AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2640-1053/164
Research and Training Center ‘Physical and Chemical Materials Science’ Under Kyiv Taras Shevchenko University and NAS of Ukraine, Kiev, Ukraine.
*Corresponding Author: Anthony Kodzo-Grey Venyo. Research and Training Center ‘Physical and Chemical Materials Science’ Under Kyiv Taras Shevchenko University and NAS of Ukraine, Kiev, Ukraine.
Citation: Grey Venyo AK (2023), Leydig Cell Tumour of Testis and Scrotal Contents: A Review and Update, J. Cancer Research and Cellular Therapeutics, 7(5); DOI:10.31579/2640-1053/164
Copyright: © 2023, Anthony Kodzo-Grey Venyo. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 20 October 2023 | Accepted: 25 October 2023 | Published: 31 October 2023
Keywords: leydig cell tumour; testis; benign; malignant; diagnosis; histopathology; immunohistochemistry; electron microscopy; biopsy; radiology imaging; orchidectomy; frozen section biopsy; follow-up; lymph node dissection; tumour markers
It has been iterated that even though testicular cancers are relatively uncommon, and they account for only 1% to 2% of global male cancer diagnoses, it is the commonest malignancy in men who are aged between 15 years and 44 years. It has also been iterated that testicular tumours may originate from any of the cell types that are present within the testes and the on the whole fall into the two competing categories of germ cell tumours, of which approximately 95% of testicular cancer is comprised of, and sex cord-stromal tumours which constitute the remaining 5% of testis tumours in in adults. Out of the 5% of sex cord-stromal tumours, Leydig cell tumours are regarded to be commonest tumour which is derived from the same Leydig cells which normally reside within the interstitium of testis and secrete testosterone in the presence of luteinizing hormone. Leydig cell tumours of the testis are generally known to be benign tumours and only 5% to 10% being of Leydig Cell Tumours of the testis are considered to be malignant or portend malignant features. Leydig Cell Tumours of the testis have a bimodal distribution with peaks in the prepubertal age group and between the ages of 30 to 60. Apart from this Leydig Cell Tumours of the testis, had been reported in all age groups of males including young males and the over 90-yearl old male individuals sporadically. Due to Leydig cells' hormonally active properties, Leydig Cell Tumours might manifest with precocious puberty, breast tenderness, or gynecomastia, as well as infertility problems. Leydig cell tumours are derived from Leydig cells, which are histologically packed between the seminiferous tubules of the testis and they are physiologically responsible for testosterone secretion in response to luteinizing hormone. Apart from Leydig cell tumour afflicting the testis or rare occasions, Leydig Cell Tumour may also on very rare occasions afflict the epididymis. Leydig Cell Tumour of testis may manifest in different ways including: painless mass in the testis or intra-scrotal mass, precocious puberty, including early development of pubic hair as well as penile and musculoskeletal growth beyond that expected for the child’s age. Diagnosis of Leydig Cell Tumour of Testis whether benign or malignant is made based upon the histopathology examination and immunohistochemistry staining features of the tumour. Traditionally, Leydig cell tumours of the testis had been treated by the undertaking of radical orchidectomy based upon the provisional diagnosis of a malignant tumour only to find that the pathology examination of the orchidectomy specimen has confirmed features of Leydig Cell tumour of the testis which most often is benign. Radical orchidectomy alone has generally been curative for clinically benign Leydig cell tumours of testis. Testis-sparing surgery could be considered if the clinical suspicion of Leydig cell tumour is high, and pre-operative testicular serum tumour marker levels are within normal limits, and the size of the tumour is less than 2.5 cm. An intra-operative frozen section should always be undertaken to confirm benign Leydig Cell tumour of the tests, and a radical orchiectomy undertaken if the tumour is reported to be malignant. Leydig cell tumours of testis, do depict malignancy by metastasizing. About 10% of Leydig cell tumours in adults exhibit malignant biological behaviour. The only treatment for malignant Leydig cell tumour of testis is retroperitoneal lymph node dissection, in addition to the undertaking of radical orchidectomy due to the fact that they are known to be resistant to chemotherapy and radiotherapy. Because of the possibility of local recurrence of Leydig Cell Tumour of Testis, it is important for clinicians to ensure their patients undergo regular clinical and radiology imaging follow-up assessments for a long time.
Leydig cell tumours are rare tumours of the testis of the gonadal interstitium which may be hormonally active and which may lead to feminizing or virilising syndromes. Leydig cell tumours do comprise about 4% of adult testicular tumours [1] [2] and 3% of testicular tumours in infants and children. These Leydig Cell Tumours of testis could be pure tumours or they could be mixed tumours with other sex cord–stromal or germ cell tumours. Leydig cell tumours usually have a local manifestation; metastases of Leydig Cell Tumours had been stated to occur in about 2.5% of cases. [1] [2] The commonest sites for metastases are lymph nodes, lung, liver, and bones. [1,3] It has been pointed out that as with germ cell tumours, the route of spread of Leydig cell tumour of testis is via hematogenous and lymphatic spread to the retroperitoneal lymph nodes. Unlike germ cell tumours; nevertheless, it has been pointed out that Leydig cell tumours of testis and scrotal contents do portend a relative lack of sensitivity to radiotherapy and chemotherapy agents. [1,4]
Clinical presentations of Leydig cell tumour of testis include the following: [1]
Diagnosis of Leydig cell tumour of testis is confirmed by the histopathology and immunohistochemistry staining study features of the testis tumour. In cases of Leydig Cell Tumour of testis, Serum testosterone levels usually tend to raised; nevertheless, serum oestradiol levels might also be increased, especially when feminization is evident in the tumour. The results of the ensuing laboratory studies tend to be normal in patients who have pure Leydig cell tumours of testis: [1]
Ultrasound scan of scrotal contents and testis is stated to be a radiology imaging option which confirms the diagnosis, especially when clinical examination findings of patients are equivocal [1,5,6] Magnetic resonance imaging (MRI) scan could demonstrate small nonpalpable Leydig cell tumours which are not visible upon ultrasound scan. Computed tomography (CT0 scanning of the abdomen and chest radiography is stated to be indicated when malignancy is suspected. Radical orchidectomy was previously the primary treatment for Leydig cell tumours of testis, and radical orchidectomy remains in use for malignant cases. Nevertheless; the prognosis is poor for metastatic Leydig Cell tumour of testis and there are no standard treatment recommendations. Even though complete or partial remission following chemotherapy had been reported, it has been found ineffective in the majority of cases. [1,3] Testis-sparing surgery with the undertaking of enucleation of the mass is stated to be increasingly reported in the scenario of benign cases of Leydig cell tumour of testis, in both the adult and paediatric populations. [1,7,8] [9]
It has been pointed out that when Leydig cell tumours of testis are diagnosed and treated early, testicle-sparing surgery had proven to be feasible and safe and could be regarded as first-line option of treatment. Nevertheless, because of the rarity of Leydig cell Tumour of testis, and non-availability of frozen section pathology examination facilities in many hospitals in the world, it would be envisaged that many cases of Leydig Cell tumours of the testis would continue to be diagnosed pursuant to the undertaking of radical orchidectomy based upon a provisional clinical diagnosis of malignant tumour of the testis. A high index of suspicion is required to establish a pre-operative diagnosis of Leydig Cell tumour of Testis. The ensuing article on Leydig Cell Tumour of testis and Scrotal contents has been divided into two parts: (A) Overview which has discussed general miscellaneous aspects of Leydig Cell Tumour and (B) Miscellaneous Narrations and Discussions from Some Case Reports, Case Series, And Some Studies related to Benign and Malignant Leydig Cell Tumours of Testis and Epididymis.
To review and update the literature on Leydig Cell Tumour of Testis and Epididymis.
Internet data bases were searched. The search words that were used included: Leydig cell tumour of testis; Testicular Leydig cell tumour; Leydig cell tumour of testes; Intra-scrotal Leydig cell tumour; Leydig cell tumour of epididymis; and epididymal Leydig cell tumour. Seventy-one (71) references were identified which were used to write the article which has been divided into two parts: (A) Overview which has discussed miscellaneous general aspects of Leydig Cell Tumour and (B) Miscellaneous Narrations and Discussions from some case reports, case series and some studies related to Leydig tumour of testis and epididymis in human beings.
Definition / General Statements Related to Leydig Cell Tumour of Testis
The ensuing generation statements had been made related to Leydig Cell Tumour of testis: [10]
Essential features
The essential features of Leydig Cell Tumour of testis had been summated as follows: [10]
Terminology
Epidemiology
The epidemiology of Leydig Cell Tumour of testis has been summated as follows: [10]
Sites
The sites within the testis that tend to be afflicted by Leydig Cell Tumour of testis include the following: [10]
Pathophysiology
The pathophysiology of Leydig Cell Tumour of testis has been summated as follows: [10]
Aetiology
The ensuing iterations had been made regarding the aetiology of Leydig Cell Tumour of Testis. [10]
Clinical features
The clinical manifestations of Leydig Cell Tumour of testis had been summated as follows: [10]
Diagnosis [10]
Laboratory tests
Prognostic factors
The prognostic factors of Leydig Cell Tumours of the testis had been summated as follows: [10]
Treatment
The treatment of Leydig Cell Tumour of the testis has been summarized as follows: [10]
Gross macroscopy Examination Features.
The macroscopy examination features of Leydig Cell Tumour of the Testis, had been summated as follows: [10]
Frozen section description of Leydig Cell Tumour of Testis
The frozen section examination features of Leydig Cell Tumour of Testis had been summarized as follows: [10]
Microscopy (histopathology) examination features of Leydig Cell Tumours of Testis description
The microscopy (histopathology) examination features of Leydig Cell Tumours of Testis had been summarized as follows: [10]
Microscopy histopathology examination of Leydig Cell Tumour of Testis does demonstrate the ensuing patterns:
Cytology examination features of Leydig Cell Tumour of the Testis had been summarized as follows: [10]
Cytology description
The cytopathology examination features of Leydig tumour of the testis had been summarized as follows: [10]
Positive Immunohistochemistry stains
It has been iterated that Leydig Cell Tumours of the Testis Cells do exhibit positive immunohistochemistry staining for the following markers: [10]
Negative Immunohistochemistry stains
It has been iterated that Leydig Cell Tumours of the Testis Cells do exhibit positive immunohistochemistry staining for the following markers: [10]
Electron microscopy description
The electron microscopy examination features of Leydig Cell Tumour of Testis had been summarized as follows: [10]
The molecular and cytogenetics examination features of Leydig Cell Tumour of Testis had been summarized as follows: [10]
Differential diagnosis
The differential diagnoses of Leydig Cell Tumour of Testis had been summated as follows: [10]
Testicular tumour of adrenogenital syndrome or testicular adrenal rest tumours:
Leydig cell hyperplasia:
Granular cell tumour.:
Large cell calcifying Sertoli Cell Tumour. [10]
Malakoplakia: [10]
Seminoma: [10]
[B] Miscellaneous Narrations and Discussions from Some Scase Reports, Case Series, And Studies Related to Leydig Cell Tumours of Testis.
Ruf et al. [11] stated that Leydig-cell tumours (LCT) of the testis are poorly understood clinically. Ruf et al. [11] analysed the clinical characteristics of LCT in a large patient sample and compared their findings with corresponding data of germ-cell tumours (GCT). Ruf et al. [11] reported that in a sample of 208 patients who had been treated during between 1995 and 2017 in 33 institutions, the following characteristics were registered: age, manifesting symptoms, primary tumour size, testis-sparing surgery (TSS) or orchidectomy, malignancy, laterality, medical history, and outcome. Ruf et al. [11] reported that their data analysis included descriptive statistical methods and logistic regression analysis. Ruf et al. [11] summarized the results as follows:
Ruf et al. [11] made the ensuing conclusions:
Pozza et al. [19] undertook a study in order to understand their question regarding when should 'not so rare' Leydig cell tumours (LCTs) of the testis be suspected, diagnosed, and treated? Pozza et al. [19] iterated the ensuing summations related to their study:
With regards to the Study design, size, and duration, Pozza et al. [19] reported that a case-cohort study of consecutive patients who were diagnosed with LCTs over a 10-year period was prospectively enrolled from 2009 to 2018 and they were compared to matched cohorts of patients with seminomas or no testicular lesions that were screened in the same timeframe. Relating to the participants/materials, setting, methods, Pozza et al. [19] reported the following:
Pozza et al. [19] summated the main results of their study and the role of chance as follows:
Pozza et al [19] also stated the ensuing:
Abe et al. [37] reported a 33-year-old man who was referred to their hospital for male infertility with painless swelling of his left scrotal content. He underwent a left high orchidectomy was based upon a diagnosis of left testicular tumour. Histologically, the testicular mass was a Leydig cell tumour.
Shiraishi et al. [38] stated the following:
Shiraishi et al. [38] reported a case study of a Leydig cell tumour in a single testis manifesting as male infertility. Shiraishi et al. [38] reported A 38-year-old male who was referred to their hospital because of a tumour within his right testis. He had undergone left orchidectomy when he was one year old because of a testicular tumour. During an examination related to his infertility, he had ultrasound scan which had demonstrated a 1 cm tumour. His serum tumour markers were all within normal ranges. The results of his hormonal examination showed that the results of his serum luteinizing hormone (LH) 30.3 mIU/ml (1.5-12.4) and follicle stimulating hormone (FSH) 11.9 mIU/ml (1.7-8.6) were higher than the normal limits, but his serum total testosterone (total T) and oestradiol (E2) were within normal ranges. He underwent firstly, testicular tumour enucleation, and then testicular sperm extraction (TESE) was undertaken from a macroscopically normal site of the testis. Histopathology examination diagnosis of the testis tumour as a benign Leydig cell tumour encompassed by Leydig cell hyperplasia. For 12 months following his operation there had not been any recurrence of his testis tumour. Even though his high serum LH and FSH had persisted, his serum total T and E2 were within normal ranges.
Komai et al. [39] reported a case of Leydig cell tumour of the post-pubertal cryptorchid testis with the main manifestation of male infertility. Komai et al. [39] reported a 36-year-old man who had consulted another clinic and his semen analysis had demonstrated oligospermia. A solid mass was found palpable within his right inguinal undescended testis. He was referred to the hospital of Komai et al. [39] for treatment of the testicular tumour. He underwent a right inguinal orchidectomy and pathology examination diagnosis was Leydig cell tumour with no malignant findings. After the surgery improvement was seen in his semen analysis. There was no evidence of recurrence of his tumour 9 months after surgery.
Madina Pérez et al. [40] reported a case of Leydig cell tumour in a cryptorchid testis. Medina Pérez et al. [40] reported a 55-year-old man who had presented with no specific scrotal symptoms. A cryptorchid testis was found upon his clinical examination and the patient underwent orchidectomy. During his operation, a solid, well-circumscribed, round nodule of 0.8 cm in diameter was found in an atrophic testis. Histopathology examination of the tumour showed a Leydig cell tumour with crystals of Reinke and immunostaining with vimentin.
Efthimiou et al. [41] 72-year -old man who had manifested with a 2-month history of painless left testicular enlargement. In the past, he had had undergone orchidopexy of the contralateral testis for cryptorchidism. His clinical examination demonstrated an irregular hard swollen left testis and a small right one. He did not have any gynecomastia. The results of his serum tumour markers (Alpha-fetoprotein, human chorionic gonadotropin, and lactate dehydrogenase) were negative for malignancy. He had ultrasound scan of testes which demonstrated an 11 cm × 6-cm nonhomogeneous testicular mass with multiple hypoechoic nodules. Metastases were not evident in the staging investigations. He underwent a left radical orchidectomy. Histopathology of the specimen demonstrated malignant LCT. Immunohistochemistry was positive for inhibin A, and Ki-67, and immunohistochemistry staining studies of the tumour had shown that the tumour was negative for pancytokeratin, cytokeratins AE1/AE3, cytokeratins 8/18, epithelial membrane antigen, carcinoembryonic antigen, alpha-fetoprotein, human chorionic gonadotropin, vimentin, CD30, and actin. His post-operative hormone profile demonstrated hyper-gonadotropic hypogonadism. The patient was placed on testosterone substitution treatment and retroperitoneal lymph node dissection was suggested, but he refused to undergo any further operation.
Michalec et al. [42] stated the following:
Michalec et al. [42] reported the rare case of leydigioma in 71-year-old man with unilateral cryptorchidism. Michalec et al. [42] stated that only a few cases had been reported as arising from undescended testis.
Taguchi et al. [43] reported an 85-year-old man who had visited their hospital with a complaint of painless swelling of his right testis. He underwent a right high orchiectomy under the diagnosis of right testicular tumour. Histopathology examination of the right testicular tumour specimen confirmed the diagnosis of Leydig cell tumour. Taguchi et al. [43] stated that they had reviewed 86 cases of this tumour that had been previously reported in Japan and to their knowledge, their patient is the oldest one who had been treated in Japan.
Harada et al. [44] reported a 63-year-old man who had visited their hospital with a symptom of painless swelling of his left scrotum. He underwent left trans-inguinal high orchidectomy since he had ultrasound scan which had suggested a testicular tumour. Histologically, the testicular mass was a Leydig cell tumour of testis.
Sugimoto et al. [45] reported a 40-year-old man who was referred to their hospital with gynecomastia and painless swelling of his right scrotum. He had ultrasound scan which demonstrated 15 mm x 10 mm mass with low echogenicity within his right testis. He underwent right high orchidectomy. Histologically, Reinke's crystals and capsular invasion by tumour cells were found. The final diagnosis, the tumour was a malignant Leydig cell tumour of the testis.
Bertola et al. [46] stated that Leydig cell tumours (LTC) are uncommon neoplasms that arise from gonadal stroma which account for 1% to 3% of all testicular tumours. Bertola et al. [46] reported a case of LCT in a 36-year-old man who had been suffering from painful bilateral gynecomastia for one year. The results of his endocrine function tests showed decreased gonadotropin concentrations, and reduction of testosterone/oestradiol ratio. He had ultrasound scan which revealed a 10 mm to 12 mm hypoechoic area within his right testis, which was not evident upon his clinical examination. He underwent right orchidectomy and histological examination of the orchidectomy specimen confirmed the supposed existence of an LCT. After surgery, the gynecomastia had completely disappeared, and his hormonal alterations had returned to normal.
Dounis et al. [47] reported a man had manifested with left painful breast enlargement and impotence as his main complaints of a right cryptorchid young man. He underwent ultrasound scan which demonstrated a hypoechoic mass which was found in his right testis. He underwent right orchidectomy and post-operatively the increased level of his serum oestrogens and the decreased levels of testosterone were normalized 6 months following his orchidectomy, his gynecomastia had subsided considerably, and his impotence had improved quite satisfactorily.
De Jong et al. [48] reported one case of a bilateral testicular Leydig cell tumour in a man of 29 years old. They stated that there are few cases of such tumours which had been reported in the literature and that Gynecomastia forced the patient to consult his doctor. His hormonal profile was found to be practically normal; nevertheless, his serum oestradiol level was at the limit superior of normal range, and his serum testosterone level was at the limit inferior (lower range). His testicular palpation was normal. He had scrotal ultrasound scan which confirmed the diagnosis of testicular tumour. They recommended that scrotal ultrasound scan should be performed in every patient who has unexplained gynecomastia. There was no metastasis in the reported patient. Before the treatment, the patient’s sperm conservation was performed (his sperm was normal). The reported surgical treatment sequence for the patient who did not have a child was the next one as follows: 1. inguinal orchidectomy was undertaken at the side of bigger tumour. Histological diagnosis was the benign Leydig cell tumour; 2. one month later, an inguinal orchidotomy at the other side was undertaken and the palpable tumour of 9 mm was removed. The extemporaneous biopsy confirmed the same diagnosis as at the other side; 3. one year later, there is no evidence metastasis, and the woman of the reported patient became pregnant.
Carmignani et al. [9] undertook a long-term evaluation of conservative surgical treatment of benign Leydig cell tumour. Carmignani et al. [9] performed a multi-centre retrospective clinical study at 6 European centres. They examined case files of all patients who were diagnosed as having Leydig cell tumour and treated with conservative surgery. Patients underwent clinical examination, hormone and tumour marker assays, scrotal and abdominal ultrasound, chest x-ray, and an endocrinological examination. Carmignani et al. [9] summarized the results as follows:
From 1987 to 2006, 22 patients who had Leydig cell tumour had undergone conservative surgery. The mean patient age of the patients was 35 years and the ages of the patients had ranged between 5 years and 61 years. The mean follow-up of the patients was 47 months and the follow-up of the patients had ranged between 1 month and 230 months. No local recurrence or metastasis was found. The patients presented with symptoms as follows: a palpable testicular nodule in 3 patients, that amounted to 13.7% of the patients or a nodule which was diagnosed by ultrasound scan on 15 patients, that amounted to 68.2% of the patients, gynecomastia by 2 patients, which amounted to 9.1% of the patients, precocious pseudo-puberty by 1 patient, which amounted to 4.5% of the patients, or scrotal pain by 1 patient, which amounted to 4.5% of the patients. Three patients were monorchid after their undergoing of contralateral orchidectomy for inguinal hernia repair (1 patient, 28 years before surgery) and nonseminomatous germ cell tumour (2 patients, 1 month and 6 years before surgery). The diagnosis after frozen section biopsy examination of the testis specimen was Leydig cell tumour in 20 of 22 cases that amounted to 91.0% of the cases. The mean histological size of the nodule was 1.11 cm, and this had ranged from 0.5 cm to 2.5 cm. Preoperative serum FSH and LH levels were high in 4 patients. Serum tumour marker levels were normal before and after surgery. Follow-up was undertaken for all patients every 3 to 6 months with clinical examination, tumour marker levels assessments, scrotal and abdominal ultrasound scan, chest x-ray. Six patients which amounted to 27.3% of the patients underwent abdominal computerized tomography. Carmignani et al. [9] made the following conclusions:
Giannarini et al. [49] stated that even though majority of Leydig cell tumours are benign, radical orchidectomy at the time of publication of their article in 2007 was considered the standard therapy. Giannarini et al. [49] retrospectively analysed the long-term follow-up of a series of patients who had Leydig cell tumours who were electively treated with testis sparing surgery. Giannarini et al. [49] reported that between November 1990 and December 2005, 17 consecutive patients with Leydig cell tumours had undergone testis sparing surgery on an elective basis. The pre-operative evaluation of the patients included clinical examination, serum markers for germ cell tumours, scrotal ultrasound, abdominal computerized tomography, chest x-ray and hormonal profile if clinically required. Testis sparing surgery was undertaken through an inguinal approach with spermatic cord clamping. Frozen section examination was undertaken in all cases, which had revealed Leydig cell tumours. Follow-up of the patients consisted of clinical examination, scrotal ultrasound scan, abdominal computerized tomography and chest x-ray every 6 months for the first 2 years, then annually. Tumour recurrence and survival were evaluated. Giannarini et al. [49] summarized the results as follows:
Giannarini et al. [49] concluded that in patients with Leydig cell tumours testis sparing surgery with frozen section examination does provide an excellent long-term oncological outcome.
Vergho et al. [50] compared retrospectively the outcome of testis-sparing surgery (TSS) to radical orchiectomy (RO) in patients who had Leydig cell tumour (LCT). About the methods of their study, Vergho et al. [50] reported that between 1992 and 2008, 16 patients with LCT of the testis were identified. All but 1 tumour could be identified by ultrasonography. Alpha-fetoprotein and beta-human chorionic gonadotropin levels were normal in all patients. Eight patients had undergone RO and their mean age during their surgical operation was 42 years and their ages at the time of their surgery had ranged between 27 years and 61 years; the median tumour size was 12.9 mm and the tumour size had ranged between 10 mm and 25 mm. and the remaining 8 patients underwent TSS and their mean age at surgery was 34 years and their ages had ranged between 18 years and 49 years; the median tumour size was 8.6 mm and the tumour size had ranged between 4 mm 23 mm. Staging (abdominal computed tomography and chest x-ray or thoracic computed tomography) was negative in all patients. , Vergho et al. [50] summarized the results as follows:
Vergho et al. [50] concluded that in the medium term, TSS is a safe procedure in patients with LCT that measure less than 25 mm.
Laclergerie et al. [51] compared the oncological outcomes of testicle-sparing surgery (TSS) and radical orchiectomy (RO) in patients who had Leydig cell tumour (LCT) of the testis. Laclergerie et al. [51] undertook a multi-centre retrospective clinical study within 12 centres in France. All the patients who had histologically proven LCT were included and analysed according to treatment (organ-sparing surgery or radical orchiectomy). The patients had undergone preoperative clinical, biological and imaging assessment. Demographic, clinical, and pathological variables were collected at baseline and compared between the groups according to surgical treatment. Follow-up was calculated utilizing the reverse Kaplan-Meier estimation and was updated at the end of 2015. Laclergerie et al. [51] summarized the results as follows:
Laclergerie et al. [51] concluded that long-term follow-up had indicated that testicle-sparing surgery does not compromise relapse-free survival in the treatment of Leydig cell tumour of the testis.
Loeser et al. [52] compared retrospectively the outcome of testis-sparing surgery (TSS) to radical orchiectomy (RO) in patients who had Leydig cell tumour (LCT). Loeser et al. [52] reported that between 1992 and 2008, 16 patients who had LCT of the testis were identified. All but 1 tumour could be detected by ultrasound scan of scrotum and scrotal contents. Alpha-fetoprotein and beta-human chorionic gonadotropin levels were normal in all patients. Eight patients underwent RO and their mean age at surgery was 42 years and their ages had ranged between 27 years and 61 years; the median tumour size was 12.9 mm and the tumour size had ranged between 10 mm and 25 mm and the remaining 8 underwent TSS and their mean age at surgery 34 years as well as their ages had ranged between 18 years and 49 years; the median tumour size was 8.6 mm and the size of the tumour had ranged between 4 mm and 23 mm. Staging (abdominal computed tomography and chest x-ray or thoracic computed tomography) was negative in all patients. Loeser et al. [52] summarized the results as follows:
Canda et al. [53] reported their experience in performing testis sparing surgery (TSS) to treat sequential bilateral testicular tumours. Canda et al. [53] undertook TSS on two patients with bilateral sequential testicular tumours. Canda et al. [53] summarized the results as follows: A 43-year-old patient (Case 1) and a 33-year-old patient (Case 2) had previous inguinal orchidectomy for seminoma. The patients were diagnosed as having secondary testicular tumours within the contralateral testes on follow up. They were treated by means of TSS after frozen section analysis of the peritumoral testicular tissue. Pathology examination of the excised tumours demonstrated immature teratoma and Leydig cell tumour. Both patients were disease free without local recurrence and did not have erectile dysfunction, and thus did not require androgen replacement therapy after a follow up of 6 months and 44 months, respectively. Canda et al. [53] concluded that TSS after frozen section analysis appears to be a safe and feasible procedure that, in carefully selected cases, offers adequate cancer control, preserves sexual function, and provides psychological benefits.
Bozzini et al. [54] iterated that the gold standard treatment for Leydig cell tumours (LCTs) is still considered to be radical orchidectomy, but testis sparing surgery (TSS) in conjunction with intraoperative frozen section (FSE) had been recently attempted with promising results. Bozzini et al. [54] identified studies by searching electronic databases. A bibliographic search covering the period from January 1980 to December 2012 was conducted utilizing PubMed/MEDLINE and EMBASE database. Bozzini et al. [54] excluded studies if they were single case reports, meeting abstracts and conference proceedings. Bozzini et al. [54] stated that their analysis was based upon a total of 13 studies which had fulfilled the predefined inclusion criteria. A total of 247 participants were included in the 13 studies examined in their systematic review. 145 cases were treated with radical orchiectomy and 102 with TSS. In the radical surgery group, the follow-up had varied from 6 months to 249 months. In the TSS group, the follow-up had varied from 6 months to 192 months. Frozen section was undertaken in a total of 96 patients. Sensitivity was 87.5%. None of the patients treated with TSS had manifested a metastatic recurrence, while in patients treated with radical orchiectomy three patients had manifested with metastatic recurrence. Bozzini et al. [54] stated that in selected cases radical surgery appeared excessive and the potential for a shift to TSS as the standard management is gathering momentum. Bozzini et al. [54] made the following conclusions:
Kong et al. [55] stated the following:
Kong et al. [55] reported a patient with azoospermia, a testicular Leydig cell tumour (LCT), and elevated plasma testosterone levels. Kong et al. [55] described the diagnostic and therapeutic experience of this case, and their follow-up of the patient's clinical indicators and fertility status. Kong et al. [55] reported that the patient was diagnosed with azoospermia and a testicular LCT. The patient underwent testicular tumour excision and long-term follow-up. After 4 months of follow-up, the patient's semen examination index had significantly- improved and his wife became naturally pregnant. At 4 months of gestation, the foetus was delivered because of a ruptured amniotic cavity. Twenty-six months after tumour removal, the patient's sex hormone levels had completely returned to normal and spermatogenic function had partially recovered, but there was no natural pregnancy with his partner. Kong et al. [55] made the ensuing conclusions:
Luckie et al. [14] stated the following:
Luckie et al. [14] reported on 12 children with LCT within 3 institutions between 2000 and 2016. Luckie et al. [14] reported that the manifesting symptoms of LCT included precocious puberty, palpable testicular mass, and scrotal swelling. Radical orchidectomy was undertaken in 9 patients. Three patients were treated with enucleation. All patients were alive at their last follow-ups without evidence of local recurrence or metastasis.
Carvajal-Carmona et al. [15] stated the following:
Carvajal-Carmona et al. [15] investigated the role of FH mutations in predisposition to LCTs. Carvajal-Carmona et al. [15] tested for pathogenic effects of the N64T mutation and screened an additional 29 unselected adult LCTs for FH alterations. Carvajal-Carmona et al. [15] also tested these LCTs for mutations in two genes, the LH/choriogonadotropin receptor (LHCGR) and the guanine nucleotide-binding protein alpha (GNAS) that had been implicated in LCT tumorigenesis. Carvajal-Carmona et al. [15] summarized the results as follows:
Carvajal-Carmona et al. [15] concluded that their study had shown that some LCTs are caused by FH mutations and represented one of the first reports of germline mutations within any type of adult testicular tumour.
Huang et al. [16] stated the following:
Huang et al. [16] reported a case of primary epididymal LCT in a 41-year-old Chinese man. The patient manifested with right epididymal swelling for 3 months without endocrine manifestations, including gynaecomastia and decreased libido. He had ultrasound scan of his scrotal contents which demonstrated a mass that measured about 1.5 cm in diameter entirely within the cephalic region of his right epididymis. No abnormality was found within his bilateral testes. The patient underwent total mass resection without any post-operative treatment. Histological examination of the excised mass had demonstrated that the well-circumscribed tumour was separated by conspicuous hyalinised fibrous stroma; the tumour cells were noted to be large and polygonal with round nuclei and abundant eosinophilic cytoplasm. Immunohistochemistry staining studies of the tumour demonstrated that phenotypically, the tumour cells had expressed four markers of sex cord differentiation which included: calretinin, melanA, CD99 and inhibin. There was no recurrence at his 2-year follow-up. Huang et al. [16] also stated the ensuing:
Al-Agha and Axiotis [17] stated the following:
Rossato et al. [56] made the ensuing iterations:
Rossato et al. [56] undertook a retrospective study evaluating the clinical and histopathological characteristics of 13 patients who were surgically treated for testicular tumour and diagnosed to be afflicted by Leydig cell tumour (LCT). Rossato et al. [56] stated that it was possible to retrieve the archived paraffin embedded tumour together with neighbouring healthy testicular tissue of all subjects who were affected by LCT (12 benign and 1 malignant form), that were analysed for INSL-3 expression. Immunohistochemistry staining studies of the tumour sections of the 13 patients affected by LCT had demonstrated constitutive expression of INSL3 protein within all LCT, irrespective of the histological pattern of each LCT and with no significant differences of staining intensity between all tumours. Particularly, no gross differences were observed between the staining for INSL3 in the 12 benign LCTs and the only one showing malignant clinical behaviour. Rossato et al. [56] made the ensuing discussing iterations:
Pozza et al. [19] undertook a study which was aimed to answer when should 'not so rare' Leydig cell tumours (LCTs) of the testis be suspected, diagnosed, and treated. Pozza et al. [19] stated the following:
Pozza et al. [19] reported a case-cohort study of consecutive patients who were diagnosed with LCTs over a 10-year period who were prospectively enrolled from 2009 to 2018 and compared to matched cohorts of patients with seminomas or no testicular lesions screened in the same timeframe. Pozza et al. [19] reported that out of the 9949 inpatients and outpatients who had been referred for scrotal ultrasound scans, a total of 83 men with LCTs were included. Pozza et al. [19] reported that the enrolled subjects had undergone medical history and clinical examination and they were asked to undergo routine blood tests, hormone investigations (FSH, LH, total testosterone, oestradiol, inhibin B, sex hormone-binding globulin (SHBG), prolactin), and semen analysis. Patients who consented also underwent contrast-enhanced ultrasound, elastography, gadolinium-enhanced scrotal magnetic resonance imaging, and hCG stimulation test (5000 IU i.m.) with serum total testosterone and oestradiol measured at 0, 24, 48, and 72 hours. Pozza et al. [19] summarized the results as follows:
Kim et al. [21] analysed the clinical and pathological features of 40 Leydig cell tumours of the testis. The ages of the patients had ranged from 2 years to 90 with an average age of 46.5 years of age. The commonest initial presentation of the patients was testicular swelling, which was at times associated with gynecomastia; 15% of the patients had manifested because they had gynecomastia and they were found to have palpable testicular tumours. All three children were brought to the physician because of the finding that they had isosexual pseudo-precocity. The tumours, one of which was asynchronously bilateral, had ranged from 0.5 cm to 10.0 cm (with an average size of 3 cm) in greatest diameter. They were usually well circumscribed, however, in seven of them the margin with the adjacent testis was noted to be ill-defined. Upon microscopy examination the commonest pattern was that of diffuse sheets of neoplastic cells, but insular, trabecular, pseudo-tubular, and ribbon-like patterns were also found. The neoplastic cells were noted to be most often large and polygonal with abundant eosinophilic, slightly granular cytoplasm; occasionally the cytoplasm was found to be abundantly vacuolated. In eight of the tumours some of the cells were found to be spindle-shaped, and in six of the tumours some had scanty cytoplasm. Crystalloids of Reinke were found in 35% of the tumours. Conspicuous nuclear atypicality was present in 12 tumours and the mitotic rate had ranged from less than 1 to 32 per 10 high-power fields. Blood vessel invasion, lymphatic invasion, or both were noted in four tumours. Follow-up information of 2 months to 22 years, with an average follow-up of 4 years was available for 30 patients. Five of the patients died as a result of spread of their tumour. A comparison of the clinically malignant tumours with those that were associated with survival for 2 years or longer than 2-years post-operatively had revealed that the former occurred in older patients and they were accompanied by symptoms of shorter duration and an absence of endocrine presentations. The malignant tumours were larger, often they had an infiltrative margin and they had spread beyond the confines of the testis, they frequently exhibited blood vessel or lymphatic invasion, and had a greater degree of cellular atypia and necrosis and a higher mitotic rate than the benign tumours.
Fankhauser et al. [22] stated the following:
Fankhauser et al. [22] analysed published case series data on Leydig cell tumours. They assessed the association between clinicopathological variables and the presence of metastatic disease was assessed using regression analyses. Fankhauser et al. [22] summarized the results as follows
Fankhauser et al. [22] made the ensuing conclusions:
Mukhopadhyay et al. [57] reported A 6-year-old boy presented with precocious puberty (see figure 1). He had isotope scan, which had demonstrated that his bone age was greater than (>) 12 years and less than (<) 14 years. He had ultrasound scan which demonstrated a heterogeneous echogenic space-occupying lesions which had involved the whole of his left testis with many micro- and macrocalcification and increased vascularity. The volume of his left testis was 12 cc. He had hormonal assay which showed his serum luteinizing hormone (LH) levels was <0>

Figure 1: Gross picture of lobulated yellow well-circumscribed mass. Reproduced from: Reproduced from: [57] under Copyright: © 2017 Journal of Indian Association of Pediatric Surgeons. This is an open access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.

Figure 2: Polygonal cells with abundant eosinophilic cytoplasm, prominent nucleoli (x 400). Reproduced from: [57] under Copyright: © 2017 Journal of Indian Association of Pediatric Surgeons. This is an open access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.

Figure 3: Immunohistochemistry positivity for calretinin (x 400). Reproduced from: [57] under Copyright: © 2017 Journal of Indian Association of Pediatric Surgeons. This is an open access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Zeuschner et al. [58] stated that Gynecomastia is a common incidental finding in males which can be caused by a variety of benign or malignant diseases and that in rare cases, it results from Leydig cell tumours, which is an uncommon clinical entity that accounts for 3% of all testicular tumours. Zeuschner et al. [58] also iterated that some of them are hormonally active but they rarely cause symptomatic endocrine disturbance. Zeuschner et al. [58] reported a 32-year-old man who had presented with gynecomastia which he had already been suffering from for the preceding two years. Even though he had been seen by three other specialists, including a urologist, none of them had identified the small mass in the upper pole of his right testis. Zeuschner et al. [58] decided to undertake testis-sparing surgery which confirmed the diagnosis of a hormonally active Leydig cell tumour. During follow-up, his hormonal status normalized, and his gynecomastia began to resolve.
Genov et al. [59] reported that in April 2019, a 45-year-old man was admitted to their Urology department with a large painless mass within his right testis of 1 year duration. The patient stated that one month preceding his admission, the lesion had commenced to grow. On his clinical examination, his right testis was found to measure 6.5 cm × 3.0 cm in size, with a palpable tumoral mass of about 3.5 cm × 2.0 cm in size, also the patient had a regular pulse of 78 beats/min, a temperature of 36.9 °C, as well as a respiratory rate of 18 -breaths per minute. No other signs were found, including gynecomastia or swelling of superficial lymph nodes. His penis and pubic hair were normally developed. The results of the patient’s laboratory blood tests such as complete blood cell count, renal function tests, liver function tests, and urinalysis were within normal ranges. The results of his serum tumour markers including: alpha-fetoprotein (AFP), β-human chorionic gonadotropin (β-hCG) and lactate dehydrogenase (LDH) were negative, and his hormonal investigations like serum testosterone, prolactin and follicle stimulating hormone (FSH) were within normal ranges. He underwent ultrasound scan assessment which revealed a mixed echogenic space occupying lesion which had involved half of his right testis with increased vascularity and some cystic areas. The patient underwent trans-inguinal radical high right orchidectomy, based upon a preliminary diagnosis of right testicular tumour and the specimen was submitted for histopathology examination. Postoperative pathology examination of the orchidectomy specimen showed that the tumour had cells in nets and trabeculae with chailinized and oedematous stroma, without haemorrhage and necrosis or vascular invasion. The tumour nuclei were noted to be monomorphic, oval-shaped with passing nucleoli, finely dispersed chromatin and no mitoses were found (see figure 5). The spermatic cord, scrotal skin, and surgical margins were free of any tumour. Immunohistochemistry staining studies of the tumour showed that the tumour cells had exhibited positive staining for inhibin and negative staining for pan-cytokeratin, calretinin and synaptophysin (see figure 6). Based upon the pathology and immunohistochemical examination features of the tumour, the testicular tumour mass was diagnosed as a benign Leydig cell tumour of testis

Figure 4: Histology image of Leydig cell tumour. Reproduced from: [59] under Copyright © 2019 The Authors. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)

Figure 4: Immunostaining image of Leydig cell tumour. Reproduced from: [59] under Copyright © 2019 The Authors. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Six months pursuant to his surgery, the follow-up CT-scan did not identify any local recurrence and distant metastases and his hormonal investigations had remained within the normal ranges.
Zhu et al. [60] stated the following:
Zhu et al. [60] highlighted the radiology-imaging phenotype, as well as the pathological findings of a case of LCT in a 62-year-old man. Zhu et al. [] reported that pre-operative non-contrast CT scan of the patient’s abdomen had revealed a 7.0 cm × 6.4 cm × 5.3 cm oval mass that had heterogeneous density, which was located within his right testis. He also had Pelvic non-contrast MRI scan which showed a heterogeneous mass on T1-weighted and T2-weighted images. The solid part of the tumour exhibited high signal on the diffusion-weighted imaging, and an obvious enhancement on the contrast-enhanced MR imaging. He had ultrasound scan examination which demonstrated a large mixed echogenic space occupying lesion which had involved the whole of his right testis with multiple cystic areas and increased vascularity. The patient underwent radical orchiectomy. The pathologic diagnosis was LCT. The patient underwent operative resection of the tumour. Due to the negative resection margins and absence of distant metastases, the patient did not receive additional radiotherapy or chemotherapy. Four months pursuant to his surgery, he had follow-up assessment CT-scan which did not reveal any local recurrence and distant metastases. Zhu et al. [60] made the ensuing additional iterations:
Justo et al. [61] reported a 91 years old man who had manifested with an increase of the volume of his scrotum for about 1 year, with local pain and hyperaemia over the preceding 7 months. He sought medical attention at the time and was treated with antibiotic therapy for epididymo-orchitis. When it was noted that his symptoms had persisted, he was then referred to the Urology outpatient clinic of the Santa Casa de Misericórdia de Ribeirão Preto. During his clinical examination, he was found to have an enlarged scrotum on the left with transillumination showing fluid, without hyperaemia. During the consultation, a scrotal ultrasound scan was requested. Upon his return, he produced an ultrasound scan report of hydrocele with fine debris within the left side, with a nodular, solid, rounded, partially defined, hypoechoic image with increased flow to the Doppler study that measured 2.0 cm x 1.4 cm x 1.1cm. The patient had complained of dysuria and polyuria, and Justo et al. [61] opted for treatment with antibiotic guided by urine culture and surgical treatment afterwards. He had a frontal chest radiography as a first radiology imaging procedure which demonstrated diffuse osteopenia and ectasia of the aorta. He had Computed tomography (CT) scan which had demonstrated a left renal cyst and infra-centimetric bilateral inguinal lymph nodes. After 3 months, he underwent a trans-inguinal left unilateral orchidectomy, with hydrocele repair. The surgical specimen was sent for histopathology examination. Macroscopic examination of the surgical specimen demonstrated a left testis which measured 5.8 cm x 2.9 cm x 2.7cm, with a smooth outer surface and cut with a yellowish, spongy parenchyma, containing a brown nodule, firm, well delimited and homogeneous, that measured 1.7 cm x 1.5cm, restricted to the parenchyma. Microscopy examination of the surgical specimen demonstrated features of a neoplasm which had consisted of cells with a hypertrophic nucleus, sometimes with evident nucleolus and broad and eosinophilic cytoplasm, all were contained within the testicular parenchyma, with no evidence of infiltration in testicular coating. Absence of invasion of vein and lymphatics. Epididymis and spermatic cord without evidence of neoplastic infiltration. The surgical resection margin of the spermatic cord was free of tumour. The pathology staging of the tumour was: pT1, pNx, pMx. Immunohistochemistry staining studies of the tumour showed that the tumour cells had exhibited positive staining for inhibin, calretinin, melan-A and KI-67. Diagnosis was reported to be consistent with the diagnosis of Leydig cell tumour. The patient returned for follow-up assessment 1 month after his operation, with the presence of hematoma within his scrotum, which was confirmed by ultrasound scan of his scrotum and scrotal contents. sonogram. Just et al. [61] opted for a conservative treatment. Justo et al. [61] made the ensuing educative detailed summating discussions:
Table 1
Scrotal Masses in elderly men.
Testicular
Primary lymphoma
Stromal tumours
Spermatocitic Seminoma
Metastasis
Epidermoid cyst
Leydig Cell Hyperplasia
Fibroma of gonadal origin
Haemangioma
Paratesticular
Lipomas
Adenomatoid Tumours
Leiomyomas
Testicular Appendage with Torsion
Fibrous Pseudo-tumour
Liposarcoma
Reproduced from: [61]
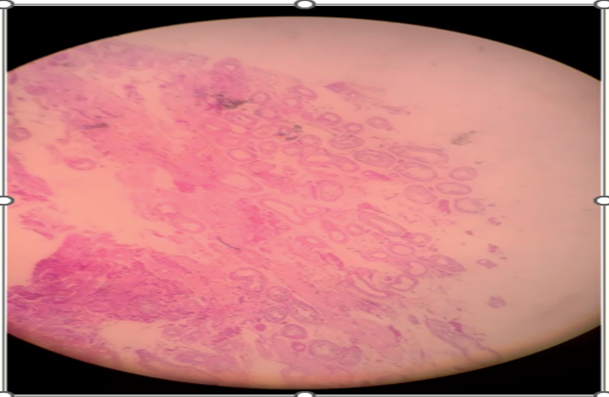
Figure 7: Epididymis (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 8: Seminiferous tubules (4x). Reproduced from [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 9: Rete testis and seminiferous tubules. (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 10: Inhibin antibody (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 11: Calretinin antibody (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 12: Melan-A antibody (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 13: Ki-67 antibody (4x). Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Figure 14: CD 117 antibody (4x), Reproduced from: [61] under Copyright: This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Conflict of interest – none
Acknowledgements –
Acknowledgements to:
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti