AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2578-8965/205
1Department of Obstetrics & Gynaecology, AIIMS Jodhpur.India
2Additional Professor, Dept. of Obstetrics &Gynaecology, AIIMS Jodhpur. Rajasthan, India.
3Professor and Head, Dept. of Obstetrics &Gynaecology, AIIMS Jodhpur.
*Corresponding Author: Manu Goyal, Additional Professor, Department. of Obstetrics &Gynaecology, AIIMS Jodhpur. Rajasthan, India.
Citation: Shafak Bhandari, Manu Goyal, Pratibha Singh, (2024), Male Infertility – An Overview of Management, J. Obstetrics Gynecology and Reproductive Sciences, 8(3) DOI:10.31579/2578-8965/205
Copyright: © 2024, Manu Goyal. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 19 February 2024 | Accepted: 29 February 2024 | Published: 07 March 2024
Keywords: follicular output rate; fort; fsh; controlled ovarian hyperstimulation / ivf-et
Male factor infertility is responsible for about 2/5th cases undergoing IVF.1,2 An exponential rise in male infertility is being driven by constantly shifting societal ideals, career demands, widespread obesity, associated stress and delayed parenthood.2,3 Semen analysis is the keystone in evaluating male fertility with WHO guidelines providing the basis for procedural standardization and reference values worldwide. They have been time and again revised since 19801,2 However, documented evidence emphasis the need of comprehensive history, physical and genital examination and hormonal analysis for complete management. FSH, LH, prolactin and testosterone are the key hormones required in evaluating between different etio-pathological categories of male infertility which are hypo gonadotrophic hypogonadism, primary testicular failure, obstructive and unexplained causes 3,4,5 Categorization of cases helps in delineating the specific management and further medical or surgical treatment required. Over the years, the role of IUI has reduced to couples with moral, ethical, physical and financial issues to IVF/ICSI.3,5 Male infertility treatment has been revolutionized by ICSI. Advancements as surgical sperm retrieval in form of PESA, TESA, MESE and TESE has allowed azoospermic men the opportunity of biological paternity. For men with complete spermatogenesis failure, only treatment option is insemination with donor sperms or adoption. 3,5,6
Infertility is defined as the “inability to achieve spontaneous pregnancy within one year of regular unprotected sexual intercourse”.1 Infertility solely due to male factor is responsible in 20-30percent cases and as contributory factor in additional 30percent of cases.1,2
Causes of male infertility can be broadly subdivided into 4 categories3,4,5
1.Hypogonadotropic hypogonadism
2.Obstructive causes
3.Primary testicular failure
4.Unexplained causes
Hypogonadotropic hypogonadism
Hypogonadotropic hypogonadism constitutes hypothalamic pituitary dysfunction, accounting upto 2percent cases.3 Causes include Idiopathic isolated gonadotrophin deficiency, Kallman’s syndrome, Single gene mutation (involving the GnRH receptors, FSHB, LHB or transcription factors involved in pituitary development), Hypothalamic and pituitary tumours (macroadenoma, craniopharyngioma) , Infiltrative diseases (sarcoidosis, histiocytosis, transfusion siderosis), Hyperprolactinemia, Drugs (GnRH analogs, opiates, oestrogen, glucocorticoids), Critical illness of injury, Chronic illness, malnutrition, Infection (eg-meningitis), Obesity. These causes are frequently correctable3,5,6,7
Obstructive causes
Obstructive causes are sperm transport mechanism failure and constitute 10–20percent cases3,4 The causes are epididymal obstruction or dysfunction, congenital bilateral absence of vas deferens, Vasectomy, Infections causing obstruction of vas deferens (eg-tuberculosis, gonorrhoea, Chlamydia), Young syndrome, Kartagener syndrome (primary cilia dyskinesia), Ejaculatory dysfunction (eg-spinal cord disease, anatomic dysfunction). They constitute frequently correctable conditions3,6
Primary testicular failure
Primary testicular failure is characterized by gonadal failure and accompanies testicular atrophy. Observed in 30-40percent total cases and 25percent are due to genetic causes3,5 Common disorders are Klienfelter’s syndrome, Y chromosome deletions, Single gene mutation and polymorphism (eg-Involving androgen, estrogen or FSH receptor), Cryptorchidism, Vasucular causes - Varicoceles, torsion, Infections (eg-viral orchitis, leprosy, tuberculosis), Drugs (eg-alkylating agents, alcohol, antiandrogens, cimetidine), Radiation, Environmental gonadotoxins (eg-heat, smoking), Chronic illness (renal insufficiency, cirrhosis, cancer, sickle cell disease, amyloidosis, vasulitis) Most of these causes are irreversible4,6
However, 40-50percent cases have unexplained or idiopathic causes [3,4]
Unexplained causes
In case of male infertility, points to emphasise are personal history, clinical examination, semen analysis and hormonal tests- FSH, LH, Testosterone, Prolactin. More detailed testing in form of genetic tests and sperm function tests may be necessary depending upon these initial results1,2,3
History
Age is usually not a significant factor till 45 years. There is increased risk of autism, schizophrenia, bipolar disorders in offspring beyond 50 years.
Occupation is important as certain chemical and radiation exposure is harmful.
General wellbeing of the patient is important. Stress on diabetes, anosmia, respiratory issues, galactorrhea.
Smoking, alcohol and drugs as anabolic steroids, chemotherapy, recreational drugs (marijuana) affect sperm quality and quantity
Past history involving infections (Mumps, STDs, prostatitis) , groin trauma, surgeries for hernia, hydrocele, cryptorchidism, vasectomy
Developmental growth pertaining to onset of puberty should be enquired. Sexual history and history pertaining to androgen deficiency are crucial. Ask regrading libido, sexual intercourse frequency, previous conceptions, previous fertility treatment,ejaculation problems, shaving frequency, loss of body hair or muscle mass, breast tissue development, voice change
Family history of hereditary disorders
Relevant history of female partner is equally important3,6,7
General, physical and genital examination
Look for built of the patient. Healthy male endocrine pattern can be identified on basis of low-pitched voice, male pattern, distribution and amount of axillary hair, pubic hair and beard. Consistently high-pitched voice, gynaecomastia and reduced masculine muscle mass are indicators of hypogonadism prior to puberty3,6,7
Local examination of male genitalia involving testis and scrotum is done. Testis are measured for their volume and consistency. Testicular volume is measured with Prader orchidometer, which is twelve numbered wooden or plastic beads string ranging in size from 1-25ml.Testicular growth indicates pubertal development. Volume less than4 ml -prepubertals, 4–15 ml peri-pubertals and 12–25 ml adults. Hard consistency signifies malignancies while soft consistency shows loss of germinal tissue. Inspect scrotum for scars of previous surgeries, palpate for any masses, varicocele and palpable epididymis. Varicocele is confirmed performing Valsalva manoeuvre. Normal adult penile length is 10-17 cm. Examine for presence of hernias and signs of STDs3,6,8
Klinefelter's syndrome is suggested by small testes, phallus and prostate, sparse hair, unusually lengthy limbs and legs and delayed epiphyseal closure3,7,8
Semen Analysis: It holds an undisputable key role in diagnostic process of male infertility. Terminology associated with semen analysis are in Table [11,2,5,6]
| Normozoospermia | All semen parameters normal |
| Oligozoospermia | Reduced sperm numbers Mild to moderate: 5-15 million/mL Severe: less than 5 million/mL |
| Asthenozoospermia | Reduced sperm motility |
| Teratozoospermia | Increased abnormal forms of sperms |
| Oligoasthenoteratozoospermia | Sperm variables all subnormal |
| Azoospermia | No sperms in semen |
| Aspermia (anejaculation) | No ejaculate (ejaculation failure) |
| Leucocytospermia | Increased white cells in semen (>1 x 10*6) |
| Necrozoospermia | All sperms are non-viable or non-motile |
Table 1: Semen analysis terminology
| Semen parameter | Normal value |
| Semen Volume (ml) | 1.4 (1.3-1.5) |
| Total sperm number (106 per ejaculate) | 39 (35-40) |
| Total motility (%) | 42 (40-43) |
| Progressive motility (%) | 30 (29-31) |
| Non progressive motility (%) | 1 (1-1) |
| Immotile sperms (%) | 20 (19-20) |
| Vitality (%) | 54 (50-56) |
Table 2: Normal semen parameters
Indian research reports 22.4- 64.2percent prevalence of deranged semen parameters amongst infertile couples. Azoospermia accounts for 1percentof all men and 15-20percentof infertile men. [3,5,10] Semen is tested after 3-5 days of abstinence. A single semen analysis has false positive rate of 10percent while two tests will falsely identify only 2percent men as having abnormal semen parameters. Ideally repeat sampling is done after 3 months. However, it can be scheduled earlier at 6-8 weeks if the delay is causing undue anxiety to patient. Repeat analysis in case of azoospermia should be performed in 2–4 weeks. [1,2,10]
Hormone analysis
FSH, LH, prolactin and testosterone are the key hormones required in evaluating between different aetio-pathological categories of male infertility [3,4]
| Endocrinology | Semen Analysis | Testicular Examination | Likely diagnosis |
Elevated FSH, Normal to high LH, Low to normal testosterone | Normal volume, Severe Oligozoopermia/ Azoospermia | Low to normal volume testis, soft consistency, usually palpable vas, +/-varicocele | Hypergonadotrophic hypogonadism |
Low FSH Low LH Low testosterone Hyperprolactinemia +/- | Severe Oligozoopermia/ Azoospermia | Low volume testis, firm consistency, usually palpable vas | Hyogonadotrophic hypogonadism |
Normal FSH Normal LH Normal testosterone | Low volume, Severe Oligozoopermia/ Azoospermia | Nearly normal testis volume Dilatation of epididymis +/- non palpable vas | Obstructive pathology Retrograde ejaculation Idiopathic male infertility |
Low FSH Low LH Normal testosterone | Normal volume, Oligozoopermia/ Azoospermia | Normal volume testis | Illicit drug abuse, Exogenous testosterone |
Table 2: summarizes the patient endocrine profile and its correlation with semen analysis and testicular examination and likely diagnosis.
Single raised FSH value holds more significance than LH value due to its longer half-life. Morning testosterone sample is preferred due to diurnal variations.Total circulatory testosterone level is dependent on sex hormone binding globulin (SHBG). Disorders such as Hyperthyroidism, liver disease and oestrogen excess have increased SHBG resulting in total circulatory testosterone values to be within normal limit. However, obesity, hypothyroidism, Type 2 diabetes and acromegaly have low SHBG, so falsely sub-normal total testosterone levels. In spite of low production of testosterone 40percentof Klienfelter’s patients may be tested to have normal range testosterone levels due to increased SHBG levels following androgen excess. [3,4,11,12]
Imaging: Routine scrotal and testicular ultrasonography is not advised.Indications include inconclusive diagnosis or clinically palpable pathology.[3,13] It helps improving detection of scrotal pathologies and information on testicular volume. Varicocele (most common), hydrocele, epididymal cyst and spermatocele are common pathologies diagnosed. [3,4,10] Suspected cases of obstructive azoospermia with palpable vas deferens calls for trans-rectal USG. Renal imaging is advised when either unilateral or bilateral vas deferens is absent (10-25percent renal agenesis). Cranial MRI is suggested for patients with hyperprolactinemia or with presence of visual symptoms. [5,13]
Genetic testing: There is 15 times higher chances of detecting chromosomal anomalies in severe male infertility compared to fertile males, signifying genetic screening in specific patients.14Genetic counselling is done for conditions with autosomal dominant inheritance pattern. Fundamental genetic tests recommended are Karyotype, Y micro-deletions (Azoospermia factor- AZF deletions) and cystic fibrosis trans-membrane conductance regulator (CFTR), if sperm concentration less than 5 million/ml [3,4]
Offer karyotype and Screening of Y chromosome microdeletion to men with non-obstructive azoospermia or severe oligospermia who are candidates for IVF with ICSI.3 Chromosomal abnormalities by peripheral karyotype testing are present in about 7percent infertile, 5percent oligospermic and 15 % azoospermic men.4 Genetic testing to diagnose FSH receptor defect and other spermatozoa epigenetic are currently used in research settings only. The most common numeric chromosomal anomaly encountered among infertile men is Kleinfelter’s syndrome affecting 1 in 600 males and accounting 14% of azoospermic cases.14 AZFa, AZFb, AZFc deletions are identified in 10percent,12percent and 2percent of oligospermic men. AZFc deletions accounts for 80% of these mutations whereas AFZa leads to complete testicular germ cell atrophy. [4,5] Prior to surgical sperm retrieval in these individuals, genetic counselling should be prioritized due to possible risk of passing on these genetic abnormalities to offsprings. These microdeletions will, through ICSI, be transmitted to sons who most probably will be infertile as well. However, ICSI children are too young to evaluate their fertility status.3,14 Upto 95percent men with clinically manifested cystic fibrosis are infertile and congenital bilateral absence of vas deferens (CBAVD) is most common underlying cause with mutations in CFTR gene. It should be presumed that CBAVD patients have mutations, so testing for carrier status in female partners should be done.[3,4,14]
Sperm function tests: help in assessing functional characteristics including sperm motility, hyperactivation, mucus penetration, zona interaction and acrosomal reaction. They are based on sperm proteomics, metabolomics, mitochondrial metabolism and signalling system essential for fertilisation. They are not used in daily practice. The common ones are Cap-score assay, sperm capacitation index, zona-free hamster oocyte penetration assay, CatSper expression testing. [4,5,6,10]
Testicular biopsy: for diagnosis in infertile males is not recommended. It can be taken in setting of surgical sperm retrieval sent for histopathological examination to evaluate spermatogenic capacity of testis. [3,4,12]
Management
Lifestyle factors
Vaginal intercourse every alternate day maximises odds of natural conception and should be recommended, as timed intercourse may be emotionally stressful. Stress can be a contributing factor to male subfertility.14 Early recognition and providing psycho-therapy is useful. Healthy diet rich in carbohydrates, fibre, folate, lycopene,fruits and vegetables with lesser fats is useful. Excessive alcohol consumption is damaging to semen quality but 3 to 4 units per day are unlikely to have adverse effect.4,5 Association between caffeinated beverages and male sub-fertility has not been proven. Although there is debate on the benefits of wearing loose-fitting underwear to increase fertility, elevated scrotal temperature is linked to inferior semen parameters.3,6,14
Obesity deteriorates semen quality by lowering testosterone levels. Studies inform that males with BMI > 30kg/m2 take longer to conceive. Improved semen scores were found in physically active men with who exercised 3-4 hours/week. [5,14]
Hypogonadotropic hypogonadic infertility
Gonadotrophins:Use Human chorionic gonadotrophin (hCG) or Pulsatile gonadotrophin-releasing hormone (GnRH) for 3-6 months followed by combination of FSH and LH to initiate spermatogenesis by stimulating Leydig cells for normalisation of intra-testicular testosterone. During treatment, measure testosterone levels every 1-2 months, target level 400-900ng/dL. The best response is seen in individuals with post-pubertal onset of gonadotrophin deficiency and testicular volume >8mL. They have good outcomes resulting in normalization of testicular function in 80percent with 50percent–70percentpregnancy rates. Start with hCG alone, in case of failed response, combined treatment with hCG + hMG or pure FSH (75-150 IU three times weekly) is advised. [4,7,11,12]
Hyperprolactinoma is a treatable cause and usually responds to bromocriptine or cabergoline.7,12 Gonadotrophins, bromocriptine, SERMs (clomiphene citrate and tamoxifen) do not confer benefit in unexplained male sub-fertility.3,11 Exogenous steroids should be stopped. After stopping testosterone use, azoospermia recovers to concentration of 6.5 million/mL after 4.5 months. Men with testosterone use < 1>
Eugondaotrophic hypogonadism: It is characterized by severe oligospermia, low serum testosterone levels and abnormally low serum testosterone/estradiol ratio. Aromataze inhibitor named Testolactone 50-100 mg twice daily or anastozole 1 mg daily is advised. [4,5,6]
Hypergonadotrohpic hypogonadism: No use of medical management. Preliminary genetic evaluation is advised. For men with complete spermatogenesis failure, only treatment option is insemination with donor sperms or adoption. [4,6,7,15]
In patients with retrograde ejaculation, Alpha agonist increasing bladder sympathetic tone, Anti-cholinergics inhibiting para-sympathetic stimulation are recommended. Imipramine is most commonly used. As second line of management, penile electro-vibration and recovery of sperms from buffered urine can be attempted. Success rate of medical therapy for retrograde ejaculation is 50% while that for anejaculation is poor. Surgical sperm retrieval is the final resort if these modalities fail. [3,6,15,16]
In Leucocytospermia with Leukocytes>1 × 106/ml of semen, send semen for culture and sensitivity testing and treat accordingly particularly if it is associated with identified infection. [4,15,16]
There is no proven medical treatment for idiopathic male infertility. Androgen therapy may be advised for stimulating spermatogenesis. Use of exogeneous FSH has conflicting results. Empirical treatment with either clomiphene citrate (25 mg daily 3-6 months) or tamoxifen (20 mg daily) are commonly offered to stimulate increased pituitary gonadotrophin secretion and spermatogenesis.3,4,15
Surgical options
Reconstructive surgery-Corrective surgical procedures including vasovasostomy and vasoepididymostomy are preferred over surgical sperm retrieval for vasectomy reversal given better pregnancy outcomes and cost benefits. Usually, pregnancy happens 24 months after reversal. Reversal results are predisposed by vasectomy site, interval between vasectomy and reversal and surgeon expertise. Within 3 years high patency rates upto 97percent and 75percentpregnancy rates have been reported while corresponding figures drop to 80percent and 55percentafter 3 years.3,4 Consider ART in couples who fail to conceive 12-18 months following vasectomy reversal. Though antisperm antibodies are seen in 60percent patients, they donot affect fecundability. Periodic semen analysis can identify re-obstruction in 3percent to 21percent cases post-surgery, depending on which segment were re-anastomosed.5,15 In cases with persistent azoospermia for 6 months post reversal, ICSI is preferred over repeat surgical reversal as the patency rates drop to 75percent and pregnancy rates fall to 43percent. consider ICSI.4 Favourable outcomes are less reported in cases with infections leading to obstruction. In such cases, cryopreservation of sperms for ART is recommended to alleviate the need of future surgical procedures. Such situations demand sperm cryopreservation for use in ART in order to reduce need for additional surgeries.3,17 Reconstructive surgery in CABVD is not indicated as major portion of vas deferens is developmentally lacking, making SSR the only viable option for this condition. [15,17]
Surgical Sperm Retrieval
The term refers to a group of surgical procedures used to give azoospermic men the opportunity to become biological fathers by obtaining sperm via ICSI.18The indications for SSR are as follow-
• To provide sperms for ICSI in azoospermic men.
• In obstructive azoospermia, by recovering sperms from epididymis. Percutaneous procedures have high success rates of 95-100percent
• In non-obstructive and idiopathic azoospermia, sperms are retrieved from testis directly. The success rate of SSR is 45-55percent4,15,17,18
Sperms obtained are managed by fresh approach or frozen approach. In fresh approach, SSR is planned along with oocyte retrieval. In frozen approach, SSR is scheduled before ICSI treatment with aim to cryopreserve sperms and to be used at later date. Both methods report similar fertilization and pregnancy rates. Frozen approach is preferred particularly in non-obstructive azoospermia. Repeat SSR is advised after 3-6 months to allow adequate healing. [3,4,18]
SSR procedures 4,17
Open surgical procedures:requires operating microscope and General or regional anaesthesia
Microsurgical epididymal sperm aspiration (MESA)Testicular sperm extraction (TESE)
Percutaneous procedures: requires local anaesthesia.Percutaneous epidydimal sperm aspiration (PESA)Percutaneous sperm fine needle aspiration (TESA), also known as fine needle aspiration (FNA)
These procedures are diagrammatically depicted in Figure 1.
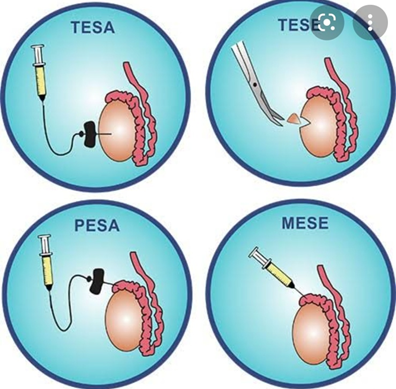
Figure 1: Surgical Sperm Retrieval procedures
Augments to ICSI
Failed fertilization occurs in 2-5% of ICSI cycles.19A sperm-specific protein is injected into oocyte during early stages of fertilisation process. This causes intracellular Ca+2 oscillations within oocyte, leading to its activation. Based on this theory, calcium ionophores have been used in lab conditions to enhance fertilization by artificially facilitating oocyte activation in PLC zeta deficiency sperm. In asthenozoospermic patients, improved sperm motility with theophylline or pentoxifylline treatment has been shown to improve implantation, fertilisation and clinical pregnancy rates. [3,19]
Donor Sperm
Using donor sperms is usually a difficult decision for the couple. They are advised for azoospermic men with failed corrective surgery/SSR, for patients with Severe sexual or ejaculatory dysfunction refractory to medical treatment, in cases with severe azoospermia with recurrent fertilisation failure or poor embryo development, when there is risk of inheriting genetic anomaly through male gamete or because of financial implications of assisted reproduction. [3,4,20]
Sperm donors are rigorously screened for STDs and genetic conditions. Before employing the sperms for any treatment, semen sample is quarantined for minimum six months if serology has been the only testing method employed, or at least for three months if nucleic acid amplification testing has been carried out in addition to serology testing. [3,4,19,20]
Proper treatment and counselling of patient and the couple are must in addition to good clinical practice before, during and after ART in cases of male infertility. The goal is informing the patient related risks, improving success rate of ART treatment and avoiding birth of children with severe genetic disease.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
