AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2641-5194/066
1 Department of Gastroenterology and Endemic Medicine.
2 Department Of surgery, Faculty of Medicine- Minia University- Egypt.
*Corresponding Author: Hala I. Mohamed, Department of Gastroenterology and Endemic Medicine, Turkey.
Citation: Hala I Mohamed, Madiha Makhlouf, Ayman Hassanin, Elham A. Mohamed, Magdy F. Shalaby. (2023), Prevention of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis using Different Drugs. J. Gastroenterology Pancreatology and Hepatobilary Disorders, 7(4); DOI:10.31579/2641-5194/066
Copyright: 2023, Hala I. Mohamed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 02 June 2023 | Accepted: 13 June 2023 | Published: 26 June 2023
Keywords: pep; non-steroidal anti-inflammatory drug; pancreatitis; indomethacin; ercp
Background: New trials with use of different pharmacological agents have been conducted in the prevention of PEP. New trials with the combination of NSAIDs and other pharmacological agents have been conducted. Our aim to determine the efficacy and optimal regimen of different drugs for preventing PEP. Eight hundred and one Patients planned for ERCP. The patients were divided into 2 groups; Placebo Group and drug group which further subdivided into 4 subgroups: Allopurinol- treated patients: allopurinol (600mg) given orally one hour; Indomethacin-treated patients: single dose of indomethacin (100mg) rectally 10-15 minutes before ERCP; 39 Epinephrine- treated patients: 20ml of 0.02% epinephrine sprayed on the papilla during ERCP and Somatostatin treated patients: 250 mcg/hour for 6 hours before ERCP by continuous infusion
Results: Post ERCP pancreatitis in the patients who received Indomethacin rectal suppository was lower 12 (11%) than that in the placebo group (52%) and other drug groups, (P<0.005) multivariate logistic regression analysis showed that Knife precut and biliary sphincterotomy are independent risk factors for PEP and use of rectal Indomethacin before ERCP was significantly associated with low incidence of PEP.
Conclusions: The incidence of post-ERCP acute pancreatitis can be reduced by giving 100-mg Indomethacin suppository before the endoscopic procedure and reach significance in univariate or multivariate analysis as a protective agent against PEP.
Acute pancreatitis is the most common event associated with endoscopic retrograde cholangiopancreatography (ERCP). Several efforts have been made to minimize the frequency and severity of this complication [1].
More than 35 pharmacologic agents have been evaluated for the prevention of post ERCP pancreatitis (PEP), with different mechanisms of action. However, no single pharmacologic agent has shown no consistent benefit or efficacy for PEP prevention.
Recently, rectally administered non-steroidal anti-inflammatory drugs (NSAIDs; indomethacin and di¬clofenac) were determined to be poten¬tially effective in the prevention of PEP in both low- and high-risk patients [2].
This study aimed to determine whether prophylactic use of drugs can reduce the incidence and severity of post ERCP pancreatitis and evaluate the efficacy of different drugs in reducing the incidence of post ERCP pancreatitis.
This double-blinded, randomized, trial performed in Minia University Hospital in Egypt. The patients were selected from Tropical Medicine Department and General Surgery Department of Minia University hospital during the period from July 2019 to November 2020.The study protocol was approved by the institutional review board of the ethics committee of Minia University School of Medicine, before initiation of the study. All patients provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Patients (age, 18–70 y) planned for diagnostic or therapeutic ERCP were eligible for enrollment in the study. The patients were divided into 2 groups ; Placebo Group ( group I) : included 375 patients were not given any drugs before or after ERCP and drug group (group II) , 426 patients which further subdivided into 4 subgroups: group IIa, included 153 Allopurinol- treated patients: allopurinol (600mg) given orally one hour before ERCP; group IIb, included 112 Indomethacin-treated patients: single dose of indomethacin (100mg) rectally 10-15 minutes before ERCP; group IIc, included 82 Epinephrine- treated patients: 20ml of 0.02% epinephrine sprayed on the papilla during ERCP and group IId, include 79 Somatostatin treated patients: 250 mcg/hour for 6 hours before ERCP by continuous infusion.
Exclusion criteria included contraindications to ERCP; as gastrointestinal hemorrhage
within the past 2 weeks, creatinine level >1.4 mg/dL) or INR (international normalized ratio) more than 1.5; acute pancreatitis within; pregnant and inability to provide consent.
At the end of the procedure, the endoscopists recorded the presence of periampullary diverticula, total procedure time (defined the time immediately before insertion of the endoscope to the last radiograph taken immediacy after withdrawal of the endoscope), cannulaion time (defined as the time from the radiograph taken immediately before the initiation of cannulation to the radiograph taken immediately after successful cannulation), and interventions such as endoscopic sphincterotomy ; stone extraction; endoscopic papillary balloon dilation (EPBD), or stenting, if performed. Difficult cannulation was defined as more than eight attempts [3].
Serum amylase levels were measured at baseline, at 6hours; at 24 hours and 48 hours after the procedure.
Triphasic computed tomography (CT) abdomen was done to all patients before ERCP to confirm data of abdominal ultrasonography and inform about size, echopattern, focal lesions of pancreas, duodenal thickening, collections, abdominal lymph nodes or other abdominal masses or malignant liver nodules
Another CT abdomen was done after ERCP to selected cases who develop upper quadrant pain with hyperamylasemia to report degree of pancreatitis according to Balthazor Score (A: normal pancreas: 0, B: enlargement of pancreas: 1, C: inflammatory changes in pancreas and peripancreatic fat: 2, D: ill-defined single peripancreatic fluid collection: 3, E: two or more poorly defined peripancreatic fluid collections [4].
Definitions and main outcome measures
The primary outcome of the study was the incidence of PEP, defined as follows: serum amylase level at least three times the upper limit of the normal range plus newly developed or worsened pancreatic-type abdominal pain and tenderness with nausea and/or vomiting for more than 24 hours after ERCP. Once PEP occurred, patients received conservative treatment for acute pancreatitis. Specifically, PEP was graded as follows: 1) mild, symptoms lasting 3 days or less and a mildly edematous appearance of the pancreas on ultrasonography and/or computed tomography (CT); 2) moderate, requiring specific therapeutic measures for 4–10 days after the procedure (Balthazar’s grade B/C on CT); and 3) severe, local or systemic complications lasting longer than 10 days after the procedure (Balthazar’s grade D/E), or death. CT findings that included the presence of either tissue necrosis involving more than 30% of the pancreatic gland or peripancreatic fluid collection were also used to classify pancreatitis as severe.
Eight hundred and one patients with Ultra-sonographic and CT evidence of extrahepatic cholestasis were recruited in this study. All the patients were subjected to diagnostic and therapeutic ERCP at ERCP unit in Minia University Hospital
The patients were divided into 2 groups: Group I (placebo group) Included 375 patients and Group II (drugs group) included 426 patients which further subdivided in 4 subgroups. Group IIa, Allopurinol- treated patients (153), Group IIb, Indomethacin- treated patients (112). Group IIc Epinephrine- treated patients (82) and Group IId Somatostatin treated patients (79). These drugs were given before the beginning of ERCP except the Epinephrine- treated patients which epinephrine sprayed on the papilla during ERCP.
In placebo group, the mean age was 46.3±10.5, 36% were females, the drug groups, the mean age was 49.9±12.9, female Sex prevalence was 35%, The range of hospital stay in placebo group was (2-15 days) and in drug groups was (2-7 days). Other laboratory investigations were demonstrated in Table 1.
The most common indication for ERCP was bile duct stones in both placebo and drug groups [76 (50%) & 59 (30%), respectively] followed by malignant obstructive jaundice [51(34%) and 87(45%), respectively]. Stricture and primary scelerosing cholangitis (PSC) were 15 (10%) 7 (5%) and respectively, in placebo group 27 (14%) and 12 (6%) respectively, in drug groups Table 1.
All patients were received antibiotics pre ERCP. The mean procedure time was
20.6± 9.1 in placebo group and 23.5± 7.1 in drug groups. Stone extraction was done to 243 (65%) patients in placebo group and 193 (45%) patients in drug groups. Mean Bile duct cannulation time and total cannulation attempts and in both placebo group and drug groups were 5.9 ± 6.3, 2.9 ± 2.3 and 6.6 ± 4.9; 3.6 ± 1.9, respectively Table 1.
Difficult cannulation recorded in 60 (16%) of patients in placebo group and 105 (25%) of those in the drug groups. Endoscopic insertion of a biliary stent was performed more frequently in drug group 316 (74%) than in the placebo group 242 (64%)). Knife precut and biliary sphinctrotomy were performed in placebo group for 108 (29%) and 122 (33%) patients, respectively and in drug groups 186 (50%) and 185 (43%) patients, respectively. Balloon dilation was conducted in 55 (17%) patients in the placebo group and in 58 (14%) patients of those in the drug groups Table 1.
| Parameters | Group I (Placebo group) (n=375) | Group II (Drug groups) (n=426) |
Age Range Mean±SD |
29-67 46.3±10.5 |
25-70 49.9±12.9 |
Sex (no, %) Male Female |
219 (58%) 156(42%) |
127(65%) 67 (35%) |
| Hospital stay (days) (Range) |
(2-15) |
(2-7) |
¥ Serum amylase level (Mean ± SD) |
72.3±24.7 |
79.6±22.8 |
| Indication of ERCP | ||
| Malignant obstructive jaundice (n, %) | 71 (19%) | 109 (26%) |
| Suspected/known bile duct stone (n, %) | 249 (66%) | 269 (63%) |
| Stricture (n, %) | 25 (7%) | 27 (6%) |
| Suspected PSC (n, %) | 15 (4%) | 12 (3%) |
| Others (n, % | 15 (4%) | 9 (2%) |
| Procedure details | ||
| Procedure time, mean ±SD, minutes | 20.6± 9.1 | 23.5± 7.1 |
| Pre ERCP antibiotics, n (%) | 375 (100%) | 426 (100%) |
| Gallstone Extraction, n (%) | 243 (65%) | 193 (45%) |
Cannulation Bile duct
cannulation time (mean ± SD, minutes)
Total cannulation attempts (mean ± SD)
* Difficult cannulation, n (%) |
5.9 ± 6.3
2.9 ± 2.3
60 (16%) |
6.6 ± 4.9
3.6 ± 1.9
105 (25%) |
| Biliary stent insertion, n (%) | 242 (64%) | 316 (74%) |
| Knife precut, n (%) | 108 (29%) | 186 (50%) |
| sphinctrotomy, n (%) | 122 (33%) | 185 (44%) |
| Balloon dilation of biliary sphincter, n (%) |
55 (15%) |
58 (14%) |
| Failed procedure, n (%) |
51(14%) |
46 (11%) |
*Difficult cannulation was defined as >8 attempts. ¥Serum amylase level was measured before ERCP.
Categorical variables are presented as n (%), Continuous variables are presented as mean±SD
-PSC: Primary scelerosing cholangitis
Table1: Base line Characteristics of all studied patients.
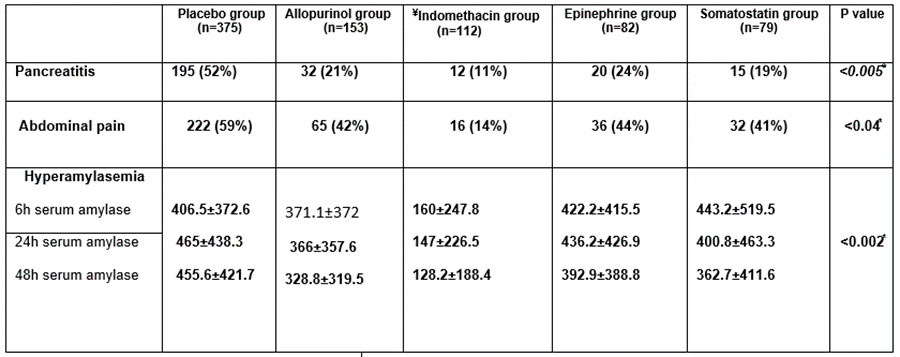
Post ERCP pancreatitis in the patients who received Indomethacin rectal suppository was lower 12 (11%) than that in the placebo group (52%) and other drug groups, (P<0>
Six hours after endoscopy, the mean serum amylase level was 406.5±372.6 IU/L in the Placebo group 371.1±372 IU/L in the Allopurinol group, 160±247.8 IU/L In the diclofenac group, 422.2±415.5 Epinephrine group and 443.2±519.5 in Somatostatin group. Twenty-four hours after endoscopy, different mean serum amylase levels in Placebo, Allopurinol, Diclofenac Epinephrine, Somatostatin were (465±438.3, 366±357.6, 147±226.5, 436.2±426.9400.8±463.3, respectively).
Forty-eight hours after endoscopy, mean serum amylase levels in Placebo, Allopurinol, Diclofenac Epinephrine, Somatostatin were (455.6±421.7, 328.8±319.5, 128.2±188.4, 392.9±388.8, 362.7±411.6, respectively). The mean values of amylase at different times (6 hours, 24 hour and 48 hour) were significantly low in Indomethacin group versus the drugs group (P > 0.002) Table 2.
Data are expressed in No (%) and mean±SD
Kruskal Wallis test for non-parametric quantitative data between the five group
¥Significant value of pancreatitis, abdominal pain and serum amylase levels in Indomethacin group versus another drug group and placebo group
*Significant level taken at P value < 0>
Table 2: Post ERCP pancreatitis, abdominal pain and hyperamylesemia in both placebo and drug groups
In placebo group the degree of post ERCP pancreatitis was mild in (51%) patients: moderate in (31%) patients and sever in (18%) patients, in the patients who received Allopurinol tablets degree of pancreatitis was mild in (63%) patients, moderate in (22%) patients, and sever (16%), in Indomethacin group was mild in (67%) patients and moderate in (25%) patients with no sever PEP recorded in this group. Patients who received Epinephrine drug PEP was mild in (27%) patients and moderate in (40%) patients and sever in (33%) patients. In somatostatin group 30% of patients had mild pancreatitis, 40% had moderate pancreatitis and 30% had sever pancreatitis Figure 1.
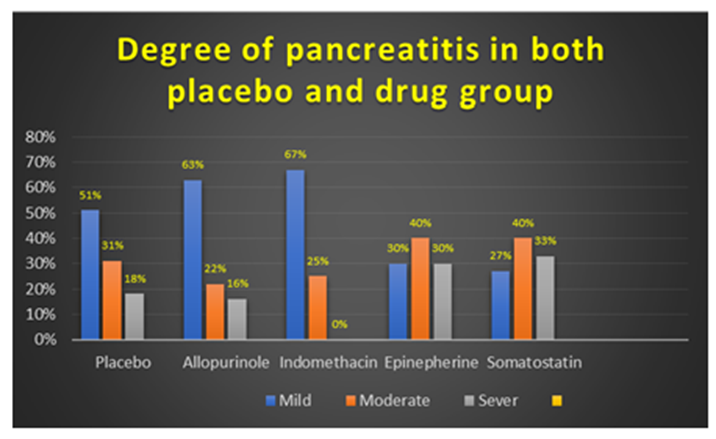
Figure 1: Degree of post ERCR pancreatitis in both placebo and all drug groups
On univariate analysis, significant patient-related factors included female sex (for female sex compared with male sex: OR 1.934, 95% CI 0.712-2.945; P<0>
The influence of pharmacological prophylaxis on PEP was estimated. Use of rectal Indomethacin before ERCP was significantly associated with low incidence of PEP (OR 0.082, 95% CI 0.016-0.406; P<0>
Of the previously mentioned risk factors, multivariate logistic regression analysis, Knife pre-cut and Biliary sphinctrotomy (OR 24.3, 95% CI 7.7-76.6; P<0>
In addition, use of rectal Indomethacin before ERCP was significantly independently effective for preventing PEP by both univariate (OR 0.082, 95% CI 0.016-0.406, P<0>
| Factors | Univariate analysis OR 95% CI | P value | Multivariate OR 95%CI | P value | |||
Age
>60 year (n=66) <60 year(n=84)> |
0.532 |
0.014-0.203 | 0.2 |
----
| |||
Sex Female (n=86)
Male (n=64) |
1.934
1 |
0.712-2.945 | 0.03* |
1.62 1.71- 0.54 | <0> | ||
Biliary sphinctrotomy Yes (n=77)
No (n=73) |
2.347
1 |
0.953- 3.675 | 0.04*
|
2.047 0.953- 3.105
1 |
<0> | ||
Knife precut Yes (n= 42)
No (n= 108) |
10.5
1 |
4.6-23.7 | 0.001* |
24.3 7.7-76.6
1 | <0> | ||
| Drugs | |||||||
| Allopurinol Yes (30)
No (120) |
0.762
1 |
0.274-2.121 | 0.6 |
-- -- | ||
Indomethacin
Yes (30)
NO (120) |
0.082 |
0.016-0.406 |
0.002*
|
0.0241 0.003-0.165 |
<0> | ||
Epinephrine
Yes (30)
N0 (120) |
0.874
1 |
0.316-2.418
| 0.7 |
--- ----
| |||
Somatostatin
Yes (30)
No (120) |
0.662 1 |
0.236-1.858
| 0.4 |
--- --- | |||
ERCP pancreatitis, OR: Odds Ratio, CI: Confidence Interval
*: Significant level taken at P value < 0>
Table 3: Univariate and Multivariate regression analysis of factors associated with post ERCP pancreatitis
Endoscopists have long grappled with PEP, which is the most frequent and threatening complication of ERCP. Endoscopists have evaluated many mechanical procedures and pharmacological prophylactic solutions for the prevention of PEP [5].
The mechanisms of ERCP-induced pancreatic injury are not clearly understood, and several proposed factors may act independently or in combination to induce PEP. Irrespective of the mechanism of injury, the host inflammatory response to endoscopic instrumentation appears to play an important role in the pathophysiology of PEP [6].
A delay of several hours (median 4.5 hours) exists between pancreatic injury during ERCP and the onset of symptoms. This “therapeutic window” invites the use of anti-inflammatory strategies to modulate the premature intracellular. Activation of proteolytic enzymes and acinar cell damage, and subsequent local inflammatory response that in turn leads to the release of chemokines and pro-inflammatory cytokines into the general circulation [7].
The ideal pharmacological prevention of post-ERCP pancreatitis should meet the following three criteria: (1) effective in patients who really risk developing post-ERCP pancreatitis; [2] not require prolonged administration in the post procedure period; and (3) be as economical as possible to make it cost effective [8].
The current study showed that the incidence of PEP was 34.2% in the overall study sample 52% in the placebo group, 21% in the Allopurinol group, 11% in the Indomethacin group, 42% in the Epinephrine group, and 19% in the Somatostatin group.
And according to the subgroup analysis, there is significantly reduction in Post ERCP pancreatitis in the patients who received Indomethacin rectal suppository than that in the placebo group and other drug groups, (P<0>
In the current trial, we demonstrated that rectal indomethacin also reduced the incidence of post-ERCP hyperamylasemia and frequency of abdominal pain and this was significantly different from the placebo group and other drugs subgroups (P < 0 P=0.002),>
In terms of effective agents for preventing PEP, NSAIDs potently inhibit phospholipase A2, which is implicated as an important player in the initial inflammatory cascade of acute pancreatitis [9].
Diclofenac is an NSAID marketed worldwide in oral, suppository, transdermal patch, gel, and intramuscular formulations. The parenteral route is often preferred due to its more rapid onset of action compared with other routes.
Several previous studies assessing rectally administered diclofenac to prevent PEP had
positive results or demonstrated a trend toward positivity [10.11.12].
one published study assessing intramuscularly administered diclofenac were negative, In contrast to our current study. However, it remains uncertain whether the route of diclofenac administration affects the clinical efficacy [13].
Khoshbaten et al., have reported a randomized controlled study that compared 100 mg rectal diclofenac with placebo in 100 patients who underwent ERCP. The incidence of pancreatitis in the placebo group was 26%, whereas the incidence of pancreatitis in the diclofenac group was 4%. This difference was statistically significant [14].
The peak plasma concentration of diclofenac or indomethacin is reached 30 min after their rectal administration. Theoretically, therefore, rectal administration appears more reasonable before the ERCP investigation than after it [15].
Diclofenac, an NSAID, inhibits phospholipase A2, which is thought to play a critical role in the early inflammatory cascade. In addition, it strongly inhibits neutrophil/endothelial attachment, thus preventing accumulation of neutrophils at the site of tissue damage, and inhibits the expression of nitric oxide synthase, an enzyme associated with inflammation and cell damage. It is a cheap, widely available agent with a short, easy method of administration [16].
The specific mechanism by which rectal indomethacin demonstrates preventive effect on PEP is that peak plasma concentration is achieved in 90 minutes after rectal indomethacin, but this peak plasma concentration is sustained for more than 2 hours and decreases slowly, compared to intramuscular administration [17].
Regarding to the dose and timing of Indomethacin administration in our study (100 mg 15 minutes before ERCP) and if that dose is sufficient to prevent PEP. The majority of published clinical trials to date have been conducted with a single 100 mg dose of rectal indomethacin or diclofenac [18].
Recently, randomized clinical trial with dose escalation of rectal indomethacin to 200 mg was reported ,43]. It was hypothesized that a higher dose might be superior to the existing standard 100 mg dose in PEP prevention. Split dose was performed to potentially lead to a higher peak serum concentration and a more sustained impact on the inflammatory Cascade [19].
Risk factors for PEP were evaluated in the current study, we found that female sex, biliary sphinctrotomy and Knife precut were significant independent risk factor for PEP. Also use of rectal Indomethacin have a definite beneficial and significant role in preventing PEP. Multivariate analysis model further showed that these two factors [female sex, Knife precut rectal) were significantly associated with PEP. diclofenac administration was the independently effective for preventing PEP.
In a systematic review included 13 clinical trials which provided data about risk factors for PEP, the results suggest that female gender, previous PEP, previous pancreatitis, precut sphincterotomy, Sphincter of Oddi dysfunction and so on were all risk factors for PEP [20]. The increased incidence of PEP in women would probably be because Sphincter of Oddi dysfunction affects women more frequently than men [21].
Endoscopic sphincterotomy is a common and essential procedure in therapeutic
ERCP. Akashi et al. [24] reported that the edema in surrounding tissues was induced because of the sensitivity of the pancreatic duct to thermal damage caused [22].
The effect of endoscopic sphincterotomy and subsequently the pancreatic duct was temporarily blocked, all of which caused the occurrence of PEP. However, in many studies endoscopic sphincterotomy was not considered to be a risk factor for PEP [23].
Theoretically, endoscopic sphincterotomy can reduce the tension at the orifice of the pancreatic duct. The incidence of post-EST pancreatitis is largely dependent upon the skill of the endoscopist, in addition to factors related to the host.
Knife precut may cause edema of the duodenal papilla, a poor discharge of pancreatic juice,and induce post-ERCP acute pancreatitis [24].
The incidence of post-ERCP acute pancreatitis can be reduced by giving 100-mg Indomethacin suppository before the endoscopic procedure and reach significance in univariate or multivariate analysis as a protective agent against PEP.
ERCP: endoscopic retrograde cholangipancreatitis, -PEP: post ERCP pancreatitis, NSAID: non-steroidal anti-inflammatory, INR: international normalized ratio, -EPBD: endoscopic papillary ballon dilatation, -CT: computerized tomography.
Ethics approval and consent to participate
The study was approved by ethics committee of Minia University Hospital, Minia, EGYPT carried out according to the Declaration of Helsinki and the guidelines of the International Conference on Harmonization for Good Clinical Practice. Ethics committee’s reference No. not applicable.
Consent for publication
Written informed consent was obtained from every participant for both participation and publication in the study. The institutional review boards approved this study.
Availability of data and material
The data of the patients participated in the current study are available with the corresponding author on reasonable request (As sharing the patient data is not applicable due to Egyptian customs and traditions).
Competing of Interests
The authors declare that they have no competing interests
Funding
All author declares that there was no fund provided to this research regarding to study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript
All authors read and approved the final version of the manuscript