AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2767-7370/051
1 Student of the Nutrition course at Paulista University UNIP, Campinas São Paulo/ SP, Brazil.
2 Nurse, Doctor, Professor of the Nursing and Medicine Course at Paulista University UNIP, Campinas, São Paulo/SP, Brazil.
3 Biologist, Doctor, Professor of the Nutrition and Medicine Course at Paulista University UNIP, Campinas, São Paulo/SP, Brazil.
*Corresponding Author: Luciana Pietro, Biologist, Doctor, Professor of the Nutrition and Medicine Course at Paulista University UNIP, Campinas, São Paulo/SP, Brazil.
Citation: Amanda de Paula Soares da Silva, João Paulo Marciano, Simone Camargo de Oliveira Rossignolo, and Luciana Pietro, (2023), Relationship of Diabetes Mellitus type 2 in the Alteration of Proteins Involved in the Incidence of Alzheimer, J New Medical Innovations and Research, 4(4); DOI:10.31579/2767-7370/051
Copyright: © 2023, Luciana Pietro. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 19 July 2023 | Accepted: 09 August 2023 | Published: 18 August 2023
Keywords: alzheimer's disease; diabetes mellitus; beta amyloid protein; tau protein
Background: Alzheimer's disease (AD) is a syndrome of chronic or progressive origin that can deteriorate neural functions, especially cognitive ones, with worsening of memory, thinking, orientation, understanding of learning capacity and judgment, usually accompanied by bipolarity.
Aim: to analyze the influence of Type 2 Diabetes Mellitus (DM2) on the alteration of proteins involved in the development of Alzheimer's disease.
Materials and methods: This is a systematic review study, in English and Portuguese, between 2010 and 2022.
Results: From the data collection, it was possible to observe the relationship of amyloid beta and tau proteins with Alzheimer's, and the direct impact that glycemic dysregulation due to insulin resistance, considered one of the main characteristics of DM2, it can cause, leading to the accumulation of beta-amyloid and Tau hyperphosphorylation, making it possible to state that there is a connection between DM2 and AD, acting as a cofactor for the development of the disease of Alzheimer's and evolution of more severe conditions.
Conclusions: we can see that there is a link between DM2 and AD, due to selective deficiencies in insulin signaling, together with the positive regulation and accumulation of beta amyloid protein and hyperphosphorylation of Tau protein, being a cofactor for the development of Alzheimer's disease and the evolution of more severe conditions.
Running title: Diabetes Mellitus Type 2 and Alzheimer
AD: Alzheimer's disease DM: Diabetes Mellitus DM2: Type 2 Diabetes Mellitus WHO: World Health Organization βA: β-amyloid protein HOMA-IR: Homeostatic Model for the Assessment of Insulin Resistance BBB: Blood-brain barrier PSD: Component of postsynaptic density CSF: Cerebrospinal fluid PET: Positron emission tomography PIB: Positron emission tomography with 11C-Pittsburgh compound B BMI: Low cognitive impairment P-tau: levels of phosphorylated tau protein T-tau: Levels of phosphorylated total tau protein | MRI: Cranial magnetic resonance imaging APOE: Apolipoprotein E CBD: Corticobasal Degeneration PSP: Progressive supranuclear palsy DFT: Frontotemporal dementia PI3K: Phosphoinositide 3-kinase EDI: Insulin-degrading enzyme CNS: Central nervous system (CNS) PLA2: Phospholipase A2 p-AKT: Protein kinase B (p-AKT) GSK3: Glycogen synthase kinase 3 (GSK3). AGE’s: Advanced Glycation End Products (AGE's) |
Alzheimer's disease (AD), or also known as dementia, is a syndrome of chronic or progressive origin that can deteriorate neural functions, especially cognitive ones, with worsening of memory, thinking, orientation, understanding of learning capacity and judgment, usually accompanied by bipolarity [1].
According to the World Health Organization (WHO), more than 55 million people live with Alzheimer's worldwide and about 10 million incidences per year [1]. In Brazil, it is estimated that the average prevalence may present the highest worldwide in elderly people over 65 years old, which will increase from 7.6% to 7.9 percentage between 2010 and 2020, totaling 55,000 new cases per year². In the ranking of the main causes of death in the world, Alzheimer's currently occupies the seventh place among all the diseases that cause disability and dependency in the elderly, with about 1.5 million per year, with the prevalence increasing more and more [1].
Dementia can affect not only the carrier of the pathologies, but also their family members and caregivers, presenting and manifesting in different ways, depending on the preceding causes, or other health conditions that would aggravate the neurological functioning problems before the person is diagnosed with Alzheimer's [1]. The apparent symptoms linked to Alzheimer's can be separated into 3 stages: initial, intermediate, and late stage, where in the initial stage the loss of simple notions of space and time is observed, such as getting lost in familiar places, forgetting recent facts, and even lose track of time. As the disease worsens, it advances to the intermediate level, in which communication begins to be affected, with difficulty recognizing family members, and the need for help with personal care. In the most advanced stage called Late Phase, the patient becomes totally dependent, needing to be always assisted, with his motor coordination to walk affected and showing the first signs of bipolarity [1,2].
According to Povova et al. [3], more than a century after the discovery of Alzheimer's disease, only two pathological processes were identified for the of the disease, which are: development deposition of β-amyloid protein (βA) and tau proteins, usually observed in the first diagnosis. However, other hypotheses are also suggested, such as molecular, genetic, and epidemiological alterations, which are currently being studied. Diabetes is one of the diseases in which it is believed that there is a relationship in which there may be a contribution to the development of AD, and there are already some studies that try to talk about the relationship between the two diseases [4].
Diabetes is part of a group of metabolic diseases that are determined by hyperglycemia, resulting from a defect in insulin action, secretion, or both. Hyperglycemia, which is directly related to diabetes, is associated with future damage, dysfunction, and the failure of numerous organs, some of which are: “eyes, kidneys, nerves, heart and blood vessels”. The development of diabetes involves many pathogenic processes, which can be “autoimmune destruction of the β cells of the pancreas with consequent insulin deficiency and even abnormalities that result in resistance to the action of insulin”, or a lower tissue response at points in the pathways of hormonal action, of so that, when there is a deficiency in the action of insulin, as in cases of diabetes, the metabolism of macronutrients does not occur normally [5,6].
Diabetes cases most often fall into two categories, Diabetes Mellitus (DM) type 1 and Diabetes Mellitus type 2, with type 1 occurring when there is a total deficiency of insulin secretion because of the destruction of pancreatic β cells, while type 2 DM is caused by insulin resistance defined by a certain deficiency in the secretion of pancreatic β cells, which can lead to hyperglycemia [7]. Among these two types of DM, the most common is type 2, which is closely associated with obesity and aging, with type 1 having its greatest development in children and adolescents [8].
In type 1 DM, only 5 to 10% of people have this form of diabetes that is characterized by autoimmune destruction by pancreatic β cells, this rate of destruction varies from fast to slow from individual to individual and is usually faster in children and adolescents and slower in adults. The first manifestation that can be represented mainly by children and adolescents is ketoacidosis (accumulation of ketone bodies making the blood acidic), while others have modest fasting hyperglycemia, but which can change to more severe hyperglycemia or ketoacidosis due to infection or some other another stress. While adults normally retain pancreatic β cell function and arrest ketoacidosis for quite some time, making them dependent on insulin to live and still at risk of ketoacidosis [5-9].
Regarding type 2 DM, about 90 to 95% of people have this form of diabetes, occurring in people who have insulin resistance and presenting a relative diabetes, usually without an insulin deficiency, which happens at the beginning or even for a long time these people do not need insulin treatment. Most cases of type 2 DM are in obese people, which already causes a degree of insulin resistance by itself, since obese patients may have a higher percentage of body fat in the abdominal region. It is rare for ketoacidosis to occur spontaneously in type 2 DM, when it happens it is usually associated with other stresses or diseases [5-10].
AD and type 2 DM share features that are common in oxidative stress inflammation and insulin resistance [11]. As already mentioned, DM 2 is characterized by insulin resistance, which is the result of the reduction or failure of the response of peripheral tissues to insulin [12], generating hyperglycemia, which can negatively affect brain functions, such as the mechanism of glucose neurotoxicity. A common way to assess insulin resistance is through the Homeostatic Model for the Assessment of Insulin Resistance (HOMA-IR), a method considered the gold standard [13].
Insulin is a peptide hormone produced by pancreatic beta cells, and in addition to the function of regulating blood glucose levels, it has a neuroprotective function, regulating synaptic plasticity, becoming essential for good cognitive functioning. Insulin transport to the brain is via receptors located on the blood-brain barrier (BBB), which are distributed throughout the brain. Insulin also has access to regions of the brain that are not protected by the BBB, such as the hypothalamus [12-14].
Most insulin receptors are located on neurons, concentrating on synapses, which is an important insulin signaling site in the brain, as a component of postsynaptic density (PSD) [12]. The pathophysiology of neurodegeneration and cognitive decline that are associated with DM involves not only hyperglycemia and deficits in insulin signaling, but also hormonal changes, inflammatory processes, activation of neurotoxic pathways and mitochondrial dysfunction, characterizing multifactorial [15].
Therefore, the aim of this study was to analyze, through a systematic review of the literature, the influence of Type 2 Diabetes Mellitus on the alteration of proteins involved in the development of Alzheimer's disease.
This is a systematic review of the literature, which was carried out from the consultation of databases such as SCIELO, PubMed and Science Direct, selecting articles published in English and Portuguese, between the years 2010 to 2022. The terms used was Alzheimer Disease, Diabetes Mellitus, amyloid beta and tau. Based on these descriptors, the following word combinations were investigated: demyelination, disease progression.
Inclusion criteria were original articles published between 2010 and 2022, addressing the relationship between diabetes mellitus and the alteration of proteins involved in Alzheimer's disease, review articles and those published outside the defined date for inclusion were excluded.
In the survey process, through the use of descriptors, the presence of 11,004 articles related to the theme was verified. Of these, the abstracts were read, and based on the inclusion and exclusion criteria, 35 articles were separated, which were carefully analyzed. Of these 35, only 10 articles presented dosages of the analyzed proteins, which were then used for the systematic review. The process of search and selection of papers is shown in the Flowchart below (fig. 1), and the main data of the articles gathered are described in (table 1).
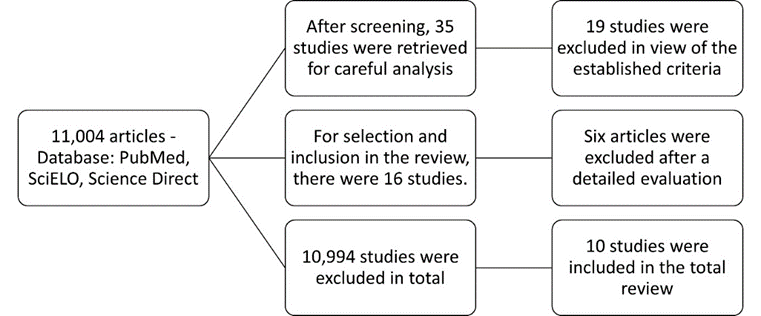
Figure 1: Search and selection process flowchart
After the search carried out in all mentioned databases, 10 original articles were selected, 4 of which analyzed Tau protein [16-19], 5 addressed the β-amyloid protein (βA) [20-24] and 1 covered both proteins, Tau and β-amyloid [25], all associated with increased or reduced expression of these proteins in the pathological condition of AD.
The study by Karikari et al. [22] analyzed the plasma expression of the p-tau181 protein in patients with Alzheimer's disease and age-matched controls, with or without varying degrees of cognitive impairment. Their results found that plasma p-tau181 expression showed gradual increases along the Alzheimer's disease continuum, allowing to distinguish Alzheimer's disease dementia from amyloid β-negative young adults and cognitively intact older adults, as well as other neurodegenerative disorders, including frontotemporal dementia, vascular dementia, progressive supranuclear palsy or corticobasal syndrome, and Parkinson's disease or multiple system atrophy. In addition, plasma p-tau181 has been associated with pathologies of brain tau as measured by PET (positron emission tomography), β-amyloid, and 1-year cognitive pathologies of decline and hippocampal atrophy [22].
Similarly, studies by Drummond et al. [17] aimed to determine which proteins pathological pTau interacts with in Alzheimer's disease. For this reason, cases of sporadic Alzheimer's disease were included in the first localized proteomic study of NFTs (neurofibrillary tangles), and 5 cases of sporadic Alzheimer's disease were included in the second pTau interactome study, with subjects with a mean age of 61-91 years. In their results, the presence of tau protein was verified in all cases, as being the most abundant in the samples. One hundred and sixty-six unique tau peptides were identified in this study (considering the presence of post-translational modifications), of which analysis of specific peptides showed evidence of all six isoforms of human tau. In the two complementary proteomic approaches, it determined the proteins present in the NFTs and the proteins that interact with pTau. Combined analysis of data generated using both approaches identified 75 proteins that are present in NFTs and significantly interact with pTau [17].
The article by Barthélemy et al. [18] determined by mass spectrometry the potential usefulness of plasma p-tau isoforms for detecting AD pathology and investigating relationships of tau isoform profile in cerebrospinal fluid (CSF) and plasma. A total of 126 individuals were evaluated in discovery and validation groups, in which plasmatic p-tau alterations were verified, especially in p-tau-217, reflecting highly specific modifications in the CSF for the detection of phosphorylation alterations in soluble tau and amyloidosis, which is consistent with that blood p-tau isoforms are potentially useful for detecting AD pathology, disease staging and diagnosis [18].
In relation to the works by Palmqvist et al. [19] examined plasma tau phosphorylated on threonine 217 (P-tau217) as a diagnostic biomarker for AD, with three cross-sectional cohorts: Arizona-based neuropathology cohort, including 34 participants with AD and 47 without AD, the Swedish BioFINDER-2 cohort (cohort 2), including cognitively intact participants (n = 301) and patients clinically diagnosed with mild cognitive impairment (MCI) (n = 178), AD dementia (n = 121) and other neurodegenerative diseases (n = 99) and an autosomal dominant Colombian AD family (cohort 3), including 365 carriers of the PSEN1 E280A mutation (mutation that causes early-onset Alzheimer's disease) and 257 non-mutation carriers, with a mean age of 34 - 84 years. Among 1402 participants from 3 selected cohorts, plasma P-tau217 discriminated AD from other neurodegenerative diseases with significantly greater accuracy than established plasma and MRI-based biomarkers, and its performance was not significantly different from leading cerebrospinal fluid (CSF) or Positron emission tomography (PET) based measurements [19].
The article Blasko et al. [20], examined the similarity of Late-Level Depression (LOD) subsequent to AD (Alzheimer's Disease) with plasma amyloid-42 Beta, in a sample of people who never had episodes of depression and without dementia in the beginning of the study. A 5-year prospective longitudinal study was performed in a nursing home, with 331 participants. After separating DA converters, regression computation showed that the highest plasma Aβ42 value at baseline was a positive predictor. Regardless of whether patients with mild cognitive impairment at 2.5 years were included or excluded in the regressions, higher plasma levels of Aβ42 were relevant predictors for the development of AD at 5 years. The highest conversion to AD was for males, but not high scores on the Geriatric Depression scale, with cases of stroke or cerebral infarction, there was no association for interaction in plasma levels [20].
The article Wang et al. [21] evaluated 24 patients with AD and 37 with normal cognitively controlled levels and underwent extensive clinical evaluations, such as: blood collection, neuropsychological tests, brain magnetic resonance imaging, measurement of Aβ42 cerebrospinal fluid (CSF) and positron emission tomography with 11C-Pittsburgh compound B (PIB). Pearson's correlation judgments between estimates of Aβ oligomer levels and other biomarkers. Analyzes were used to compare the performance of each biomarker. In the results, plasma levels of Aβ oligomers by MDS were higher in patients with AD than in control individuals [21].
In the Youn et al. [16] article, an ultrasensitive blood immunoassay was performed. Trial performance was evaluated in 4 prospective clinical cohorts. The investigated cohort consisted of patients with diagnosed Alzheimer's and age-matched controls. Two markers were used (TRIAD and BioFINDER 2), cognitively affected elderly, mean age 63 to 69 years, patients with low cognitive impairment (BMI) were entered into the study. In all tests, plasma p-tau181 showed increases throughout AD, even in smaller scales in cognitively unimpaired adults and young and elderly, and higher concentrations in elderly with beta-amyloid accumulation and cognitively compromised. The concentrations with the highest levels of p-tau181 were elderly positive for Alzheimer's, vascular dementia, supranuclear palsy and Parkinson's disease [16].
The article Stocker et al. [23], evaluates a cohort linked to the community, the misfolding of Aβ in the plasma measured by immuno-infrared sensor and APOE genotype, 770 volunteers were studied followed for 14 years. We investigated similarities between Aβ misfolding, APOE4 and other predictors with clinical incidence of AD, vascular dementia and mixed dementia. Aβ misfolding in blood plasma was aggressively and specifically predictive of risk for AD, even before diagnosis in a community setting. Incorrect folding increased the chances of a clinical diagnosis of AD by 23 times. There was no association observed with volunteers diagnosed with vascular or mixed dementia [23].
The article Wattmo et al. [25] investigated the potential associations between cerebrospinal fluid (CSF) levels of phosphorylated tau (P-tau) and total tau (T-tau) with short-term response to cholinesterase inhibitor (ChEI) treatment, longitudinal outcome, and rates of progression in Alzheimer's disease (AD). This prospective, observational study included 129 participants clinically diagnosed with mild to moderate AD who underwent a lumbar puncture. The CSF amyloid-β1–42 (Aβ42), P-tau and T-tau biomarkers were analyzed with xMAP technology. Cognitive, global, instrumental, and basic activities of daily living skills (ADL) were assessed at baseline chei therapy and biannually over 3 years. All participants had abnormal Aβ 42 (A+). 58 subjects (45%) exhibited normal P-tau and T-tau (A+ T– (N)–), 12 (9%) abnormal P-tau/normal T-tau (A+ T+ (N)–), 17 ( 13%) normal P-tau/abnormal T-tau (A+ T– (N)+) and 42 (33%) abnormal P-tau and T-tau (A+ T+ (N)+) 21) [23].
The Article Dominguez et al. [24], were evaluated in the St. Luke's Medical Center Global City from January 2018 to March 2019 for detailed neuropsychological examinations, cranial magnetic resonance imaging (MRI) and blood work. The criteria for including the patients were: adult patients with reporting cognitive decline or memory loss; diagnosed by a specialist; underwent comprehensive neuropsychological assessment at the Memory Service clinic and βMDS-OA result was available. In total there were 231 participants, among them, 84 were excluded for not meeting the required criteria, which ultimately left 147 participants in the study, predominantly female. The results showed no significant difference in sex distribution among the four clinical diagnoses. However, age group and alternation of AD stage were significantly different in different etiological diagnoses [24].
| Author / Year | Protein | Sample Group | Results (concentrations) |
|---|---|---|---|
| Blasko et al.,2010. [20] | beta amyloid. | Total: 331 individuals Group: NC Age: 75 years old Follow-up: 2 – 5 years | Higher levels of Beta-42 predict the development of AD. higher plasma A 42 levels at baseline and male gender were significant predictors for the development of probable or possible AD at 5 years |
| Wang et al., 2017. [21] | beta amyloid. | Total: 61 individuals Groups: AD and NC Age 50 – 90 years Follow-up: 6 years | Plasma levels of Aβ oligomers by MDS were higher in AD patients than in normal control subjects. AUC levels of AD: 0.844 (95% CI 0.7359-0.9539) high result within the comparison range, potential method to discriminate patients with AD from NC. |
| Karikari et al., 2019. [22] | Tau. | Total: 1131 individuals Groups: discovery cohort; validation (TRIAD), validation (BioFINDER-2) and primary care. Age: 23 – 69 years old | In all cohorts, plasma p-tau181 showed gradual increases across the Alzheimer's disease continuum. Plasma p-tau181 distinguished Alzheimer's disease dementia from amyloid β-negative young adults, from cognitively intact older adults, and from other neurodegenerative disorders, including frontotemporal dementia. Plasma p-tau181 was associated with brain tau pathologies measured by PET, amyloid β, 1-year cognitive decline, hippocampal atrophy discriminating Alzheimer's disease of cognitively intact young adults from elderly. With elevated AUC range from 76.14% to 100% |
| Youn et al., 2019.[16] | beta amyloid.
| Total: 162 individuals Groups: Normal Healthy (HNC), Subjective Cognitive Decline (SCD), Mild Cognitive Impairment (MCI) and Dementia of Alzheimer's Disease (AD). Age: 63 years | Oligomerization of Aβ in the blood can cause a pattern of AD in the brain. Characteristics of Aβ in the blood are related to changes in brain volume. MCI/light - 0.964 AD/alzheimer's - 0.993 HNC/normal - 0.852 SCD/subjective decline - 0.925 |
| Drummond et al., 2020.[17] | Tau. | Total: 12 individuals Groups: Localized proteomics of NFTs and pTau interacting Age 61 – 91 years old | Tau was the most abundant protein detected in PHF1 co-IP samples. Tau was the most significant upstream regulator of proteins present in NFTs (104 tau-regulated proteins) |
| Wattmo et al., 2020. [25] | Tau, Beta amiyloid. | Total: 129 individuals Group: AD Age: + 40 years 3 - year follow-up; | All patients had abnormal Aβ 42; CSF biomarkers: Reference: Aβ 42: < 209> 51 ng/ml - T-tau: > 100 ng/ml; Lowest quartile and quintile of Aβ 42 (≤ 104 and ≤ 106 ng/ml), highest quartile and quintile of P-tau (≥ 65 and ≥ 70 ng/ml) and T-tau (≥ 126 and ≥ 129 ng/ml) ml) |
| Barthélemy et al., 2020.[18] | Tau. | Total: 126 individuals Groups: discovery and validation Age: unknown; | Blood p-tau isoforms are potentially useful for detecting AD pathology, disease staging, and diagnosis. Aged Controls 2.21 No Alzheimer's: MCI 2.13 Preclinical AD: 2.81 AD-mild cognitive impairment: 3.28 Moderate Alzheimer's: 3.58 |
| Palmqvist et al., 2020.[19] | Tau. | Total: 1,402 individuals. Groups: Arizona Neuropathology Court, Swedish BioFINDER-2 Court, and Colombian Court, autosomal dominant AD parents Age: 34 - 84 years old; | Discriminative accuracy of plasma P-tau217 for AD (clinical or neuropathological diagnosis). WITHOUT ALZHEIMER 3.5pg/ml WITH ALZHEIMER 11 pg/ml higher concentration in patients with Alzheimer’s.
|
| Stocker et al., 2020.[23] | beta amyloid. | Total: 770 individuals Groups: AD, VD, MD, Control. Age: 52 - 75 years old Follow-up: 14 years; | Aβ misfolding was associated with a 23-fold increase in the odds of a clinical diagnosis of AD at 14 years, APOE4-positive participants had a 2.4-fold increased chance of a clinical diagnosis of AD at 14 years. Specific AUC and 95% confidence intervals (CIs) for predictors of AD were [AUC (95% CI)]: Aβ: 0-8 years, 0.82 (0.73-0.91), 8-14 years, 0.80 (0.73-0.88), 0–14 years, 0.81 (0.75 –0.87); APOE4 : 0–8 years, 0.59 (0.48–0.69), 8–14 years, 0.62 (0.53–0.71), 0–14 years, 0.61 (0.54 –0.67); Aβ + APOE4: 0–8 years, 0.85 (0.77–0.94), 8–14 years, 0.84 (0.77–0.92), 0–14 years, 0.85 (0 .79–0.90). High values within the comparison range. |
| Dominguez et al., 2022.[24] | beta amyloid. | Total: 147 individuals Groups: Alzheimer's disease (AD), Non-Alzheimer's disease (Non-AD), Alzheimer's disease of mixed etiology with vascular cognitive impairment, subcortical ischemic vascular dementia or cerebrovascular disease (mixed AD-VaD) and without cognitive impairment (NCI/SCI) Age: 38 - 94 years old | MDS-OA β can differentiate between AD versus non-AD dementias. The level of OA β can provide valuable information regarding the stage or progression of AD. Consistently, mean levels of oligomerized amyloid-β (OA β) were highest among patients diagnosed with AD, followed by mixed AD-VaD, and lowest in the NCI/SCI group |
Aβ: Amyloid beta, MDS: Multimeter detection system, NC: Cognitively normal control subjects, PHF1 CO-1P: widely used antibody that recognizes pTau species that are abundant in NFTs and dystrophic neurites in Alzheimer's disease, NFTs: tangle neurofibrillary CSF: cerebrospinal fluid, MDS-OA β: Oligomeric-β amyloid-multimer detection system.
Table 1: Selected scientific articles on proteins related to Alzheimer'
Among the factors involved in the development of Alzheimer's disease (AD), we can mention the deposit of beta amyloid protein (βA) and Tau in specific regions of the brain, forming plaques and tangles that prevent the neurological synapses that are so necessary for the normal functioning of the brain. According to studies, it appears that the beta amyloid protein is the main factor for AD to develop, but not the one, since this protein accumulates on the surface of neuropils (where glial cells, compacted dendrites and axon branches are found), expanding into the extracellular medium. The behavior of the Tau protein is similar, involving the cell body and the dendrites of neurons forming tangles, preventing them from connecting with the microtubules of the axon, thus causing permanent damage to the brain and even modification at the cellular level [26].
According to studies, beta amyloid and tau proteins were initially identified by Blasko et al. [20], who were the first to associate high levels of these proteins with the development of AD, mainly the β-42 protein. Similarly, Youn et al. [16] found that the oligomerization of βA proteins in the blood can cause a pattern in AD, with the concentrations of these proteins in the blood related to changes in brain volume.
Another study that also showed a direct relationship between βA proteins and AD was Stocker et al. [23], who observed that an incorrect folding of βA proteins was directly associated with a 23-fold increase in the chances of clinical diagnosis of AD, as well as increased levels of APOE4, in patients with lipid alterations, who showed a 2.4 times greater probability of clinical diagnosis of AD in 14 years. Apolipoprotein E (APOE) is the main producer of high-density lipoproteins (HDL) [27], and in addition to its relationship with lipid metabolism and associated pathologies, APOE is one of the main genetic risk factors for late-onset AD [28,29], as it is associated with the formation of amyloid plaques and neurofibrillary tangles, components of brain amyloid plaques. In the case of APOE4, considered a variant of Apolipoprotein E, it triggers in vivo and in vitro fibrillogenesis of the Beta-amyloid peptide, accelerating the development of AD. In contrast to this assertion, the study [30] examined the effects of APOE4 on human brain cell types, showing that APOE4 neurons exhibited an increased number of synapses and increased secretion of βA 42. In addition, when analyzing these brain cells by immunostaining, the authors demonstrated an increase in the number of βA protein aggregates and also in elevated levels of p-tau protein in organoids (a type of 3D cell culture) in cases of elevated APOE4 during six months, compared to their APOE3 equivalents, another variant, that was being analyzed in comparison in the study [30].
In the study by Drummond et al. [17] tau protein was the most abundant protein detected in samples of PHF1 co-IP, a widely used antibody that recognizes species of pTau protein, which are abundant in NFTs and dystrophic neurites in Alzheimer's disease. AD is part of the group of taupathies that also includes Corticobasal Degeneration (CBD), progressive supranuclear palsy (PSP) and the different subtypes of frontotemporal dementia (DFT), including Pick's disease, and in all of them there is a large amount of accumulated tau protein. Therefore, the intracellular accumulation of hyperphosphorylated Tau protein in neurons or glial cells is an important biological marker for the detection of tauopathies that include AD [31].
In view of this, numerous works show and confirm the direct relationship between the increase in amyloid beta and tau proteins with Alzheimer's, however, there are also other factors that can and are involved with them, which may potentiate the action of these proteins, and thus favor the AD development.
One of these factors is the glycemic decompensation caused in Type 2 Diabetes Mellitus due to insulin resistance, which favors the accumulation of beta amyloid proteins and Tau phosphorylation, which are characteristics of AD [4].
According to studies [32], insulin is responsible for driving the βA protein into the neurons, favoring its accumulation inside the neuronal cell, and consequently the development of AD. According to this study, this decrease in insulin signaling that occurs in DM2, which is associated with a genetic predisposition, leads to a lower activity of Phosphoinositide 3-kinase (PI3K), causing the synthesis of the insulin-degrading enzyme (EDI) is not promoted, and consequently the degradation of the βA protein does not occur, thus triggering an accumulation of amyloid, and favoring the development of AD [33].
According to studies, in acute cases of hyperinsulinemia, there is an increase in insulin signaling in the brain, thus increasing the deposition of βA proteins, however in chronic cases, there is a downregulation of the insulin transporter to the brain, triggering a decrease in the insulin CSF/insulin blood ratio [33], insulin is a hormone that acts in the brain and also in our peripheral tissues, transport to the brain is through a process regulated by receptors, carried out through the blood-brain barrier (BHE). However, it was shown that, because the cerebrospinal fluid (CSF) has an insulin level that is not proportional to the level found in the blood plasma, this means of transport can be saturated [33], so it is also explained by saturation of the insulin transporter across the Blood-Brain Barrier (BBB), so that low levels of insulin in the central nervous system (CNS) lead to neurodegeneration, cognitive deficits and decreased IDE levels, with a consequent decrease in the degradation of βA proteins [33].
In addition, studies have also shown that in DM2, hyperglycemia induces the activation of cells of the immune system, with the production of pro-inflammatory cytokines that stimulate the amyloidogenic pathways of APP, amyloid precursor protein, the βA peptide is produced from the cleavage abnormality of this precursor protein [36], thus increasing the formation and deposition of beta amyloid protein. As a result, there is accumulation of βA proteins and mitochondrial dysfunction, which may contribute to the activation of phospholipase A2 (PLA2), an enzyme involved in the inflammatory process, leading to anomalies of the white matter, characteristic of AD [33].
It is known that defective insulin production by pancreatic β cells, or decreased insulin sensitivity, affects the functioning of essential organs for metabolism, such as muscle and liver, which are the most affected by T2DM. This is because, within normal limits, insulin participates in the regulation of synaptic and neuronal function within the cerebellum, hippocampus and cortex, so that its action also leads to the protection of neurons from cell death [37].
In DM2, in addition to alterations in glucose metabolism, there are also high levels of LDL and low levels of HDL, which are associated with greater production and deposition of beta amyloid [33]. The central nervous system is the organ that presents the most lipids, and about 25% of the total cholesterol in the human body is found in the central nervous system, especially in the myelin sheath, in the membranes of astrocytes and neurons. Elevated cholesterol has been described as responsible for increased amyloid plaque formation in different in vivo models, while lowering cholesterol has also been shown to affect beta amyloid production. HDL prevents the aggregation and polymerization of beta amyloid protein, in addition to influencing the risk of dementia through its anti-inflammatory and antioxidant effect. Furthermore, in a brain imaging study, HDL decrease was associated with smaller hippocampal volume, even in non-demented individuals and regardless of APOE and coexisting cerebrovascular disease [38].
Regarding the Tau protein, in healthy individuals it appears little phosphorylated, because the low level of phosphorylation ends up attributing greater stability to neuronal microtubules, facilitating the transport of substances and chemical signaling between nerve cells. However, under pathological conditions, there is tau hyperphosphorylation, characteristic of AD [33]. Similarly, studies also show the association of DM2 with the phosphorylation of the tau protein, since the deficiency in insulin signaling leads to an increase in the phosphorylation of this protein, stimulating neurodegeneration [33].
This occurs, since in insulin resistance, due to the decrease in insulin signaling, there is a decrease in protein kinase B (p-AKT) signaling, resulting in non-phosphorylation of glycogen synthase kinase 3 (GSK3). Consequently, with the non-phosphorylation of GSK3, it remains active, promoting the phosphorylation of the tau protein, thus favoring its aggregation in nerve cells [33].
Another factor also associated with tau protein phosphorylation is the action of protein phosphatase 2 (PP2A). This is because a decrease in PP2A, associated with insulin resistance, triggers an increase in GSK3β, leading to an increase in tau protein hyperphosphorylation [33]. As well as the inhibition of the p62 protein, responsible for leading to the polyubiquitination of the tau protein, and the degradation in the proteasome and autophagosome. This is because, with the lack of p62 protein, the degradation of phosphorylated tau proteins is blocked, thus increasing tau protein hyperphosphorylation, also characteristic of AD [33].
Faced with this increase in phosphorylation of tau proteins, as a result of DM2, there is an increase in reactive species of Advanced Glycation End Products (AGE's) [33], known as advanced glycation, whose final result is heterogeneous molecules resulting from non-enzymatic products of glucose or other derivatives of saccharides with proteins or lipids [39], which occurs rapidly in the hyperglycemic state of diabetes, also increased oxidative stress, mitochondrial dysfunction and increased levels of intracellular Ca2+; and pro-inflammatory cytokines such as IL-1β, IL-6 [33].
Thus, it is possible to affirm that there is a strong connection between DM2 and AD, since DM2 causes neurodegeneration, inducing changes in vascular function and structure, in glucose metabolism, in insulin cell signaling, as well as changes in metabolism beta-amyloid protein and/or hyperphosphorylation of tau protein, promoting the development and worsening of AD [33].
However, further studies are needed, since the pathophysiological mechanisms related to the cause and the exact mechanism that triggers changes in Alzheimer's disease are still not well understood, although most studies suggest that the deposit of beta-amyloid peptide caused by an abnormal processing of the beta-amyloid precursor protein (hypothesis of amyloid cascade) may initiate and/or contribute to the pathogenesis of Alzheimer's, as well as, influence the systemic glucose metabolism, inducing behavioral changes, memory disorders, hypothalamic dysfunction, fragility, among others.
Taking into account the results presented, we can see that there is a link between DM2 and AD, due to selective deficiencies in insulin signaling, together with the positive regulation and accumulation of beta amyloid protein and hyperphosphorylation of Tau protein, it can be concluded that conclude that it cannot be said that DM2 has significant conditions to cause AD, but that it is a cofactor for the development of Alzheimer's disease and the evolution of more severe conditions.
Given the importance of the subject, in relation to two diseases that are so present in society today and their consequences, we emphasize why our work has a significant contribution, but we also ask that it is necessary to study with more collection the common mechanism between DM2 and AD and from this, it is possible to think about possible drugs that can be positive from DM2 to AD.
We declare that the article has not been submitted for publication in another journal; and that there is no conflict of interest regarding the publication of this work. We also declare that each author contributed significantly to the conception and design of the study and/or to the analysis and interpretation of its results; (b) substantial contribution to the production of the article, or critical review of its intellectual content, and (c) approval of the final version to be published.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
