AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2692-9406/145
1 Pediatric Nephrology Unit – Centre of Nephrology - Santa Casa de Belo Horizonte Hospital, Minas Gerais, Brazil.
2 Pediatric Nephrology Unit, Department of Pediatrics, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
3 Department of Internal Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
*Corresponding Author: Maria Goretti Moreira Guimarães Penido, Centro de Nefrologia da Santa Casa de Belo Horizonte R. Piauí, 420 - Santa Efigênia, Belo Horizonte – MG.
Citation: Mariana G. P. de Paula, Maria G. M. G. Penido, Milena M. M. Guimarães, Dirceu B. Greco, et al., (2023), Osteometabolic Disorders of HIV Infection: A Review. Biomedical Research and Clinical Reviews. 8(1); DOI:10.31579/2692-9406/145
Copyright: © 2023, Maria Goretti Moreira Guimarães Penido, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 20 January 2023 | Accepted: 31 January 2023 | Published: 10 February 2023
Keywords: HIV; antiretroviral therapy; bone mineral density; osteoporosis; osteopenia; vitamin D
The antiretroviral therapy (ART) has improved the prognosis of HIV patients, but has also revealed complications related to this therapy. Some bone-related abnormalities such as low bone mineral density (BMD), osteoporosis, osteopenia, osteomalacia, fractures and other bone disorders have appeared in HIV-infected individuals. The risk of osteopenia/osteoporosis in HIV patients is not only due to ART. Both ART and chronic HIV infection have been implicated in reducing BMD. Therefore, the pathogenesis of reduced bone mass is multifactorial: including the contribution of the virus, immunosuppression, ART, drug toxicity, in addition to traditional risk factors for the development of osteoporosis (female gender, yellow and white ethnicity, advanced age, early menopause, heredity, family history of osteoporosis or osteoporotic fracture, low calcium intake, poor food absorption, sedentary lifestyle, use of medications (glucocorticoids and anticonvulsants), hypovitaminosis D, and systemic inflammatory diseases. HIV infected patients are more likely to have fragility fractures as a consequence of reduced BMD. A moderate increase in prevalence of fractures and increased propensity to fall in these patients have been reported.Considering the involvement of HIV/AIDS infection on bone mineral metabolism, knowing the profile of markers of its metabolism and body composition in these patients before starting ART would be of great value as well as supplementation of vitamin D and calcium with the initiation of ART.This review will address bone metabolism disorders associated with HIV infection and use of ART in HIV-infected patients.
Acquired Immunodeficiency Syndrome (AIDS) was first described in the United States of America in 1981 in homosexual individuals with recurrent pulmonary infections by Pneumocystis carini [1]. Since then, and in a relatively short period, much has been done by the community to expand knowledge regarding this infection and with the intention of preventing its spread.
Despite enormous efforts, the human immunodeficiency virus (HIV) epidemic has spread at alarming rates. During the first years of the epidemic most patients progressed inexorably towards a state of almost complete destruction of immune functions. In 1987, less than 4 years after the isolation of HIV-1, zidovudine was approved by the Food and Drug Administration (FDA). However, this drug, as well as others that appeared soon after, did not achieve satisfactory control of viral replication. The patients had high morbidity and mortality and the prognosis of those with HIV infection was dismal. In developed countries, the trend towards a decrease in AIDS-related morbidity and mortality had been observed even before the emergence of highly potent antiretroviral therapy (ART), as a result of prophylaxis and better management of opportunistic infections. With the advent of protease inhibitors (PI) and combination antiretroviral therapy, great advances have been made in reducing mortality [2].However, the epidemiological profile of HIV infection has undergone major changes in recent years. People living with HIV/AIDS are aging, thus becoming more exposed to the complications of its chronicity and the prolonged use of antiretrovirals. In addition, infected patients are mostly young and have a life expectancy similar to that of uninfected patients [2].
The introduction of ART has improved the prognosis of HIV patients, but has also revealed complications related to the therapy. Previous studies have shown metabolic changes that contribute to increased cardiovascular risk, such as dyslipidemia, insulin resistance and lipodystrophy [3]. After that, there were reports of changes in mineral and bone metabolism [2]. The evidence of a high prevalence of reduced Bone Mineral Density (BMD), osteopenia and osteoporosis in the HIV population when compared to the control population has been studied [4,5].
Vitamin D deficiency, a substance with immunomodulatory properties, has reached epidemic proportions, involving even healthy individuals in tropical regions. Possibly there is an association of its deficiency with metabolic syndrome, diabetes, arterial hypertension, autoimmune diseases and neoplasms [2]. Studies suggest that in HIV-positive patients, factors linked to the virus itself and the use of ART may be added to the other causes of hypovitaminosis [2].
In this sense, the reports of alterations in the mineral and bone metabolism of HIV-infected patients and the possibility of abnormalities in the serum levels of vitamin D and calcium, deserve to be highlighted [2,6,7]. It is important to investigate the profile of markers of mineral and bone metabolism and BMD in these patients before starting ART, as well as describing them and evaluating the possibility of some clinical or laboratory parameter working as an early marker. With this, therapeutic strategies can be evaluated, with gains for the health of them.
Considering the potential risks of these alterations, it is important to know them well in order to better approach these patients' mineral and bone metabolism disorders and, consequently, their quality of life.
general population [8]. Osteopenia and osteoporosis are more common with an estimated prevalence three times higher than in individuals without HIV [9]. Both ART and chronic HIV infection have been implicated in reducing BMD.10 Pinto Neto et al., demonstrated that BMD was reduced in individuals who had classic risk factors, such as low body mass index (BMI) and postmenopausal women. The authors also observed that male individuals had lower BMD than females [11].
Clarifying the causes of bone changes in patients with HIV/AIDS is a challenge, due to their multifactorial nature, including the contribution of the virus, immunosuppression, ART, drug toxicity, in addition to traditional risk factors for the development of osteoporosis (female gender, yellow and white ethnicity, advanced age, early menopause, heredity, family history of osteoporosis or osteoporotic fracture, low calcium intake, poor food absorption, sedentary lifestyle, use of medications (glucocorticoids and anticonvulsants), hypovitaminosis D, and systemic inflammatory diseases [12,13,14].
Patients with HIV infection are more likely to have fragility fractures as a consequence of reduced BMD [15-19]. However, data on the prevalence of fragility fractures in the HIV population are poor and often based on retrospective studies. A moderate increase in prevalence of fractures in those patients compared to the general population have been reported by some studies [17-21]. Other studies, however, show a significant increased prevalence of fractures and increased propensity to fall [21]. Recently, Dalla Grana et al., concluded HIV patients suffer from bone fragility, particularly at spine, independently by the level of BMD [5].
Vitamin D is essential for maintaining serum calcium levels. It has been suggested that vitamin D plays a key role in several extraskeletal processes, such as cardiovascular disease, diabetes, muscle weakness and falls, autoimmune disorders and neoplasms [22]. The deficiency of this vitamin has reached epidemic proportions and, according to the position of national and international medical societies, the recommendation is to maintain 25(OH)vitamin D (25OHvitD) levels greater than 30 ng/ml in specific conditions, including patients over 65 years of age or pregnant women, those with recurrent falls, a history of fragility fractures, osteoporosis, secondary hyperparathyroidism, chronic kidney disease or cancer, and individuals who use drugs with the potential to affect vitamin D metabolism [2,22-24].
Observational studies have determined high rates of vitamin D deficiency in HIV-positive patients, a finding that suggests that it is a combination of traditional risk factors, factors directly related to the virus, and factors related to ART [25,26]. As described by Lake & Adams and Escota et al., this deficiency in HIV-positive patients is defined by serum levels of 25OHvitD below 20 ng/ml and its insufficiency by levels between 21 and 29 ng/ml [11,22].
Vitamin D metabolism
Vitamin D3 (cholecalciferol) is photosynthesized in the skin upon exposure to ultraviolet B radiation. Vitamin D2 (ergocalciferol) can be ingested through food (fish oil, cod liver oil, shitake mushrooms, egg yolks) and supplements. Both forms of vitamin D are converted in the liver and other tissues to 25OHvitD. It is then transformed into its active metabolite, 1,25(OH)2vitaminD by the enzyme 25-hydroxyvitamin D 1-α-hydroxylase, mainly in the kidney. Its catabolism is carried out by 25-hydroxyvitamin D 24-hydroxylase [11,22]. The main function of the active form of vitamin D is to maintain normal serum calcium levels through increased renal calcium absorption, suppression of PTH secretion, and increased intestinal absorption of calcium and phosphorus [11,22].
Epidemiology of vitamin D deficiency/insufficiency
It is estimated that one billion people have vitamin D deficiency or insufficiency. In the USA, data from the National Health and Nutrition Examination Survey (NHANES) 2003 and 2006 estimate a prevalence of vitamin D deficiency of 38%, whereas these figures rise to 79% when assessing the adult population with deficiency and insufficiency together [26,27].
Vitamin D deficiency is also common among HIV positive patients. Among 253 ART-naïve HIV-positive patients in London, Gedela et al., reported 58% vitamin D deficiency, with 13% of them having severe deficiency (25OHvitD<10ng>
There is no consensus on what is the ideal serum level for vitamin D. There are many suggestions for values that define the lower limit of normality, ranging from 20 to 37 ng/ml. However, what is known so far is that this lower limit should be the one that maintains normal serum calcium levels and, consequently, does not induce PTH release [2,31].
Factors associated with vitamin D deficiency/insufficiency
Hypovitaminosis D in the HIV-positive population has its genesis based on traditional risk factors, which affect the general population, and specific risk factors, related to the infection and its treatment. Among the traditional risk factors there are dark skin pigmentation, obesity, winter, low sun exposure, low dietary intake of vitamin D, injecting drug use, sedentary lifestyle, smoking, kidney disease, liver disease and malabsorption syndrome [22]. HIV-positive patients have specific risk factors for reduced serum levels of 25OHvitD due to the complex interaction of the host, chronic infection and inflammation, and the consequences of metabolic disorders resulting from ART [11].
Studies suggest that factors linked to the virus itself and the use of antiretrovirals may be added to other causes of hypovitaminosis D. The virus reduces vitamin D levels through the action of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), inhibiting renal hydroxylation. There is consumption of 25OHvitD by macrophages and lymphocytes as soon as the disease progresses and the type of antiretroviral used influences vitamin D levels. PIs block 25(OH) hydroxylation vitamin D and the bioactivation of 1,25(OH)2vitamin D in the kidneys; non-nucleotide reverse transcriptase inhibitors (NNRTIs) increase catabolism of 25(HO) and 1,25(HO)2vitamin D by inducing 25-hydroxyvitamin D 24-hydroxylase [11,22]. However, HIV/AIDS infection and the action of ART on vitamin D metabolism require further studies. The available data come from reduced samples and short follow-up time, requiring further research to define the benefits of vitamin D on the cardiovascular, immune and endocrine-metabolic systems of patients infected with HIV.
Consequences of vitamin D deficiency/insufficiency
The main consequences of vitamin D deficiency/insufficiency are associated with reduced BMD, which are fragility fractures, muscle weakness and risk of falling. Observational studies in the general population showed that vitamin D deficiency was associated with risk of fragility fracture, as well as risk of falling and muscle weakness, which could be explained by the effect of vitamin D on skeletal muscle [22,32].
BMD reduction is prevalent in the HIV-positive population when compared to the general population and more common among those individuals who have hypovitaminosis D [33]. Prolonged vitamin D deficiency would cause hypocalcemia, and consequently secondary hyperparathyroidism and bone demineralization. However, most studies in the HIV-infected population did not establish an association between vitamin D and BMD [22,23].
Screening and treatment recommendations
The European AIDS Clinical Society recently published guidelines recommending screening for vitamin D status in HIV-positive patients and treatment. This assessment should be performed in case of a history of fractures or reduced BMD, in those at high risk of fractures, and in those with factors associated with vitamin D deficiency. For individuals with serum 25OHvitD levels <10>
McComsey and colleagues have developed recommendations for HIV bone disease, including vitamin D deficiency. The authors recommended 50,000 IU of vitamin D weekly for 8 to 12 weeks, then monthly. Another recommendation could be 2,000 IU daily for 12 weeks, then 1,000 to 2,000 IU daily for those diagnosed with vitamin D deficiency. The target would be serum 25OHvitD levels greater than 32 ng/ml, which should be evaluated after replacement.35 However, there are many controversies in the literature regarding replacement and the best regimen for it.
As aforementioned, BMD reduction is prevalent among individuals with HIV. These patients present osteopenia or osteoporosis in a proportion that varies from 28 to 50%, against 16% expected for the general population. It has also been shown that HIV-positive patients have significant changes in biochemical markers of bone metabolic activity, such as pyridinolines, alkaline phosphatase, N-telopeptide, hydroxyproline, osteocalcin, among others [5].
The etiopathogenesis of reduced BMD in HIV-positive patients is multifactorial. Traditional risk factors such as hypogonadism, smoking, alcoholism, decreased organic capacity, low body mass index (BMI), and vitamin D deficiency contribute to the increased risk. Among the non-traditional factors, we can highlight the direct effects of ART and the chronic activation of the immune system determined by the infection.4 Furthermore, it is known that HIV-infected patients have osteopenia or osteoporosis in a proportion that varies from 28 to 50%, against a percentage of 16% expected for the general population [4,5].
Randomized controlled trials comparing BMD during PI and non-PI regimens have shown mixed results. Some studies have shown that PI-containing regimens lead to a decrease in spine BMD. Studies have shown no difference in total body or hip BMD between treatment in the two groups [10,36]. Despite mixed effects on BMD, an association was found between cumulative PI exposure and an increased risk of fracture (RR: 1.11; 95% CI: 1.05-1.18; p=0.001). Treatment with lopinavir/ritonavir led to a 17% increase in the risk of incident hip, vertebral, or wrist fracture [36].
Randomized clinical trials comparing BMD in patients using tenofovir vs patients not using tenofovir found that tenofovir use is associated with a significant decrease in BMD in the hip and spine [38,39]. Study to define the cumulative impact of tenofovir therapy vs other antiretroviral therapy (ART) and its relationship to the risk of osteoporotic fractures in HIV-infected patients during pre-highly active ART ) periods (1988-1995) and ART (1996–2009) showed that cumulative exposure to tenofovir was associated with an increased risk of incident fracture (RR: 1.16; 95% CI, 1.08-1.24; p=0.0001) [37].The mechanism to explain the effect of tenofovir on bone is not yet clear. Experimental models have shown that tenofovir impairs bone mineralization through its effects on renal function and phosphate handling, which results in increased bone remodeling and osteomalacia [40,41]. These effects may be exacerbated by concomitant vitamin D deficiency [42].
The impact of starting ART on BMD was evaluated and the authors showed a loss of 2% to 6% of bone mass after 48 to 96 weeks of therapy, regardless of the type of ART started.10 This degree of bone loss is greater than would be expected with aging alone and was comparable to bone loss seen in women aged 50-59 years [43,44]. Importantly, a low CD4 cell count prior to initiation of ART is associated with greater decreases in BMD.45 Probably, the loss of BMD with the initiation of ART can be explained by a rapid increase in bone remodeling.
Studies have shown a significant increase in osteocalcin (bone formation marker) and c-telopeptide (bone resorption marker) after starting ART [39]. Markers of bone resorption are already elevated with untreated HIV infection, and they increase earlier and to a greater extent than markers of bone formation, creating a "catabolic window" during the first six months after initiation of ART [46]. Although treatment is associated with significant bone loss, longitudinal studies have shown that with continued ART, BMD stabilizes over time [47,48].
Direct effects of the virus and inflammation promote an imbalance between bone formation and resorption. Studies in vitro showed that the viral proteins Vpr and gp120 stimulate osteoclastic activity, whereas p55-gag suppresses osteoblastic activity and induces its apoptosis, thus observing, an imbalance between bone formation and resorption [49,50].
Furthermore, the immune response of HIV infection produces activation of pro-inflammatory cytokines that alter the relationship between osteoblasts and osteoclasts. Authors have shown that cytokines released by the inflammatory immune response, such as interleukin (IL) 1, IL3, IL4, IL6, IL11 and TNF-α, are implicated in the control of osteoclastic differentiation and activation, increasing bone resorption. Another mechanism proposed to explain the increase in osteoclastic activity involves the overexpression of cytokines and specific growth factors. It has been demonstrated that the increase of IL4 or transforming growth factor-β (TGF-β) in bone can result in loss of bone mass that is probably due to primary effects on osteoblasts [4,25,49]. According to Hileman et al., the activation of pro-inflammatory cytokines, such as IL1 and TNF-α, plays an important role in the pathogenesis of AIDS [50]. This pro-inflammatory activation contributes to viral replication, the development of immunodeficiency and the appearance of some clinical manifestations, especially endocrine abnormalities [51].
Cytokines and growth factors are involved in the modulation of osteoblasts and osteoclasts. It has been shown that the TAX viral protein could play an important role in bone pathologies by inducing genes responsible for multiple cytokines, such as IL6, IL2, TNF-α, which modulate osteoclasts and increase bone resorption [52].Defining whether these alterations originate from an increase in osteoclastic activity (resorption) or inhibition of osteoblastic activity (formation) is essential for efficient therapeutic strategies. However, most studies did not demonstrate sufficient statistical power for definitive conclusions [5].
Sclerostin and Sirtuin 1 (SIRT1) were recently identified as important for bone metabolism [53]. Sclerostin is encoded by the SOST gene e plays a key role in bone turnover by reducing osteoblasts’ differentiation and mineralization. The SOST gene is an epigenetic target of SIRT1 in osteoblasts. It is negatively regulated by SIRT1 [54] and SOST knockout mice exhibit an increased bone mass phenotype [55]. The treatment with the SIRT1 activator SRT3025 in mice reduces the expression of sclerostin in bone and increases the periosteal cortical mineralizing surface and the marker of bone formation pro-collagen type I. The SRT3025 also downregulates sclerostin in a murine osteocyte. Consequently, it plays a key role in bone turnover by reducing osteoblasts’ differentiation and mineralization. As a result, there is an increased bone mass phenotype [55].
Silent information regulator 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, which deacetylates histone and non-histone proteins [56-58]. It is a cellular regulator that has received attention recently and is involved in the regulation of physiological functions, including endocrine, metabolic regulation, immune response, oxidative stress, inflammation, and ageing [59-63].
Studies have demonstrated a relationship between SIRT1 and the HIV virus, due to its ability to modulate viral replication. SIRT1 recycles HIV-Tat protein, which is critical for transcriptional activation of HIV-1 provirus and induces T-cell hyperactivation [64,65].
Sirtuins have the ability to control numerous cellular pathways during the viral life cycle, however, their exact role in viral infections is still not fully understood.66 It is known that they can affect viral factors and influence the course of an infection. One example is the deacetylation of the Tat HIV viral protein by SIRT1, affecting the transcription efficiency of the integrated viral genetic material [65]. Viral Tat protein influences chromatin modifying factors favoring viral replication (histone acetyltransferase 1 recruitment, increased Tat acetylation, increased RNA transcription efficiency, and increased viral load [67,68]. Tat deacetylation by SIRT1 promotes its reconstitution and recruitment of host proteins, preventing the end of the transcription process in the elongation phase and the beginning of the next transcription cycle. Without this protein, the elongation process is ineffective, resulting in significantly higher levels of altered virus [69]. Tat is also a factor that affects SIRT1, binding to its catalytic domain and thus blocking the deacetylase activity towards NF-kB, p53, p21 or BCL2-associated X protein (BAX), causing an HIV-specific status of chronic immune activation [70]. This state of chronic immune activation due to HIV infection favors the heightened production of transcription factors and pro-inflammatory interleukins, which allows the integration of newly formed viral DNA into the host genome [71,72].
Studies on the relationship between SIRT1 and HIV Tat protein have shown that IL-2 and NF-kB activities are important in the course of infection. Activation of IL-2 gene expression occurs via the T-cell receptor (TCR) and the CD28 co-receptor. The Tat protein of the virus is involved in the process of activating the IL-2 gene and regulates the expression of host genes in infected T cells [71]. As a result, activation of T cells via TCR receptors causes cleavage of NF-kB at the from NF-kB Inhibitor (IkB) inhibitory factor, its translocation to the nucleus and activation due to post-translational modifications, including lysine acetylation by SIRT1 [73]. In vitro studies have shown a reduction in NFkB-p65, a deacetylation of the subunit in the presence of Tat, as well as in the presence of nicotinamide factor (NAM), limiting the activity of SIRT1.
Bone changes are common in HIV-infected patients. A prospective open cohort study (n=5826) from 2000 to 2006, showed that fracture rates and the relative proportion of fragility fractures were significantly higher in outpatient HIV patients compared to the general population.74 Gibellini et al., and Womack et al., showed that in the plasma of HIV-infected men, levels of osteoprotegerin (OPG), NF-kappa receptor activator b-ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL) were significantly higher in the HIV-infected group compared with the control group [75,76].
The reduction in bone density is mainly caused by the negative impact of Vpr on RANKL. The upregulation of RANKL increases the formation of osteoclasts and lowers the levels of OPG (RANKL inhibitor), the main determining factors of bone metabolism and the degree of bone resorption [74,77]. Cross-sectional study showed that OPG levels produced by T cells were significantly lower and that those of RANKL were higher in HIV-infected individuals compared to healthy ones [77]. Titanji et al., showed lower OPG expression and higher RANKL expression in B cells from HIV positive patients compared to negative controls. The authors also found a correlation between B-cell RANKL/OPG ratio and T-score, Z-score, and total bone mineral density (BMD) in areas at highest risk of fracture: hip and femoral neck [77].
Osteoporosis is also associated with highly active antiretroviral therapy (HAART). A cohort study by Womack et al., with 40,115 individuals, found significant positive correlations between increased risk of fragility fracture and PI use among HIV-infected patients.76 The decline in BMD is independent of the type of PIs used. Patients treated with these drugs had a significant reduction in lumbar spine BMD after one year compared to other therapeutic regimens [78,79]. In vitro studies in cultures of human osteoblasts showed that PIs alter the expression of some genes responsible for bone calcium deposition, they reduce alkaline phosphatase (ALP) and the transcription factor runt-2 (Runx-2) [79]. Cozzolino et al., showed a reduction in BMD of more than 5% in 31% of the analyzed HIV cases, after 4 years of virological suppression, as a result of combination antiretroviral therapy (cART). In the National Health and Nutrition Examination Survey (NHANES) a significantly higher incidence of reduced BMD was demonstrated in the femoral neck compared to controls (47% vs. 29%). It is likely that PIs have a negative effect on vitamin D3 metabolism [80].
Another mechanism of bone resorption would be the proximal renal tubular damage caused by tenofovir (TDF), leading to hypophosphatemia and increased parathyroid hormone. This drug also interacts directly with osteoblasts and osteoclasts, contributing to increased bone resorption [81]. Negredo et al., showed a significant reduction in BMD in HIV-infected patients after 48 weeks of switching from TDF to abacavir (ABC), with changes in serum bone markers, especially with increased sclerostin [81].
Choi et al., showed that SIRT1 is a deacetylating agent of SRY-Box transcription factor (SOX2), the main factor maintaining the self-renewal and ability to differentiate mesenchymal stem cells (MSCs), that is also observed in osteoblasts. SOX2 maintains stem cells by regulating the expression of Dickkopf-related protein 1 (DKK1). The SIRT1/SOX-2 axis regulates the differentiation or regeneration of MSCs. Deacetylation of SOX2 by SIRT1 inhibits its transport from the nucleus to the cytosol, degradation by proteasomes and ubiquitination, as demonstrated in in vitro studies on bone marrow mesenchymal stem cells (BM-MSCs) [82].
SIRT6 is another sirtuin that participates in bone remodeling disorders. Zhang et al., demonstrated abnormal bone remodeling and resorption in SIRT6 knockdown mice. Genes in osteoblasts are indicated as possible changes in the mechanism in the expression of transcription factor 2 related to runt (Runx2) and Osterix (Osx). In the absence of SIRT6, there is increased acetylation of histone H3K9 in the promoter region of Runx2 and Osx, responsible for inhibiting blastogenesis and the transition from osteoblasts to osteocytes [83]. The authors suggest that SIRT6 is considered as an important determinant of osteoblastogenesis. Increased expression of OPG and DKK1 were also observed in SIRT6 deficiency, causing alterations in the differentiation of osteoblasts and osteoclasts. Studies show that SIRT6 is a positive regulator of osteogenic differentiation. SIRT6 also regulates osteogenic differentiation, and controls IGF-1-mediated bone resorption, affecting hypoxia-induced osteoblastic apoptosis. Under hypoxia, there is increased production of pro-inflammatory cytokines and increased glycolysis. Studies conducted on human osteoblastic cells have shown that SIRT6 inhibits the above processes confirming its role in preventing inflammatory bone resorption [84].
HIV-infected individuals on chronic use of HAART are more likely to develop adipose tissue and metabolic disorders such as lipodystrophy and metabolic syndrome. SIRT1 may influence the outcome of these changes due to its role in regulating transcription factors involved in energy regulation. Tagliari et al., genotyped four polymorphisms located in SIRT1 in 832 HIV-infected patients receiving this kind of therapy by real-time polymerase chain reaction. The authors concluded that none of the investigated genetic variants are predictive factors for the development of lipodystrophy and metabolic syndrome in HIV-infected individuals in Brazil [85].
Cross-sectional study carried out in our service with 60 people living with human immunodeficiency virus, both sexes between the ages of 18 and 55 years (median: 34 yr) prior to the start of ART drugs demonstrated changes in 25-OH vitamin D levels. The authors observed changes in 36 (60%) patients, of which 14 (23.3%) showed deficiency and 22 (36.7%) showed insufficiency. Concerning the BMD, there were prevalence of spinal BMD (L1-L4), femoral neck BMD, and total femur BMD deficiencies as well as the bone markers evaluated in the study population. The prevalence of low spinal BMD (L1-L4) and total femur BMD were 16.7% and 7.1%, respectively (Tables 1 and 2) [86].
Brown et al., studied 33 HIV-infected individuals who were not using ART and found a lower prevalence of low BMD than that found in our study [87]. One possible explanation for the difference between these findings may be that viral proteins are able to directly stimulate osteoclastic activity and inhibit osteoblastic activity. In addition, HIV-positive patients show elevated levels of inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, which are capable of promoting osteoclast formation, and thus, contribute to bone reabsorption [22].
Hileman et al., demonstrated decreased BMD in 33.3% of their patients with a median age of 40yr (25-50yr), which is higher than the median age in our study [50]. The authors also did not find an association between vitamin D status and alterations in the BMD, although their study population had a high prevalence of vitamin D deficiency. The data evaluated in our study emphasized that it is a population with a recent diagnosis of HIV infection and the evaluated patients were not receiving ART [86].
In clinical practice, it is necessary to consider the serum vitamin D levels and make the decision to use supplements. The description of biomarker profile is necessary for establishing the prognosis of these patients with low bone mass. Our results revealed that, although young and unexposed to ART, the study population presented with compromised bone health, low BMD, and low levels of 25OH vitamin D.
Table 1: Bone metabolism’ markers and BMD in adults with HIV not yet exposed to ARV (n=60)
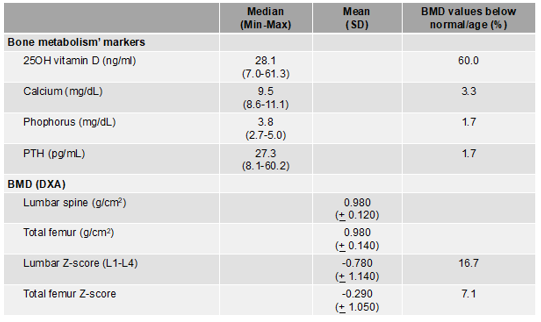
HIV: human immunodeficiency virus; ART: antiretroviral Therapy; SD: standard deviation; BMD: bone mineral density; DXA: dual-energy X-ray absorptiometry; PTH: parathyroid hormone. Cut off points: <29ng>
Table 2: Univariate analysis of 25OH vitamin D and BMD in adults with HIV not yet exposed to ARV (n=60)
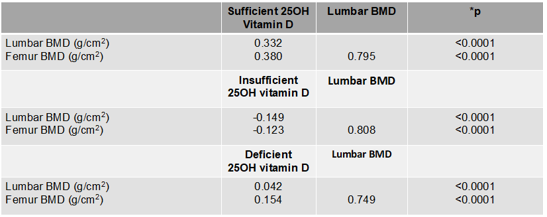
HIV: human immunodeficiency virus; ART: antiretroviral; BMD: bone mineral density. *Spearman test
Due to the strong association between HIV infection and reduced BMD, McComsey et al., recommended screening using dual-energy X-ray bone densitometry (DXA) in all postmenopausal women, men aged ≥ 50 years, and in individuals with a history of fragility fracture regardless of age and gender.35 Once BMD reduction is confirmed in these patients, the investigation of secondary causes is necessary. The main causes are endocrine disorders (vitamin D deficiency, hyperparathyroidism, subclinical hyperthyroidism, hypogonadism), renal disorders (phosphate wasting, idiopathic hypercalciuria), gastrointestinal disorders (celiac disease) and hematological disorders (multiple myeloma, mastocytosis) [4].
Osteoporosis is characterized by compromised bone strength that predisposes to an increased risk of fractures. Its diagnosis can be based on a history of fragility fracture or on BMD assessment by DXA. The World Health Organization (WHO) classifies BMD as normal, osteopenia or osteoporosis according to the number of standard deviations below the mean BMD of the reference population (healthy, young between 25 and 35 years old, paired for sex and ethnicity) by T-score. T-scores ≤ -2.5 define osteoporosis for women who are in menopause and for men > 50 years. Osteopenia was defined by T-scores ≤ -1.0 to ≥ -2.5 for women who are already in menopause and for men > 50 years. For individuals younger than 50 years of age and women in pre-menopause, the Z-score (standard deviation related to age-matched populations matched by sex and ethnicity) is used to further define changes in BMD. Osteoporosis was defined by Z-scores ≤ -2.0 associated with fragility fracture. Z-scores ≤ -2.0 in the absence of fractures defined reduction in BMD for age [4,88,89].
Osteomalacia is the impairment of bone matrix mineralization caused, most often, by severe vitamin D deficiency [35]. In HIV-positive patients, reduced BMD is associated with low weight, hypogonadism, malabsorption, long-standing HIV infection, use of glucocorticoids, lipodystrophy, insulin resistance, and hyperlactatemia. Other factors such as vitamin D deficiency, hyperparathyroidism, subclinical hyperthyroidism, Cushing's syndrome, phosphate wasting, hypercalciuria, celiac disease, multiple myeloma and mastocytosis are associated with reduced BMD not only in the HIV-infected population, but also in the general population [4].
Studies have shown the usefulness of DXA as an instrument for assessing body composition (fat mass, fat-free mass, BMD and bone mineral content). It is considered a tool that presents validity, precision and reliability, in addition to being a non-invasive technique for collecting information about the different tissues that make up the human body [89].
The pharmacological treatment for osteoporosis should be considered for: postmenopausal women and men aged 50 years or older who have a history of hip or vertebral fractures, associated with T-score for BMD ≤ -2.5 or low BMD characterized by BMD T-score between -1.0 to -2.5 associated with 10-year probability of hip fracture ≥ 3% or probability of hip fracture associated with osteoporosis in 10 years ≥ 20
Bone formation markers
a) Total alkaline phosphatase
Total alkaline phosphatase (TAF) is an enzyme that catalyzes the hydrolysis of phosphate esters and has a half-life in the blood of 24 to 48 hours. Its serum concentration has been used as a marker of bone turnover. Although the measurement of TRF activity involves a wide variety of isoenzymes that originate from the intestines, kidneys, pancreas, placenta, liver and bones, the two major sources of this enzyme are bone (osteoblast) and liver (endothelial cells). Thus, TAF is non-specific tissue and changes in any disease that affects one or more of these sources, reducing its value as a marker of bone turnover [93].
b) Bone-specific alkaline phosphatase
Bone-specific alkaline phosphatase (BEAF) is located in the plasmatic membrane of osteoblast and is released into the circulation as a dimer. In vivo it is involved in bone formation and mineralization and predominates in childhood until the end of longitudinal growth. It is the most used bone formation marker.93 BEAF has advantages over TAF because it does not suffer hepatic influences and it is more sensitive to small variations in the circulating pool. BEAF correlates with TAF and osteocalcin, and its advantage is that it is relatively independent of diseases that affect other sources of TFA [93].
The bone phenomena associated with elevations in TAF need to be of great intensity, as occurs in fractures, Paget's disease, fibrous dysplasia, hyperparathyroidism with advanced bone disease, and osteomalacia or rickets. In these situations, it can be used as a marker of disease activity or as a parameter of response to established treatment. However, when greater sensitivity and specificity are needed, BEFA is the most indicated.
c) Pro collagen type I peptide
About 97% of the organic matrix produced by osteoblasts consists of type I collagen, which is synthesized as a large protein: procollagen I. During the conversion of procollagen to type I collagen, the amino (N) and carboxy (C) terminal fragments of the procollagen molecule are broken and released into the circulation. The amino and carboxyterminal propeptide separates from the procollagen molecule in a 1:1 molar ratio in relation to newly formed collagen. They are not incorporated into the bone, being released into the circulation where it reflects bone formation without being affected by resorption. However, the carboxyterminal propeptide is not specific for bone and is produced by other tissues that synthesize type I collagen, including the skin [93].
None of the assays developed for the analysis of the carboxyterminal or aminoterminal propeptide were more sensitive than BEAF or osteocalcin for differentiating between normal and pathological conditions of bone formation. In part, this fact occurs as a result of the inability of the assays used to distinguish between bone and soft tissue contributions to circulating levels of this peptide [93].
d) Osteocalcina
Osteocalcin (OC) is the most abundant non-collagenous osteoblast-specific protein in the body and is initially synthesized in osteoblasts as a pre-promolecule. Next, it undergoes vitamin K-dependent modifications and intracellular cleavages to produce the mature OC, which will be secreted. These modifications in its molecule allow it to present high affinity for the hydroxyapatite present in bone, thus regulating bone mineral maturation. However, OC is also present in the systemic circulation, and its serum concentration has been correlated with bone formation and osteoblast numbers, thus being used as a serum marker of bone formation [94].
Bone resorption markers
FGF-23 plays a special role in mineral metabolism and is indicated as a marker of bone mineral disease. It is a molecule produced mainly in bone tissue by osteoblasts and osteocytes and is an important regulator of mineral metabolism, especially phosphorus [60,61].
FGF-23 is predominantly expressed in bone, but it is also expressed in brain, thymus, small intestine, heart, lung, liver, kidney, thyroid/parathyroid, lymph node, skeletal muscle, spleen, skin, stomach, and testis [95]. In this way, the osteocyte lacuno–canalicular system should be viewed as an endocrine organ regulating phosphate metabolism. This hormone causes a decrease in phosphate reabsorption, causing phosphaturia and hypophosphatemia, by downregulating the sodium phosphate co-transporters in the proximal tubule. Consequently, there is a decrease in phosphate reabsorption causing phosphaturia and hypophosphatemia. By inhibiting the activity of renal 1α-hydroxylase and stimulating that of 24-hydroxylase, it diminishes the production of 1,25OH vitamin D resulting in low serum levels of calcitriol [95,96]. In humans, FGF-23 also rises after prolonged phosphorus overload even in individuals with normal renal function [97].
The serum concentration of FGF-23 is elevated in patients with CKD contributing to the reduction of active vitamin D levels in these patients.98 The possible causes of the increase in this hormone in CKD population would be the stimulation of hyperphosphatemia, the low production of active vitamin D, the alteration of its metabolism and the lower renal excretion of phosphorus. Previous studies have shown that FGF-23 appears to be prognostic for the onset of secondary hyperparathyroidism and the response of PTH levels to calcitriol therapy [99].
Animals deficient in FGF-23 present, in addition to hyperphosphatemia, bone alterations, ectopic calcifications and hypoglycemia. These changes are reversed when these animals have vitamin D receptor (VDR) deletion, suggesting that some of the actions of this hormone are mediated by its action on 25-hydroxyvitamin D 1-α-hydroxylase [100]. Hypoglycemia and increased insulin sensitivity seem to be mediated by vitamin D production, corroborating the fact suggested in other studies, of the interaction between the regulation of bone metabolism and glycemic levels. Vitamin D appears to participate in the regulation of peripheral insulin sensitivity [98].
It is evident that FGF-23, calcitriol and PTH are all involved in controlling the mineral metabolism of the intestine, bone, kidney and parathyroid gland in order to regulate calcium and phosphorus homeostasis [93]. Data suggest an association between circulating levels of FGF-23, fat mass and alterations in the lipid profile in the general population. It is known that FGF-23 regulates the metabolism of phosphorus and vitamin D, in addition to being positively correlated with cardiovascular risk [101].
In the HIV-positive population, FGF-23 has only been studied in patients on HAART, mainly in those using tenofovir. This medication induces bone, kidney and endocrine alterations of unknown causes. The main changes related to mineral and bone metabolism are phosphaturia, hypophosphatemia, increased levels of 1,25(HO)2vitamin D and FGF-23 [102,103,104].
b) Osteopontin
Osteopontin (OPN) was first described as a circulating protein involved in several physiological and even pathological processes. Data showed that it can be found in the cytoplasm and nucleus of several cell types, such as: pre-osteoblasts, osteoblasts, osteocytes, chondrocytes, fibroblasts, dendritic cells, macrophages, T cells, hepatocytes, smooth muscle cells, musculoskeletal cells, endothelial cells, kidney cells, among others. The extracellular functions of OPN occur through its interaction with multiple cell surface receptors, such as integrins and CD44, regulating cellular processes such as bone mineralization, tissue remodeling and immunity [94,105].
One of the main functions of OPN is the control of biomineralization, due to its ability to directly bind to apatite crystals and inhibit mineralization, being considered a marker of bone resorption. Furthermore, it is not only important for bone mineralization, being strongly elevated in sites of ectopic and/or pathological calcification (vascular calcification, valve calcification, among others) [95].
c) Osteoprotegerin
Osteoprotegerin (OPG) is a glycoprotein described in the 1990s, produced by various tissues such as bone, vascular, heart, lung, kidney and placenta, in addition to circulating freely in the plasma. It serves as a soluble receptor for two members of the TNF superfamily: TNFSF11 and TNFSF10. TNFSF11 stimulates bone resorption through the differentiation and activation of osteoclasts, however, its neutralization by OPG prevents bone resorption and loss of bone mass [106].
d) Tartrate-resistant acid phosphatase and C-telopeptide
Tartrate-resistant acid phosphatase (TRAP) and type I collagen carboxyterminal telopeptide (C-telopeptide) are markers of bone resorption measured in blood. Acid phosphatase is a lysosomal enzyme present in bone, prostate, platelets, erythrocytes and liver. Osteoclasts contain TRAP which is released into the circulation. However, it is not osteoblast specific and is relatively unstable in the frozen sample. For these reasons, its use has no significant application in the clinical control of patients [107].
e) Calcium
Bone resorption includes the dissolution of calcium salts and enzymatic fragmentation of the organic matrix of bone, which is mainly composed of type I collagen. Thus, until now, the parameters used as bone resorption markers come from collagen degradation. The measurement of calcium in 24-hour urine or in a single morning urine sample collected with fasting is considered a clinically useful marker of bone resorption. However, although widely available, calciuria is not specific for bone turnover, but has considerable value in clinical situations and for calcium metabolism [108].
f) Hydroxyproline
Urinary hydroxyproline is a product resulting from collagen degradation. It is released into the circulation in free form and bound to peptide buffers. It is found in connective tissues other than bone, in the C1q complement system, and in the N-terminal propeptide of type I collagen.
Hydroxyproline is not a good marker of bone resorption because only 40% of it is derived from bone. Furthermore, 90% of circulating hydroxyproline is metabolized by the liver in normal individuals. Its levels are largely influenced by a diet rich in hydroxyproline, by renal excretion and also by the circadian rhythm. When compared to histomorphometric studies, its excretion is weakly correlated with bone resorption. In this way, the variability of hydroxyproline measurements limits its use in clinical practice, especially for measuring small changes in bone turnover [93].
g) Hydroxylysine
Hydroxylysine is the result of collagen degradation and is eliminated in the urine during bone metabolism. Unlike hydroxyproline, it is not influenced by diet. However, to date, its use as a bone resorption marker is not widespread, and there is no test available for its dosage in daily practice [93].
h) Pyridinoline and Deoxypyridinoline
Pyridinoline (Pyr) and deoxypyridinoline (D-Pyr) are products of collagen degradation by collagenase and are excreted in the urine without being metabolized and linked to pyridine cross-links. The “cross-links” are links that connect the collagen molecules and increase the tensile strength between the fibers. These connections are made between the non-helical portion of a collagen molecule with the helical region of another molecule. As bone is the most abundant source of collagenous matrix and its turnover rate is higher than other connective tissues such as cartilage, the dosage of Pyr and D-Pyr in biological fluids reflects the bone source. Pyd differs from D-Pyr only by the presence of a hydroxyl group, with the former having wide tissue distribution, while the latter is more specific to bone tissue and correlates better with calcium kinetics and bone histomorphometry [108]. Pyr is present in various connective tissues such as bone, cartilage and others. D-Pyr is present only in bone tissue, dentin and tendons, being considered for that reason, a more specific marker of bone resorption. They are not influenced by diet, and urine collection to measure these compounds can be performed without dietary restrictions.
Pyr and D-Pyr levels are increased during childhood, in young adults and especially after menopause. The correlation observed between the levels of Pyr and D-Pyr with the results obtained by bone histomorphometry suggest the accuracy of these markers in measuring bone turnover and reabsorption [93,109].
As mentioned above, the non-helical region of the collagen molecule is called carboxyterminal telopeptide (C telopeptide) and aminoterminal telopeptide (N telopeptide). During bone tissue degradation, these regions are broken and telopeptides C and N are released into the circulation as intact molecules. When bone is resorbed by osteoclasts, fragments of type I collagen are released into the circulation and filtered by the kidneys.
It has been shown that the dosage of these fragments in urine would reflect the level of bone reabsorption in bone-metabolic diseases [109]. However, little is known about these fragments, their measurements and possible relationships with the degree of osteoclastic activity.
The products resulting from the degradation of collagen in the urine may have a non-osseous origin, since collagen fibers are distributed throughout the connective tissue of the human body. However, the most modern methods for assess telopeptides (ELISA) measure the products derived from the urinary degradation of telopeptides (carboxyterminal or aminoterminal α 1 chain of type I collagen), which is a specific amino acid sequence that is only found in bone collagen. Thus, the measurement of telopeptides is currently a very specific marker of bone resorption.
a) Interleukin 1-β
Interleukin 1-β (IL1-β) is a polypeptide produced during infections, injuries and exposure to antigens. Macrophages are its main source, but it is also synthesized by epithelial, lymphoid and vascular tissues. It determines cell proliferation, activation and chemotaxis of leukocytes, cytotoxicity, metabolic alterations, vascular tissue alterations such as proliferation of smooth muscle cells, pro-inflammatory and degradation effects, such as bone resorption by acting as an osteoclast activating factor [110].
b) Interleukin 6
Interleukin 6 (IL6) is a pleiotropic cytokine that plays an important role in ordering the immune system in the face of injuries. It also acts by regulating the inflammatory response, the synthesis of acute phase liver proteins and bone metabolism (Lotz 1995). Produced by various cell types, such as T lymphocytes, macrophages, smooth muscle cells, fibroblasts, skeletal muscle cells, osteoblasts, and osteocytes. It plays an important role in bone resorption, as it indirectly activates osteoclasts through increased expression of the Rankl gene by osteoblasts [111].
c) Tumor necrosis factor α
Tumor necrosis factor α (TNF-α) is a cytokine that plays a fundamental regulatory role in the inflammatory response. It interacts with two different receptors, TNFR1 and TNFR2, which are expressed differently in cells and tissues, in addition to initiating distinct intracellular signaling pathways. These different signaling pathways lead to different cellular responses, such as: cell death, survival, differentiation, proliferation and migration. Endothelial cells respond to stimulation by TNF-α through pro-inflammatory changes, increasing leukocyte adhesion, transendothelial migration, increased vascular permeability, promoting thrombosis [112].
In this scope, and accepting that HIV infection and its treatment are associated with numerous comorbidities that lead to hypoactivity of the musculoskeletal system with decreased organic capacity and increased catabolism, the assessment of these patients' body composition, especially bone mass, could provide valuable information. Long-term clinical studies are not yet available to serve as a reliable source for establishing guidelines for approaching HIV-associated metabolic bone changes and their treatment.
Considering the involvement of HIV/AIDS infection on bone mineral metabolism, knowing the profile of markers of its metabolism and body composition in these patients before starting ART would be of great value as well as supplementation of vitamin D and calcium with the initiation of ART.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti