the impacts of ablation zone volume following Radiofrequency ablation (RFA) of an ex-vivo bovine liver to correlate the impacts of thermal ablation with target organ perfusion; by exploiting the unique properties of Hyperspectral Imaging (HSI).where, Vessels may source cooling in the adjacent tumor target (heat‑sink‑effect) with risk of cancer recurrence and the infiltration profundity estimations consider the lessening of the tissue.
Materials and Methods: Radiofrequency ablation was perfused on ex-vivo bovine livers at peripheral and central‑vessel‑adjacent locations, and monitored by HSI with a spectral range from 400 to 1000 nm. The system contains k-means clustering (K=8) algorithms combining spectral and spatial information. Labeled spectral signatures datasets were used as training data. Statistical analysis (10 samples) was computed to calculate the highest variance between six spectral images for determining the optimum wavelength for discrimination between the affected regions after thermal ablation (normal, thermal, and ablated liver tissue regions).
Results: The change of the optical properties of ex-vivo liver tissues provides different responses to light transmission, scattering, absorption and particularly the reflection over the spectrum range. The spectral reflectance signatures were measured and evaluated using designed K-mean clustering algorithm after image reconstructed. Trials showed that spectral region 650~650 nm was proposed as optimum spectral range. Where, these results successfully distinguishes the Surface Thermal ablation region (x,y-axis),as well as the Thermal penetration Depth (z-axis) for Tissue characterization and Contour mapping for the unwanted thermal damage.
Conclusions: Hyperspectral imaging is a powerful tool in real-time monitoring the thermal ablation and more accurate compared to the conventional imaging modality.
Liver malignancy is the 5th most common tumor and the 2nd most prominent reason for tumor-related passing around the world, and more than (800,000) deaths are accounted for globally every year [1], [2]. In Egypt, there has been a noteworthy increment in the extent of hepatocellular carcinoma (HCC) among interminable liver disease patients [3]. Hepatocellular carcinoma (HCC) is the most well-known essential dangerous tumor in the liver, particularly in patients with cirrhosis[4]. Confirmed patients with HCC follow a specific system for stage classifier and best treatment opportunity, such as the Barcelona Clinic Liver Cancer (BCLC) stage system [5].
Liver successful transplantation is considered the best option for HCC treatment for eligible patients [2], [6], [7]. However, transplantation is constrained by the accessibility of liver contributors and by the available budget [8]. Another great restorative alternative for initial-phase HCC patients with the non-cirrhotic liver is hepatic resection[9]. Fractional hepatectomy gives shorter holding up times and practically identical 5-year endurance rates (60~70%) contrasted with transplantation [10].On the other hand, the number of colorectal metastases (CLM) patients qualified for resection is less than 20% because of incompatible tumor areas and worsened residual liver function [11].
Negligibly intrusive thermal ablation, including laser interstitial thermotherapy [12], radio-frequency Ablation (RFA) [13], microwave Ablation(MWA)[14], [15], and high-Intensity ultrasound (US) (interstitial [16], focused [17], and unfocused [18]) ablation. All these modalities is a significant treatment way to deal with unresectable essential and secondary liver tumors (e.g., HCC and CLM) [13], [18], [19].
Thermal ablation may likewise be utilized to help careful resection to diminish intraoperative blood loss and postoperative hospitalization [13]. While it is considered being the most fitting alternative track for HCC long-suffering patients who are unqualified for both transplantation and resection. This track is usually used for the patients who have tumor size ≤ 3 cm, in light of the Barcelona Clinic Liver Cancer (BCLC) classification framework [5], [13].
Even-though, RF ablation is more affordable, less obtrusive, and with lower difficulty rates than incomplete resection in early hepatocellular carcinoma (HCC) and small colorectal metastases [20], [21]. However, it is not the ideal modality due to the inability to monitor the thermal effect with the conventional image-guided techniques, such as the Computed Tomography (CT) and ultrasound due to the struggle of separation of feasible and destructed tissue and distinguishing proof of the tumor edges, as a result of tissue scarring and gas bubble fact which emerge during the thermal procedure [22],[23], [24].
Imaging modalities are vital for real-time observing of thermal tissue variations throughout laser interstitial thermotherapy, RFA, MWA, HIFU, or bulk thermal ablation [25], [26]. MRᶃ-HIFU has been utilized for observing and control of thermal removal utilizing real-time MR thermometry[27], [28]. Knowing that MRᶃ-HIFU escalations treatment therapy is expensive and difficult [29], [30]. USᶃ-HIFU is more convenient and low-cost, while its high edge rate, constant ability conceivably makes it increasingly equipped for ablating versatile organs[31], [32].
Recently, many researchers have developed several imaging methods to guarantee suitable removal of liver malignant and metastases utilizing non-intrusive or negligibly obtrusive systems with respect to the conventional excisional biopsy. Of these imaging techniques, infrared thermography monitoring [33], [34],contrast‑enhanced ultrasound [35], real‑time ultrasound elastography ,high resolution US [36], [37] and electrode vibration elastography[38].This exploratory investigation was the leading at the attainability of HSI observing in RFA .On the other side hyperspectral imaging (HSI) method provides a specific wavelengths (spectral image) across the electromagnetic spectrum as it collects spatial and spectral information for the specimen under investigation [39], [40].
Spectral imaging is otherwise called imaging spectroscopy, which introduces the innovation that incorporates conventional imaging and spectroscopy techniques to acquire both spatial and spectral data of an object [41]. It was at first described in the late 1980s for remote distinguishing of the Earth [42]. Spectral imaging can be separated into multispectral imaging, hyperspectral imaging (HSI), as per its unearthly goals, the number of groups, width, and continuity of groups[43], [44]. Multispectral imaging frameworks, for the most part, gather information in few and generally noncontiguous wide ghastly groups, regularly estimated in micrometers or several micrometers. These ghastly groups are chosen to gather power in explicitly characterized pieces of the range and advanced for specific classes of data generally clear in those groups. While HSI frameworks can gather many object groups [40], [41].
HSI is a rising imaging methodology, and promising outcomes have been appeared as for malignant growth identification in the brain. Where, two HS camera is used covering the spectral range of 400–1700 nm, the system is able in ~1 min to discriminate between normal and malignant tissue in the brain tissue thru neurosurgical procedures [45]. Study on gastric tumors in 10 human subjects. Where, the spectral monograms were obtained and evaluated in normal and Tumor tissue. Processing methods with the standard deviation of the spectral graph, support vector machine, and the 1st derivatives were recommended to improve and detect the Tumor regions. The 1st derivatives in spectral region between 1226~1251 nm and 1288~1370 nm were suggested as criteria that successfully discriminate between normal and Tumor tissue[46].
Moreover, in the HSI in medical applications, ultrasonic thermos-elastic emissions were produced in breast tissue by the inclusion of nanosecond laser pulses at 1064 nm generated by a Q-switched Nd: YAG laser. Where the differences in photoacoustic reaction signatures of normal and malignant breast tissue were found to be highly enhanced[47]. In the prostate tumor, the HSI framework was utilized to catch in-vivo pictures of rats influenced by human prostate tumors. The results upon 11 mice proved that the sensitivity and specificity of the HSI classification technique are 92.8% to 2.0% and 96.9% to 1.3%, respectively [48].
This pilot study demonstrates the objective of building and implementation a custom optical imaging system and associated K-mean clustering algorithm using to characterized and evaluate different thermal effects of investigated ex-vivo bovine liver samples. This non-invasive system can provide virtual information for a medical doctor, helping in early perception during surgery by improving tissue visibility and an early admonition to maintain a strategic distance from tissue fibrosis and thermal impact utilizing electrosurgical generator or any other Surgery/Thermal ablation modality [47][49].
Materials and Methods
In this sector, exploratory arrangement and techniques for a progression of examinations on controlled thermal ablation of ex-vivo Bovine liver tissue are talked about. In these analyses, where all the pieces of the framework and their interconnections are exhibited in Figure 1. Seven different elementscompose the system acquisition platform for tissue characterization (Hyperspectral camera / CCD microscopic camera / Thermal camera / RF generator / Light source / Computer & its Image processing software / Liver sample posed on the white optical board).
Experimental under test samples (Bovine Liver Tissue) A freshly cut out bovine liver was attained from a local abattoir in EGYPT and placed in ice-box with deionized saline, Liver tissue samples were cut with measurements (5 cm × 6 cm), Analysis were performed at room temperature 25 °C, with a normal liver temperature of 23°C~ 25°C estimated before every preliminary and stored in refrigerator up to –70°C.
Optical imaging system for liver sample ablation monitoring:
To capture the necessary images Hyperspectral (HS) Camera was utilized (Brand: Surface Optics –Model: SOC710) this model has spectral range (400 to 1000 nm), 5 nm spectral resolution, pushbroom (line‐scanning) and 10 degrees field of view. The camera incorporated with a lens (Schneider KREUZNACH XENOPLAN 1.9/35 CCTV-LENS 400 bis 1000nm). For each sample, the spectral cube image composed of 128 frames, and each frame represented a spectrum image of a specific wavelength.
Thermal Ablation Set-up:
Radiofrequency Generator (RF) (Brand: Bowa, Model: ARC303) was used to generate the necessary heat in the liver tissue simulating equivalent case as the surgical theatre for liver cancer thermal ablation and have been continuously monitored the temperature by the Non-Contact thermal camera (Brand: FLIR, Model: ETS 320), where the ground plate have been cut and customized by the sample size and area under the liver tissue and the active electrode fixed with articulating arm with a specific inserting depth (2 cm) inside the tissue, where the sample dimension is (5×6 cm).
The experimental setup have been repeated by changing the RF Tip to measure initially the Surface Thermal (x,y axis) Tissue characterization as illustrated in Figure 1-a,as well as to Monitor the Thermal Depth (z axis) for Tissue characterization thermal penetration depth evaluation in the (Z-axis) as shown in Figure 1-b.
HS cameras require solid and exact brightening of the scene to be caught so as to stay away from outside obstructions created by the ecological light where the catch is being performed. The enlightenment framework utilized right now dependent on a polychromatic light (Brand: Derungs –Model: 20P SX -20 Watt) this model has spectral range (348 to 820 nm). This sort of light is reasonable for HS applications because of the high homogeneity of its range over the whole spectral range.
Several experiments were repeated and implemented to reach the desired results needed to execute the necessary image analysis and signal processing upon the various sources of captured images. Revealed the RGB images with the CCD microscopic camera (500X) to monitor and record the thermal ablation process, additionally the thermal images by thermal camera (FLIR -Model: ETS 320) for monitoring the elevation of the thermal effect in the sample tissue during the ablation, supplementary to be used in image verification with our algorithm.
HSI sensors produce a three-dimensional (3D) information structure, called HS block, where the spatial data is contained in the initial two measurements, while the third measurement incorporates the spectral data [50], [51]. In a hyperspectral picture, every pixel has an arrangement of reflectance in various spectral frequencies that can show the spectral outline of that pixel [45]. On one hand, every pixel of the HS picture contains a full otherworldly mark of length equivalent to the quantity of spectral groups of the HS cube. On the other hand, a dark scale picture of the caught scene can be gotten utilizing any of the unearthly groups that shows the spatial data gave by the picture sensor at a specific frequency[52][50].
Hyperspectral picture conquering:
Six individual arrangements of hyperspectral pictures were gotten for each liver sample, each arrangement consisting of a (5 cm × 6 cm) of the liver sample arrangement on a lined tray. Line-scan images were captured for exposure times of 6 msec at 3.12 nm intervals, and each set consisted of 600 line scans corresponding to 600 × 502 pixels per spectral band. The hyperspectral images were composed of a total of 128 spectral bands in the range from approximate 379 to 1050 nm, incorporated with a lens with range (400 to 100 nm).
The drove light enlightens the liver tissue region of interest (ROI) and the camera target focal point gathers the radiation from the tissue and shows a picture on the passage way cut plane. The cut decides the field of imaging in spatial ways. The radiation from the cut is anticipated to the prism-grating; along these lines the bearing of engendering of the radiation changes on its wavelength. Each purpose of the tissue is represented on the lattice detector by an arrangement of monochromatic focuses that makes a consistent range toward the unearthly hub.
Records standardization:
The issue with the spectral non-consistency of the light system and the impact of the dim current are wiped out through standardization of the picture information to discover the standardized reflectance of the sample. A standard reference white board was set in the area of imaging and its information were used as the white reference[44], [51].This white reference is a standard reflectance that ought to be utilized for information standardization which shows most extreme standard reflectance in every frequency and in catching time temperature. The reflectance from the board gives a gauge of the occurrence light on the organs at every frequency and standardizes the temperature changes, which is utilized in standardization of the range. The dull current was caught by keeping the camera shade shut. At that point the information was standardized to locate a relative reflectance, as clarified in equation (1):
RFλ=Rcrudeλ-RblackλRwhiteλ-Rblackλ
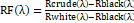
(1)
Where RFλthe determined relative reflectance esteem is for every Wavelength,Rcrudeλ
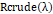
is crude information brilliance estimation of a given pixel,Rblackλ
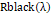
and Rwhiteλ
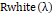
are, correspondingly, the dull current and the white board brilliance obtained for each line and otherworldly band of the sensor.
Spectral mapping and Acquisition:
The captured HSI cube is analyzed upon the 128 frames regarding the spectral range to select the optimum contrast wavelength, after The selective image at wavelength 900 nm have been normalized, then applying moving average filter with kernel value =10, afterward K-mean clustering up to 8-clusters for the contour display of the ablation and thermal regions and color mapping to the sample tissue, finally overlay of the images to produce the final algorithm for the thermal detection to the sample.
Conversion of the hyperspectral image to the RGB image segmentation by normalization of the value of R, G and B in the range of [0, 1],as more explained with its conditions in equations (2-7), lastly these values is multiplied by 255 (8-bit conversion)[53].
n=N×π 180

(2); M=m 100
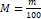
(3); i=I 255
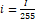
(4)
x=i ×(1-m)

(5);y=i×1+s×cos(n)cos(π3-n)

(6)
z=3i-(x+y)

(7)
If n≤2π3 then R=y;G=z and B=x
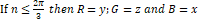
If 2π3≤n<4π3 then n=n-2π3; R=x ;G=y and B=z

Then, applying the moving average filter, likewise well-known as arithmetic mean filter at kernel value 10 for noise removal and image enhancement [54].
fx,y=1hn+k,L∈W∞φk,L
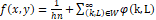
(8)
Where ‘φ’ is the noisy image, fx,y
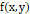
is the restored image, and ‘k’ and ‘L’ are the row and column coordinates respectively, within a window ‘W’ of size ‘h×n’ where the operation takes place.
Next, applying the K-mean clustering (KMC) up to 8-clusters with variable threshold value from 0.2 up to 0.9 for variable thermal display, k-means is one of the least difficult unsupervised learning algorithms that take care of the clustering problem. We can formulate a general equation for the clustering algorithm; we can define the center-based clustering. Where variables define as N=ni…nj
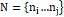
is the set of (ń) data points to be clustered in the d-dimensional, S=si...sj
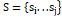
is the set of (Ƙ) center points in the d-dimensional. The membership function Msjni
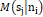
describes the proportion of the data set ni that related to the centroid sj with limitation Msjni≥0
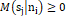
and jƘMsjni=1
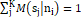
.A weight function ŵ=ni
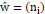
demonstrated how much effects data set ni in recalculation the new center with limitationŵ=ni≥ 0.
We can define the general model for the center-based clustering steps, as follows:
sj= i=1ńMsjniŵ=ninii=1ńMsjniŵ=ni

(9)
K-mean clustering (KMC) algorithm improves is:
KMCN,S=i=1n j∈{1…K}min ni-sj2
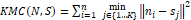
(10)
This demonstrable function in equation (10) provides an algorithm which reduces the within-cluster variance.
x+an=k=0nnkxkan-k

(11)
The weight and membership functions concerning KMC are: Msjni
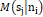
=1 ;if x=argminjni-sj20 ; otherwise
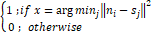
(12)
wKMCni=1
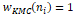
(13)
Regarding the hyperspectral image justifications, each point in the liver tissue has a succession of relative reflectance in various wavelengths that makes the spectrum outline or unearthly mark of a point. The reflectance spectra of the pixels made out of thermal and ablated zones of a liver tissue surface were separated and used to compute a normal reflectance range. The degree of the thermal effect was divided into three levels (ablated, thermal influenced, and typical tissues), where these spectrum outlines have been assessed to discover a distinction between them in the tissue.
Capturing the images with the Hyperspectral camera and processing the image before and after thermal ablation. Image normalization, moving average filter and K-mean clustering were utilized as channels to upgrade the thermal and ablated district in images caught with the hyperspectral camera. The capturing time for each image is about 5 ~ 12 sec and calculation time is < 20-sec using DADiSP/SE software (DSP Development Corporation,6.7 NI student edition B02, 2017) on a computer with processor Intel core i7 @1.8GHz with a 16 GB RAM. A conventional philosophy for target the identification and execution assessment was applied. The edges were balanced so as to expand the ablated recognition to guarantee identification of all the thermal /ablated districts. The exhibition was assessed utilizing the nature of discovery regarding the pathology.
After ablation, each sample was captured with the Hyperspectral camera, CCD microscopic camera (500X), and the thermal camera respectively thru several positions and at the same time for data recording purposes. Next, the liver sample was scanned at 1200 dpi to produce a high-resolution image to be used in image processing. Consequently, splitting it from the point of thermal ablation to explore more overall information about ablation and affected lateral cells to compare it with the captured Hyperspectral data. Thermal ablation continuously captured by a thermal camera for temperature monitoring, as shown in Figure 2.
The captured image of the ablation zone in ex vivo bovine tissue have been scanned at 1200 dpi for high image resolution shown in Figure 3 to compare it with the contour mapping analysis of the system algorithm to differentiate the unwanted thermal damage that was produced by heat conduction from the ablation tool, where heat conduction from the hot ablation zone along the ablation tool shaft have been more clearly appeared in the cross section View for the liver tissue and the penetration depth is shown by ablation tool tip which is approximately 2 cm long. Right and left piece for the bottom and top piece respectively in Figure 3-e.
The imaging method utilized with this camera is a pushbroom scanner; the cut passageway restricts the imaging field. By moving the camera between consequent pictures, inevitably all locales and wavelengths of the item are caught [52]. By reproducing the resulting pictures, a monochromatic unearthly picture for every wavelength can be developed, demonstrating the role of the wavelength to Spectacles or conceal specific information.
The deliberate optical spectra of biological tissues from 400 to 1000 nm spread the noticeable and can be separated at the atomic level, which extraordinarily adds to the reflectance esteems estimated in specific reaches. The test was performed utilizing the ex-vivo bovine liver tissue, where captured HSI is scanned and segmented, selecting specific points to measure the diffuse reflectance of the ablation region and two different positions around it for measuring the thermal effect with respect to the normal tissue, as illustrated in Figure 4-a.
These signals were drawn in the graph illustrating the unique spectral signatures of the normal, ablated and thermal regions, as demonstrated in Figure 4-b.the spectral reflectance between the normal signal (Green solid line) and the ablation region (Blue solid line) is more identified in wavelength ≥ 600 nm. However, to identify between the normal (Green solid line) and thermally affected tissue (Brown and Red solid line) it was more recognized in wavelength ≥ 500 nm.
we had used these spectral signatures as a guide for selecting the optimum spectral images which can be discriminate between the Four regions (Normal, Thermal#1, Thermal#2 and Ablation), to reduce the time of image processing for 128 frames to be only five spectral images starting from 500 nm up to 650 nm.
The Captured spectral images of Bovine liver tissue after thermal ablation have been analysis and processed at various wavelengths to differentiate the pixel reflectance of the liver tissue. Spectral images within 400~800 nm addressed the discrimination between the ablated region, the normal region and the thermal effect degree on the liver tissue. The spectral image at wavelength range 600 ~ 650 nm is more distinguishable for the Thermal / Ablated zones, as previously clarified in Figure 4-b and more identified in the worksheet of Figure 5.
The various wavelengths in nanometers and the vertical hub of the demonstrating HSI segmentation where standardized reflectance of the liver tissue utilizing hyperspectral camera; the signal reflectance of the normal tissue is coded with (N) and plotted in Figure5-b; the signal reflectance of the Ablation Region is coded with (A) and plotted in Figure 5-c; the signal reflectance of Thermal #1 and Thermal#2 Regions is coded with (T1) and (T2) respectively plotted in Figure 5-c,e. According to the analysis of the signature signals in Figure 5-f which highlight that it’s clearly identifying the thermal and ablated regions more at the wavelength region (600 ~ 650 nm).
The captured images of five spectral images starting from 500 nm up to 650 nm of the liver sample utilizing hyperspectral camera have been segmented for more image data, as shown in Figure 6-f. Then, applying normalization and Moving average filter (K=10) for noise removing.Finally, perform the Contour mapping with the K-mean image clustering (K=8) illuminating the thermal region and overlaying the contour mapping according to the threshold value over the original HSI to distinguish the selective surface thermal region in the liver sample for the surgeon, as illustrated in Figure 6-k and L respectively.
The experimental trails results that the Surface Thermal (x,y axis) Tissue characterization could be identified by the HS imaging with more details versus the visual inspection as illustrated in Figure 6,eventhough the thermal penetration depth evaluation in the (Z-axis) could not be visually identified but with the HS imaging it was clearly identified as shown in Figure 7.
Thermal ablation is the dominant modalities for ablative treatment of un-resection liver tumors, where monitoring the ablation process with the right image‑guided inclusion of terminals is vital to accomplish a fruitful removal of tumors with minimum thermal damage of liver [34]–[37]. The removal procedure is routinely observed with computed tomography, or b-mode sonography to guarantee fruitful tumor removal with fitting wellbeing edges; however, tissue scarring or gas bubble development may hamper translation of sonographic pictures[20], [21], [33], [35].
Other imaging modalities like infrared thermography [33], [34], contrast‑enhanced ultrasound [35], real‑time ultrasound elastography, high-resolution US [36], [37], and electrode vibration elastography [38], which have been inspected to screen the ablation procedure. Anyway, all checking modalities have their issues to give an exact proclamation about the achievement of the ablation. On the other side hyperspectral imaging (HSI) method provides specific wavelengths (spectral image) across the electromagnetic spectrum as it collects spatial and spectral information for the specimen under investigation[39], [40].
Because of considerable between quiet spectral changeability which in turn is reflected on the ex-vivo living tissue samples the developed algorithm encountered two main challenges: the high conditionality and the predetermined number of samples. Finally, the utilization of high spectral resolution HS cameras for thermal tissue examination permits analysts to concentrate in detail the optical properties of the tissues, distinguishing the most significant spectral diverts that are engaged with a specific application. The utilization of high spatial resolution HS cameras allows the examination of tests by joining the spectral and the morphological properties of the tissue, as illustrated in Figure 4 and Figure 5, respectively.
The work introduced in this paper with the objective of building up a custom optical imaging system and associated K-mean clustering algorithm to characterize and evaluate different thermal effects of the investigated ex-vivo beef liver sample before and after Radiofrequency ablation (RFA). The results demonstrated that the optimum wavelength range (600~650 nm) differentiated between the normal region, thermal, and ablated regions of the investigated samples.
However, Hyperspectral imaging is a powerful tool in monitoring the thermal ablation and more accurate compared to the conventional imaging modality, but it is very expensive regarding the commercial CCD camera and it can`t work in real-time as the spectral cube need to be analyzed for the 128 frames and consume more time for image processing, and to avoid that topic we had to discriminate the optimum wavelength (600~650 nm), which decrease the time consuming and provide precise information to demonstrate significant monitoring of the ablation process to accomplish a fruitful ablation of tumors with minimum thermal damage of liver..
Our study was limited by ex-vivo liver tissue utilizing HS camera; further investigations will be carried out in the future work for validation using commercial CCD camera attached with the optical filter (720 nm) and several types of tissues with different properties.
The authors declare no conflict of interest and Stated that they have no competing interest. There are No funders and No one had any role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results .This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions:
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Giselle Pentón-Rol.
