AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2692-9392/147
1College of Medicine, University of Florida.
*Corresponding Author: Brandon Lucke-Wold, Department of Neurosurgery, University of Florida, Gainesville.
Citation: Elizabeth Klaas, Shahd Mohamed, Jordan Poe, Ramya Reddy, Abeer Dagra and Brandon Lucke-Wold, (2022) Innovative Approaches for Breast Cancer Metastasis to the Brain. J. Archives of Medical Case Reports and Case Study, Doi:6(4); DOI:10.31579/2692-9392/147
Copyright: © 2022 Brandon Lucke-Wold, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 22 August 2022 | Accepted: 03 September 2022 | Published: 22 September 2022
Keywords: breast cancer; brain metastasis; emerging treatments; surgery; pre-clinical data
Breast cancer metastasis is a continued concern for patients with recent development in our understanding of disease progression. In this paper, we highlight the pathophysiology behind breast cancer metastasis. Blood brain barrier disruption plays a critical component in progression. We then investigate the current treatment strategies and recommended guidelines. This focuses on radiation and medical management. Finally, we address the role of surgical intervention. The data is organized into tables and figures to highlight key components. Finally, we address emerging treatments and pre-clinical data. The paper will serve as a user-friendly guide for clinicians and researchers to help formulate a strategy to manage breast cancer metastasis patients sufficiently.
Breast cancer is among the most commonly diagnosed cancers globally, and 10-15% of women with stage IV breast cancer are estimated to have metastasis to the brain [1]. Younger age at the time of breast cancer diagnosis, tumor size, nodal involvement, histological grade, and aggressive breast cancer subtypes, including human epidermal growth factor receptor 2 (HER 2) positive breast cancer and triple negative breast cancer, are risk factors associated with breast cancer metastasis to the brain [2,3].
Brain metastasis is a multi-step, highly dynamic process, and many aspects of the pathophysiology of breast cancer metastasis to the brain are still unknown. Similar to breast cancer metastasis to other sites, including bone, liver, and lungs, a series of key steps are involved including local invasion from the primary tumor, intravasation, and survival of tumor cells in circulation to the escape of cells from the circulatory system, extravasation, and formation of metastatic lesion following adaptation to the local microenvironment [4].
However, the brain blood barrier (BBB), a selective diffusion barrier characterized by non-fenestrated epithelium and tight junctions, is a distinct feature of brain metastasis. Key molecular features that mediate extravasation across BBB play a key role in metastasis. Cyclooxygenase 2, α2,6-sialyltransferase ST6GALNAC5, and the epidermal growth factor (EGFR) ligand HBEGF have been identified as mediators of extravasation as well as VEGF and CXCR4/CXCL12 [4,5]. Tumor cells also interact with supporting cells in the CNS microenvironment, including glial cells, microglia, and astrocytes, and activate pathways that create a favorable microenvironment for colonization [6].
Although advances in breast cancer diagnosis and treatment have increased life expectancy among patients, the incidence of breast cancer brain metastasis (BCBM) continues to rise resulting in poorer prognosis, lower quality of life, and shorter survival. A multidisciplinary, individualized approach that involves systemic and local treatment options or a combination of therapies is necessary. However, limited penetration of therapeutic agents through the blood brain barrier further complicates treatment [7].
Surgery, stereotactic radiosurgery, and whole brain radiotherapy are current mainstays in local management of intracranial disease. Systemic treatments may include targeted therapy, immunotherapy, and chemotherapy. Hormone therapy may also be used to treat hormone receptor positive breast cancer metastasis [8].

Figure 1: Breast cancer may metastasize to other organs mostly via hematogenous spread. Metastasis to brain require intravasation to blood vessels and extravasation through the BBB mediated by various molecular markers. [Adapted from “Breast Cancer to Brain Metastasis”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates]
Current treatment modalities
Breast cancer accounts for at least 30% of cancers in females, occurring in approximately 12.8% of the female population, or about 1 in every 8 women [9]. In late stages of the disease, tumors can metastasize in the lungs, liver, bone, and brain. Breast cancer brain metastasis (BCBM) is the most fatal of metastatic breast cancer and occurs in between 10-30% of metastatic breast cancer cases [6,10]. Breast cancer metastasis detection largely relies on clinical manifestations of disease spreading to other organs, including biopsies, imaging, and serum tumor markers. Earlier diagnosis usually gives a better survival rate for the patient as the disease can be treated faster, but current diagnostic tools are unable to detect the earliest stage of metastasis- when tumor cells are circulating in the bloodstream [10]. This makes predictions of disease progression difficult and can affect the outcome of the patient’s survival. Once lesions are detected, current therapeutic options are largely limited to chemotherapy, radiation therapy, or surgical intervention. However, these interventions leave a lot to be desired in terms of increasing life expectancy in patients with brain metastases due to the limiting blood-brain barrier that prevents chemical agents from reaching the brain and increases resistance to treatment.
Radiation
Radiation therapy works by radiating the DNA of tumor cells, breaking the DNA, and preventing cell replication and further tumor progression and growth. These cells die and result in shrinking of tumor size [11]. Patients who receive radiation therapy may receive it in combination with surgery or chemotherapy, or as a standalone treatment. Radiation for brain metastases is typically given via external beam radiation therapy. This means there is an instrument that sits outside of the patient’s body and delivers a beam of radiation to the designated treatment area [6]. There are a few types of external radiation therapy, but the most commonly used are stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT).
There are three types of radiation beams that may be used, including photon, particle, and electron beam therapy [11]. Photon beam radiation therapy is the same type of radiation that is used when x-ray imaging is taken but at a higher concentration. This type of radiation is beneficial for its ability to reach deeper in the body, but also has higher potential for damaging healthy tissue surrounding the tumors. Both particle beams and electron beams are separate units of energy that are released in a stream of high-energy particles. These beams can travel deep into the body and release the radiation energy at a calculated distance. This results in delivering more precise radiation to tumors with minimal effect on normal tissue surrounding it. Both stereotactic radiosurgery and whole brain radiation therapy use photon beams to apply radiation from various angles to the metastases [11].
Patients with limited metastases in the brain can be treated with stereotactic radiosurgery, but it is standard practice if a patient has greater than 4 lesions then whole brain radiation therapy is recommended [12]. Whole brain radiation is used to deliver a uniform dose of radiation to the entire brain, with a typical minimum of at least 10 daily treatments [12].
Whole brain radiation therapy was first found to be efficacious in a study performed by Chao et al in the 1950s, who found that 63% of patients who received therapy had alleviated symptoms. They also found that there was no difference in response to the therapy for tumors stemming from radiosensitive tumors to radioresistant tumors, and that there was minimal toxicity and decreased morbidity associated with radiation therapy [13]. This study set the foundation for WBRT being implemented for treatment of brain metastases and becoming the gold standard therapy by the 1970s. Whole brain radiation therapy is currently used as a monotherapy for brain metastases, when best suited for the patient based on the number of lesions and location within the brain, or if SRS or surgery interventions are not sufficient [9]. One major drawback to this approach is that WBRT tends to have a higher risk for neurodegenerative effects, including decreased cognitive capabilities in patients [12].
Adverse effects of WBRT may be present in both the short term and the long term. Some acute toxicity effects include fatigue, decreased appetite, nausea, radiation-induced alopecia, and cerebral edema [8]. These symptoms may present within a few days after radiation therapy, but generally are self-limiting and resolve with minimal intervention or with the use of corticosteroids in the case of cerebral edema. Some delayed or long-term toxicities may include behavioral changes, memory loss, and other degenerative neurocognitive effects, which may resolve over time or may become permanent effects of radiation therapy. One study found that at one year post WBRT treatment, 48-89% of patients showed a decline in neurocognitive function [14]. Another study by Kocher et al showed that patients who receive WBRT after SRS or surgery have reduced relapse rate compared to an observed group of patients post SRS or surgery (59 to 27%). The risk of neurological decline must be considered while also considering that WBRT can reduce intracranial failure and neurological death by more than 15% [15].
Stereotactic radiosurgery uses targeted doses of high radiation to individually focus on each lesion. The center of each metastasis can receive up to twice the prescription dose given when given at a low isodose surface. This means that the center of the beam has the highest concentration of radiation and can be calculated to reach the center of a lesion to give the greatest dose possible while the outer edges receive a less concentrated amount. The benefit of using SRS is that normal brain tissue receives minimal radiation, up to 50% less than the prescribed dose. However, the greater number of lesions that are present in the brain also means more normal healthy tissue will be receiving radiation as well. This becomes a concern as healthy tissue may receive overlapping doses of radiation if the metastases are in close range of each other, compared to a single uniform dosage that is given with WBRT [12].
A study comparing dosimetry data in 5 patients with brain metastases who received radiation treatment showed that the biologically effective dose for SRS was around 3 times higher than for WBRT [12]. The average effective dose for normal tissue was lower in SRS (1.3-34.3%) than WBRT (<10>
Radiation may be used in combination with surgery to shrink the tumor to a reasonable size for surgical removal [11]. Regardless of the type of radiation therapy strategy that is used, this form of treatment has proven to be effective in treating brain metastases no matter the histology of the primary tumor and its radiosensitivity status [17]. This makes radiation therapy unique and especially useful in treating the disease, reducing symptoms caused by the tumors present in the brain, and increasing chances of survival as well as quality of life.
Chemotherapy
Chemotherapy is a longstanding systemic treatment for various cancers. While systemic therapy is not the initial choice for brain metastases, chemotherapy may be used if the primary tumors of the cancer are sensitive to it [16]. Another consideration of systemic treatment is the ability of the chemotherapeutic agent to cross the blood brain barrier (BBB). Both factors are important when contemplating chemotherapy as a treatment for brain metastases stemming from breast cancer.
The blood brain barrier is always a consideration when it comes to using chemical compounds as therapeutics in the brain. The BBB consists of 3 key components: a layer of endothelial cells, astrocytes, and pericytes. There are also tight junctions, upregulated by factors secreted by the astrocytes, embedded in the endothelial cell layer to create a diffusion barrier to prevent most substances from reaching the brain [18]. Pericytes compliment the endothelial cell layer, further regulating permeability of the BBB [19].
In brain metastases, it is possible that the tumors cause a degree of BBB breakdown (Figure 1), which can be detected via MRI imaging with contrast dye [16]. This breakdown occurs by breaking the tight junctions, making permeabilization of the endothelial layer possible [19]. While this disruption of the BBB allows for malignant cells to circulate in the vasculature of the brain to metastasize, it can also make it difficult to accurately predict drug concentrations that will pass the barrier to reach the tumors in the brain. Even some water-soluble drugs have been able to pass the BBB when normally they are incapable of doing so in a healthy person [16]. Some of these compounds include cisplatin and etoposide, which have been proven to be effective against metastases stemming from breast cancer [13]. In some cases where the drug may not be able to pass the BBB, a catheter may be surgically inserted for direct delivery to the brain [7].
Once the drug passes the BBB, it must still reach the tumor site to be effective. Transmembrane efflux pumps and high interstitial fluid pressures will affect the drug’s ability to reach the target [20]. Some experimental approaches have been tested to directly deliver the therapeutic to the tumor by using stereotactically placed catheters and hydrostatic pressure.
Current chemotherapeutic agents include cytotoxic drugs such as anthracyclines, taxanes, and 5-fluorouracil [10]. However, recent studies by Von Hoff et al. have found anthracycline to be associated with cardiac dysfunction21. Recently in 2010, epothilones and ixabepilone were implemented as new cytotoxic agents that are efficacious against metastatic breast cancer in patients that previously received anthracyclines and taxanes [10]. Other cytotoxic agents that have been shown to have activity against brain metastases include high-dose intravenous methotrexate and temozolomide [22]. Breast cancer metastases are best treated when considering the molecular subtype of the primary tumor. More aggressive subtypes like HER2- or triple negative cancers will respond well to temozolomide or capecitabine. Less aggressive subtypes like luminal A or luminal B breast cancer are more likely to respond better with hormonal therapy, so cytotoxic agents may not be the best choice for treatment [20].
It is possible that using primary tumors to predict susceptibility of brain metastases to chemotherapy is not the most reliable method. This is due to the possibility of the metastases mutating to have significant genetic differences from the original tumor, potentially causing them to be resistant to chemotherapy [16]. Chemotherapeutic options are best chosen when considering the primary tumor, as BBB penetration is a lesser concern if it is disrupted. When considering the nature of the primary tumor, it is important to consider not only the type of cancer and how aggressive it is, but also what the microenvironment of the tumor is. The process of cancer metastasizing to the brain requires the primary tumor to invade local tissue, which leads to malignant cells circulating throughout the bloodstream to reach further sites in the body [23]. This means that other normal organ-specific tissues and cells in the brain, such as stromal and immune cells, interact with the cancerous cells, creating a tumor microenvironment. The microenvironment of the cancer malignancies is what regulates the tumor’s biology and therefore it’s susceptibility to specific therapeutics.
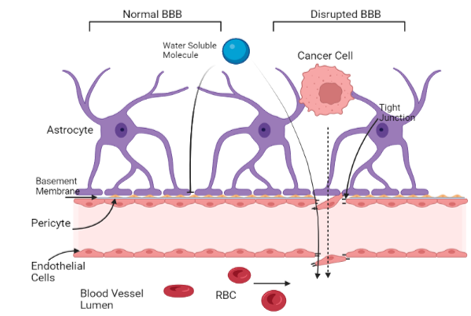
Figure 2. Malignant cancer cells alter the blood brain barrier to reach circulation in the vasculature of the brain. The disrupted BBB allows for more water-soluble molecules and cytotoxic agents to breach the barrier and reach the tumor site. [Adapted from “Breast Cancer to Brain Metastasis”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates]
Recent studies have also found that tumor-infiltrating lymphocytes can be used as a reliable and independent prediction of how the malignancies in the brain will respond to chemotherapy [23]. One study by Duchnowska et al. found that these lymphocytes were present in over 90% of breast cancer brain metastases [24]. This further strengthens the theory behind how cytotoxic agents are effective in the brain and can successfully treat metastatic tumors when the microenvironment of the primary tumor is considered.
Surgical Considerations
Surgical intervention is an approach used on patients that have few isolated and more easily accessible tumors. Tumors that produce clinical symptoms for the patient are also considered for surgical removal. Technological advances have minimized how invasive surgery may need to be to safely remove the tumor. Intraoperative imaging-guided neuro-navigation and brain mapping have allowed surgeons to safely remove tumors and resection where needed at even deeper and more delicate areas of the brain than previously possible.
In addition to imaging, advances in methods of tumor removal have advanced. Now, only a small burr hole through the skull is necessary instead of larger incisions, where a laser catheter is inserted for pinpoint stereotactic laser ablation or laser interstitial thermal therapy7. These techniques are useful for tumors that are not easily accessible or in patients who previously received radiation and have experienced toxicities associated with it. Other minimally invasive techniques involve a ‘keyhole’ approach, where the incision site is a fraction of what was previously used and only requires a small keyhole craniotomy20. These new advances minimize risk of exposure to infection, subsequently making recovery after surgery much faster with fewer side effects.
Surgery can be used as the primary treatment followed by other therapies or can be used after them if the tumor reaches a smaller size suitable for removal. When surgery is implemented following radiation therapy, patients are more likely to show improved symptoms and higher survival rates compared to patients who only receive radiation treatment. On the other hand, if surgical intervention is the primary treatment, patients will still need either radiation or chemotherapeutic treatment to eliminate potential remaining or circulating malignant cells [25]. A study performed by Patchell et al. showed that patients who received WBRT after initial surgical intervention had a significantly reduced recurrence rate from 46% to 10% [26].
Removal of the tumors also allows for better analysis of the genetic profile of the malignancies [20]. The histopathology of the malignancies may be studied and used to create a more personal therapeutic approach for the patient. This additional diagnostic method is beneficial since it may not only alleviate symptoms caused by the tumor, but it also provides insight into which therapy would be best suited for treatment on a molecular level.
The future of treatment for brain metastases from breast cancer is rapidly expanding in development of new approaches. Potential new pathways such as molecular targeted therapy, immune checkpoint therapy, or using novel targets for therapeutic drugs are closer to implementation with every clinical trial.

Emerging Approaches for Breast Cancer Metastasis to the Brain
Medical management of breast cancer brain metastasis (BCBM) is a challenging task and further compounded by the genetic heterogeneity of breast cancer (BC), which limits viable treatment options. The probability of developing a brain metastasis (BM) varies with the BC’s molecular expression pattern [27]. which is not always identical to cells of the metastatic tumor [27-29]. It is well established that patients with human epidermal growth factor receptor-2 positive (HER2+) and triple negative (TNBC) breast cancers are more likely to develop BCBMs compared to other subtypes [30]. Despite advances in our understanding of BC subtypes and their varying probabilities of metastasizing to the brain, current guidelines discourage MRI screening of asymptomatic BC patients for BMs [Lewin]. Screening for advanced disease is one of the earliest modifiable approaches to treating BC. There is a growing body of evidence that may support earlier BM imaging in patients with certain subtypes of BC, potentially at the time of their initial diagnosis if other metastases are present [3,31,32]. However, further evidence is needed to justify MRIs imaging based on tumor subtype, as MRIs are
expensive and expose patients to possibly unnecessary radiation.
Research has identified several potential biomarkers associated with BCBM, and their quantification may be a reliable indicator to assess the appropriateness of MRI imaging in BC patients with asymptomatic BM [33-35]. Beyond imaging, these biomarkers are increasingly being implicated in a wide array of diagnostic, prognostic, and therapeutic applications, constituting an exciting new realm of opportunity for the earlier detection and treatment of BCBM [36,37]. Circulating tumor cell (CTC)-specific nucleic acids, particularly single-stranded, noncoding RNA molecules known as microRNAs (miRNAs), have been the subject of several investigations looking for predictable molecular patterns in BC that could be used for earlier brain metastasis detection [38-40]. Other studies have examined differentially expressed genes between primary BC tumors and BCBMs; one bioinformatics study demonstrated critical involvement and prognostic value of the ANLN, BUB1, TTK, and SKA3 genes in the progression of BCBM [41]. A 2017 study found a long noncoding RNA (termed Lnc-BM) associated with enhanced progression of BCBM via overactivation of the tyrosine kinase JAK2 signaling pathway [36].
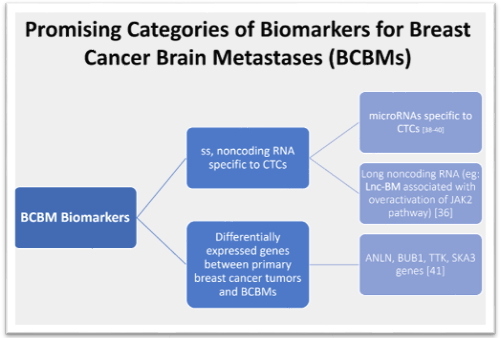
Figure 3: This figure shows the broad categories of potential biomarkers that are being further studied for therapeutic and prognostic use for BCBMs.
A long-standing barrier to treating BCBMs is the impermeability of the blood-brain barrier (BBB) to most drug therapies, which becomes the blood-tumor barrier (BTB) following metastasis [42]. The BBB acts as a physical and metabolic barrier between systemic circulation and the brain, and infiltration is a crucial step for circulating tumor cells to establish a metastatic lesion in the brain. This is a complex and multistep process that has been well described, although significant unknowns remain [43,44]. The previous belief that the BBB was completely impermeable led to the exclusion of BC patients with BMs from clinical trials for many years, resulting in a knowledge gap in the treatment of BCBMs. The composition of the BBB and later BTB have since been examined, and a 2016 study identified desmin-overexpressing pericytes and laminin alpha-2 as potential targets to alter permeability [45]. Some later studies of xenograft mouse models reported success in coupling chemotherapeutics with novel therapies aimed at manipulating the BTB’s permeability to increase the available concentration of the drug in the brain [46,47]. Other drugs designed specifically to penetrate the BBB have demonstrated promise in treating BCBMs [48]. Drugs aimed at preserving the integrity of the BBB by inhibiting its interactions with CTCs are a promising model for preventing metastasis to the brain altogether.
Discoveries about the BTB and differential expression patterns of BCBMs have resulted in numerous avenues to develop novel therapies, as well as improve upon pre-existing ones. The monoclonal antibody trastuzumab has been a longstanding treatment option for HER2+ metastatic breast cancer, but its capacity to cross the BBB alone is poor [49,50]. Antibody-drug conjugates like trastuzumab deruxtecan (T-DXd) have demonstrated increased efficacy and enhanced patient outcomes [51]. Other targeted therapy options for HER2+ BCBM have appeared over the years, far outpacing the number of available treatments for the TNBC BM subtype. Research studies examining the efficacy of novel therapies in patients with TNBC BM are urgently needed, as this subtype has the worst overall prognosis.
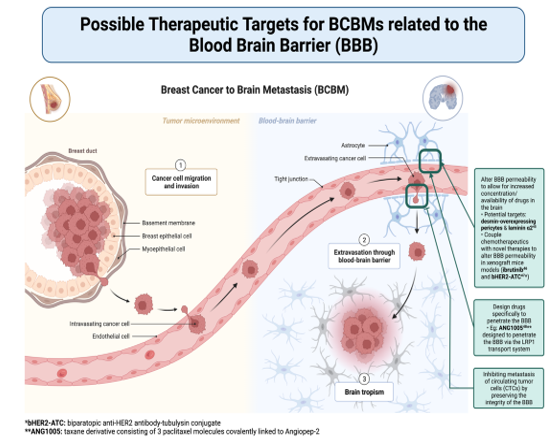
Figure 4: Figure 4. This figure shows a general overview schematic of how breast cancer can metastasize to the brain. It further highlights broad categories of therapeutic targets for BCBMs based on the role of the blood-brain barrier in metastasis. [Adapted from “Breast Cancer to Brain Metastasis”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates]
Breast cancer brain metastasis (BCBM) continues to contribute towards the poor prognosis for many patients with breast cancer. However, the further understanding and identification of the pathologic progression along with advancement in more-precisely targeted therapeutic modalities offer a gateway for improved outcomes in this population as well. Local therapies such as surgery and radiotherapy are becoming less invasive, which allows for improved outcomes by improving retention of cognitive function and hence patient quality of life. With new treatment trials seeking to examine the survival benefit in these individuals as well, immunotherapy and newer drug delivery systems (nanoparticles) may enable enhanced therapeutic efficacy. Numerous clinical trials are now underway and are anticipated to improve patient survival in the future for those who have BCBMs. Additionally, gathering more information on the receptor status and genomic profiling of the brain metastasis may be beneficial in identifying potential novel therapeutic targets for patients with treatment resistant BCBMs and would facilitate personalized therapy. Further research on various molecular processes, including as lncRNA, miRNA, and ctDNA, have recently been understood in the context of BCBM environment and are currently being explored to discover effective therapeutic targets as we understand the significance of the roles that they play in improving future diagnosis and treatment.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.
