AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2640-1053/166
Retired in Hematology Department, Iran University of Medical Sciences, Tehran, Iran.
*Corresponding Author: Ahmad Reza Rahnemoon, Retired in Hematology Department, Iran University of Medical Sciences, Tehran, Iran.
Citation: Ahmad R. Rahnemoon, (2023), Leukemia initiating cells and their niche, J. Cancer Research and Cellular Therapeutics, 7(6); DOI:10.31579/2640-1053/166
Copyright: © 2023, Ahmad Reza Rahnemoon. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 07 November 2023 | Accepted: 15 November 2023 | Published: 23 November 2023
Keywords: leukemia initiating cell; leukemia stem cell; malignant hematopoietic microenvironment
Leukemia stem cells (LSCs) possess several key properties of normal cells including self -renewal, unlimited proliferative potential, infrequent or slow replication. Leukemia initiating cells (LICs) refer to all cells with leukemia- initiating potential and/or LSC capacity. Actually, our definition for leukemia initiating cells requires that those cells should be capable of self-renewal which resulting in the appearance of progeny that share the ability of self-renew as well.
Hematopoietic cells have an essential role in the niche for self- renewal and differentiation of HSCs in vivo and their hematopoietic microenvironment show to generate functional hematopoietic stem cells. Niches provide special support for cell viability or those are for specific cell populations, varied growth factors, extra cellular matrix components and cell adhesion molecules. On other words, when tissue damage occurs, for back to the common goal, niches are as a feedback system for communicating information in pass on the disease, namely try to go to the state of tissue back in the connect with stem cells which means understand and accept the position and try to solve the problem as well. In reality, HSCs provide homeostatic maintenance of the blood system through their ability to differentiate and generate the hundreds of millions of erythrocytes and leukocytes needed per day. All told, in the microenvironment and stem cell niche unit, HSC self- renewal, control in balance between HSCs self- renewal, their differentiation and maturation can be important as well [1-6].
LSCs infiltrate the bone marrow (BM) and interfere with the normal HSC microenvironment hemostasis. In reality, pre-leukemic/leukemic stem cell model proposes a non-genetic mechanism in leukemic process which harmonized with the genetic model associated with epigenetic deregulation, contribute to leukemia heterogeneity. In addition, LICs are different from LSCs which LICs are only defined by their abilities to start working of leukemia initially into new group better introducing but with or without self-renewal ability. (18-19)
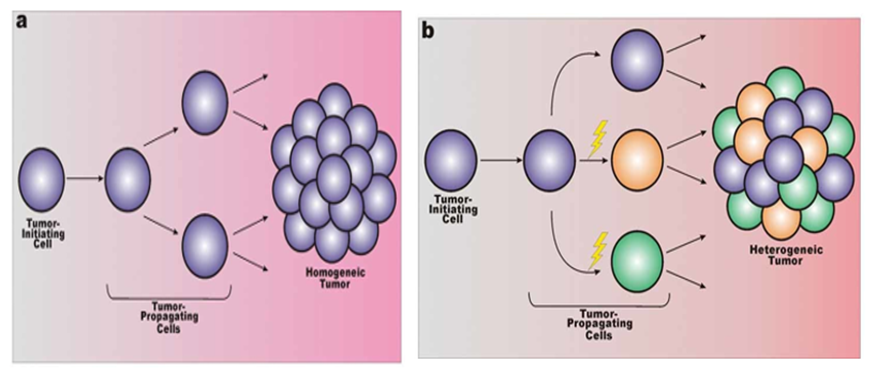
Figure 1: a) In the malignant cellular homogeneity, the tumor initiating cell and tumor propagating cell are the same entity and genetically approximately. b) In this model, some genetic aberrations can be happened, so any further potentiate malignancy leads to cellular heterogeneity. [1-2,17]
We know acute myeloid leukemia (AML) leukemia initiating cells reside within the CD34+ CD38- compartment suggested that AML HSCs are rare cells which resemble normal HSCs sharing a common limited immune-phenotype with rare population. In fact, the origin cell of any myeloid malignancy is dictated by the specific genetic and epigenetic changes together which can go to severe alterations. Moreover, in this way, the marrow microenvironment retains the ability to supply the growing tumor (tumor initiation) with malignant hematopoietic progenitors that go subsequently to more tumor propagating cells and to acute leukemia as well (figure 2).

Figure 2: The distinction between cancer stem cells (CSCs) and cancer-initiating cells: Cancer-initiating cell (blue color) undergoes oncogenic transformation in order to develop a tumor, or CSCs is not necessarily the transformed tissue-specific stem cell, but rather gives rise to the malignancy.
Based on the facts, LICs may be defined for specific genetically like translocation, etc. but sometimes demonstrated with normal karyotype or without any genetic alterations as well. On the other hand, we know that the prenatal origin has been proven for some fusion genes such as ETV6/RUNX1, BCR/ABL1, TCF3/PBX1 (E2A/PBX1), hyper-diploidy and KMT2A are the most translocations or aberrations which can occur in some children but the question is: why a part of carriers develops to ALL? In response, infection and delayed infection can be as a possible cause for this leukemic transformation. We know HSC niche provides essential micro-environmental cues for the production and maintenance of HSC in the BM as during the inflammation hematopoietic dynamics which can be perturbed. In this way, notably the mixing of populations has been postulated that can be as a causal factor in the appearance of leukemic transformation after HSC- niche microenvironment deteriorating which means the role of infection can be exposed as a trigger in start of LICs activation in the pathway and in progression from pre-leukemia to leukemia as well.[3-9,17-19]
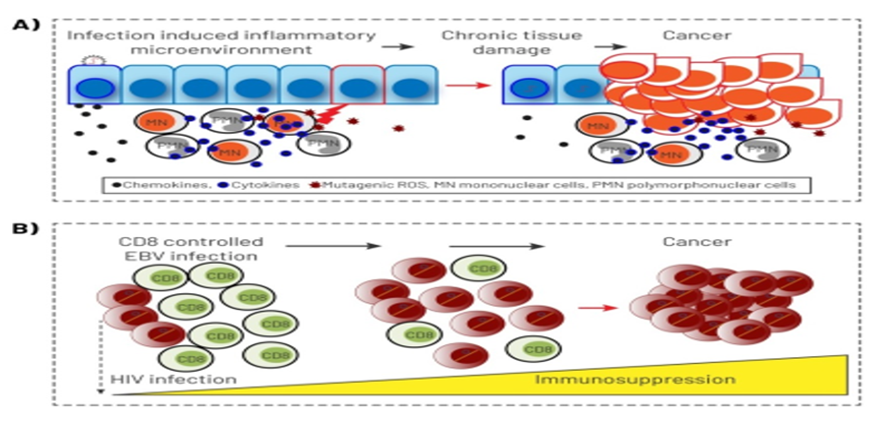
Figure 3: Infection provides a suitable model that can directly perturbs cells of the hematopoietic system which affect undifferentiated stem cell and other progenitor cells (cancer world media).
As we know blood cell production is not static and the BM has evolved to sense and respond to infection that has deleterious effects injuring HSC, inefficient hematopoiesis, and eventually can go to remodeling (malignant environment potential) which resulting is in the destruction of hematopoietic microenvironment as well (the microenvironment remodel). In this regard, notably some studies demonstrating that the stromal compartment of the BM is highly heterogeneous and suggesting that particular components of the microenvironment provide unique niches supporting some lineages differentiation too (populations mixing). The other studies stated some cases such as B cell infection with EBV that is latent, and the virus does not undergo replication. These latently infected B cells can then go on to produce proteins which function to promote cellular growth through normal signaling pathways modification and changing as well. Then they can promote into LIC appearance with the abnormality of niche and go to malignant B cells via proteins that limit apoptosis in cells which had the c-MYC translocation (figure 3). Apoptosis is limited by EBV through various means like the EBNA1 protein, BHF1 protein, EBER transcripts, vIL-10, BZLF1, and LMP1. It is mentionable that the dynamic interplay among leukemic cells and their microenvironment, also plays a necessary role in the development of the malignant B-cells. Hence if the LIC is the apex of the leukemic hierarchy, LSCs can be isolated from the reminder of malignant hematopoietic cells based on specific cell surface markers. [3-6,10-15]
After the producing of induced HSCs or HPCs, so there may be more than one way to reprogram cells in the hematopoietic lineage which means LIC can appear in the position possibly and so it will be a next way for understanding of LSCs biology in leukemia therapy. In this pathway, these cells notably play the essential role for self- renewal and differentiation of HSCs in vivo and hematopoietic microenvironment show to generate functional hematopoietic stem cells as well. That`s why, if undesirable position happened, then a subpopulation of HSCs are likely to leave their current niche and explore larger regions of BM space, hence in this condition (like some infections, figure 3) HSCs migration may benefit for an individual by corresponding to a simple bet-hedging strategy which HSCs enable to move from deteriorating niches to other more supportive niches for find a better position, so it is a robust strategy. For example, if the signals passed among the stem cells and the niche, now be blocked, thus stem cells must choose to either cling on or go in search to find a better microenvironment, otherwise if could not, so gradually go to ruined microenvironment and to malignant position possibly. Now, here, the role of LIC is prominent because all agents can help to destroy the micro-environment generally. Therefore, after the cells are perturbed at the hematopoietic system, may be directly effect on undifferentiated stem cell and other progenitor cells which lead to upset and unexpected results in these cells [1,2,11-17] (figures 3&4).
Here, the role of LICs can be embossed because firstly, many of random mutations that occur in hematopoietic cells affect non-critical genes (passenger genes) providing no growth advantage to the stem cells, now if bad luck happens when a mutation occurs in a gene that provides a proliferative advantage to the hematopoietic cells which the cells resulting clone has a greater chance to acquire mutations additionally that might evolve to leukemia frankly (figures 1&2). Secondly, in animal models, leukemia is initiated by a rare population of cells called LICs. Meanwhile, some groups have shown that LSC is able to differentiation into some different lineages which the ability of cells to trans-differentiation likely has little relevance with regard to the malignancy (figures 3&4) which describe the plastic nature of these stem cells and their ability to generate the heterogeneous cellular population that demonstrate some minor sub-populations as well. [10-15,18,19]

Figure 4: Peripheral blood film of a patient with CML with thrombocytosis and basophilia (L García Alonso, GECH Atlas).
In better understanding, some examples are necessary as follows: 1) myeloproliferative neoplasms (MPNs) are clonal disorders of hematopoiesis that lead to increase one or more mature blood cell progeny (populations mixing) which a minority of cases of myelodysplastic syndrome (MDS) can fit with MPN in being associated with increased numbers of mature cell progeny but MDSs cases are with dysplasia and cytopenia as well. In better words, MPN is the stem cell disorder characterized by the involvement of all three major myeloid cell lineages, namely they are clonal disorders of pluripotent hematopoietic stem cell with varying degree the potential (leukemia initiating potential) that can transform into acute myeloid leukemia. Actually, during MPN, after LIC appearance, then the activation of LSC with overproduce of leukemic myeloid cells as well as secrete high levels of pro-inflammatory cytokines, which resulting is the development of MPN in the time limit. So, the results demonstrate that MPN development remodels the endosteal BM niche into self-reinforcing leukemic niche which impairs normal hematopoiesis, favor LSC function including leukemic myeloid cells that can stimulate MSCs to overproduce functionally altered OBCs which accumulate in the BM cavity as inflammatory myelofibrotic cells and then can go to BM fibrosis. [4,5,11,18]
2] Polycythemia Vera (PV) leads to excessive of erythroid, myeloid and megakaryocytic elements within the bone marrow (populations mixing) with leukemia initiating potential that the cells can go to MPN- blast phase because these data indicated the existence of malignant and non- malignant populations of hematopoietic progenitor cells (HPCs) in BM of PV which including a normally EPO-responsive population and a population of cells similar in proliferative and maturational behavior in vitro with no EPO as well ( autonomous population with leukemia initiating potential). In this case, how single cell is subverted and can change to neoplasm and then to drive into leukemia? In fact, the power of single cell biological approaches to uncovering novel molecular networks in heterogenous cell population, that`s why LIC is at the apex of the leukemic hierarchy, namely although LIC and LSC being used interchangeably for leukemia, but their concepts are not the same, or in better words, LIC more appropriately denote the leukemic cells of origin, whereas LSCs are the subpopulation with the self-renewal capacity and long term clonal maintenance in the later stage. As we know PV is a clonal and chronic with driver mutations affecting JAK2. In this case, we cannot forget the important role of hematopoietic microenvironment on the neoplastic cells with or without JAK2 as an essential and original cause in PV existence.
Generally, in leukemia, LICs divide asymmetrically resulting in LIC self-renewal and production of a daughter cell known as a transient amplifying cell (transgenic model) which these cells is not thought to possess self-renewing capabilities but instead divides to contributes to leukemia development indefinitely, so in the case the structure of hematopoietic microenvironment is ruined. For example, in CML, transgenic models demonstrated that BCR/ABL1 expressing in the myeloid progenitor cells are sufficient to drive to leukemic progression (the essential role of leukemic cells including committed stem cells, other progenitor cells into maturation cells) even if BCR/ABL1 be absent in the HSCs (the important role of malignant hematopoietic microenvironment) (figure 4).
Also, in B-cell ALL with rearrangements of the mixed lineage leukemia gene is a highly aggressive and chemotherapy-resistant cancer in babies; LICs can restart it and cause relapse. [1,2,10-12,16-19]
The LIC process is including one cell that is capable of self-renewal and also, more differentiated daughter cell such as leukemia initiating cells in AML with CD34+ CD38-. Hence, the more differentiated progeny that are no longer able to undergo self-renewal after asymmetric division which often referred to as progenitor cells or transient amplifying cells that can be as the support tumor growth. Thus, LSCs are different from LICs which means LIC is only defined by their abilities to initiate leukemia, namely the cell or all cells with leukemia initiating potential and the carrying of leukemic power. It is mentionable LICs are formed and maintained at the leukemic niches, which in turn affect on stem-ness as well as lineage plasticity. On the other hand, in the process of leukemia progression, perhaps leukemic cells (LSCs) remain in contact with multiple micro-environment such as spleen, central nervous system (C.N.S) or thymus as well.