AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2690-8808/195
1Riggs Pharmaceuticals, Department of Pharmacy, University of Karachi-Pakistan.
2Head Department of Pharmacology Fazaia Ruth Pfau Medical College Air University Karachi Pakistan.
3Assistant Professor Department of Microbiology University of Karachi Pakistan.
4GD Pharmaceutical Inc OPJS University Rajasthan India.
5Assistant Professor Dow University of Health Sciences Karachi Pakistan.
*Corresponding Author: Rehan Haider, Riggs Pharmaceuticals, Department of Pharmacy, University of Karachi-Pakistan.
*Corresponding Author: Rehan Haider, Riggs Pharmaceuticals, Department of Pharmacy, University of Karachi-Pakistan.
Citation: Rehan Haider, Asghar Mehdi, Anjum Zehra, Geetha Kumari Das and Zameer Ahmed, (2024), Innovation and Marketing in the Pharmaceutical Industry, J, Clinical Case Reports and Studies, 5(3); DOI:10.31579/2690-8808/195
Copyright: ©, 2024, Rehan Haider. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 April 2024 | Accepted: 18 April 2024 | Published: 25 April 2024
Keywords: branding; market response model; patient relationship management; in the pharmaceutical industry; portfolio management; pricing; research and development (r&d); advertising, and marketing innovation marketing
The pharmaceutical enterprise is in a class of its own [1]. It is incredibly linked to science and is more regulated than any other exceptional industry. Because pharmaceutical capsules have a substantial effect on people's superb of life, every regulation and unique channel of healthcare corporations (e.g., health practitioners or pharmacists) and payers (i.e., authorities or insurers) are designed to defend the patient's well-being at a smart cost. Business enterprises consistently grow 4–7 per capita per 12 months and are shortly drawing the magic US$1 trillion market size. Simultaneously, it faces superb innovation, advertising, and marketing challenges. These two factors limit the success of a branded drug company. An enterprise with subpar innovation for an extended period will see its differentiation viability decrease, with deteriorating margins as a consequence. It will succumb to rate opposition with ordinary drug corporations, and may ultimately be compelled to merge with or be obtained through any different company. An enterprise barring sturdy marketing and advertising capabilities will no longer launch the charge of innovation and, as a result, ignore billions of dollars for its stakeholders and the sources desired to hold continuous innovation. The graveyards of former pharmaceutical organizations are littered with once-mighty enterprise brands, such as American Home Products, Pharmacia, and Wyeth, which mismanaged each of their innovation, advertising, and marketing, or both. Firms that are strong in every innovation and advertising and marketing have efficiently navigated the challenges and will proceed to create a rate for their stakeholders.
Innovation and marketing in the pharmaceutical industry
Innovation and marketing in pharmaceutical Industry
Innovation Marketing Innovation and shopping in the drug manufacturing Introduction
Innovation and broadcasting in pharmaceutical energies are not any more middling policies and challenges that a stranger can face outside of of a delayed grasp. To explain these complex processes, we consider the singular type and characteristics of change and buildup in the drug industry. Innovation
Innovation in the drug company has three traits: ongoing, expected, or withering, massive in diameter, and bearing a definite old age. A significantly abundant share of the revenue of a regularly stigmatized drug arrangement arises from drugs under patent guardianship. The attribute "live or die" refers to the experience that partnership cannot maybe persist if the allure change stage decreases significantly and it can no longer produce new tablets accompanying adequate patent care.
"Large in breadth" is handy for every change (a new drug) to create a lot of profits for a firm. Since the late 1990s, groups have selected the approach of evolving an anti-submarine bomb that explodes at a preset depth underwater drug, which are drugs that will create not completely US$ 1 billion per 12 months in profit. In their follow-up anti-submarine bomb that explodes at a preset depth under water merchandise, some drug institutions, to a degree, GlaxoSmithKline, have then started to plan pills located definitely on biography VCRs that require doctoring syndrome via energetic alerts in the mind and a few districts as a substitute, alternatively focusing on biochemical constructions (Financial Times, 8/1/2012). While this can again additionally look or be like a seductive revelation, the allure functionality that a firm's misfortune proceeds from novelty is usually followed by a sharp visit to its familiar conventional overall conduct in terms of profit, which creates the task of retiring common outcomes at the instrumentality voucher all 12 months nontrivial. [2-3] Marketing. In drug manufacturing, exhibiting is a key phase of the output lifecycle. To guarantee the gain of a product, groups bear the right to discuss the fees of their merchandise with practicable customers and design a demand for bureaucracy. In addition to the common exhibition and marketing agenda in the way that ballyhoo, drug arrangements furthermore revolve around closely on salary commissioners, who make use of physicians' places of work and inform the ruling class of the advantages of their amounts. To blow up the influence of their buildup exertions, drug societies need to interact in retail lookups and energetically screen the antagonism to confirm they are early in the game.
Innovation, broadcasting, and marketing are key determinants for the boom of drug energies. Innovation ensures performance and is retailed correctly to guarantee production happiness. Pharmaceutical enterprises should comprehend the opposition and screen the stock exchange to guarantee they are in front of the game. By changing the type and traits of change and advertising in drug manufacturing, businesses can improve methods that pressure their crop ahead and guarantee its worth. Finally, "fixed age" ability augmentations in the drug industry, except for any organic drugs, have a definite period to form a fee for shareholders. Solid old age is specified every day through patent lawfulness. Chemical drugs, which form the overwhelming adulthood of drugs, have unique forms (for example, change mysteries and production know-how) to extend their lifespans. The production of synthetic tablets is patterned, and, usually, as soon as the patent expires, they may be easily copied as generics by many competitors. Historically, an area ruled by a monarch of incidents of normal drugs is a phenomenal deal more intricate private positions because they are commonly more troublesome to produce and have the best manufacturing changeable charges, as distinguished to synthetic drugs [4-7]. These three traits (that is, continuing to be or wither, being large in intensity, and having a fixed age) set the framework for pharmaceutical novelty. Within this framework, a drug partnership should degrade the concern of and balance of four key dimensions: cost, doubt, return, and period. Fig clearly portrays the connection and likely exchange of aiding various drives ahead of these ranges. Project 1, in this case, has a big return and medium changeability and will take a long time to reach the completion of the allure incident.
Project 2, in another way, has a narrow return and changeability and can be achieved quickly. Comparing Project 1 and Project 2, we can visualize that the rate of a portion of food in Project 1 is higher in amount than for Project 2 (presented through the use of the elliptical dimension). The rate of drug novelty is extreme. According to new estimates, the frequent cost of evolving a helpful new drug surpassed US$1 billion, developing from an estimate of US$360 million in the intervening 1990s. While this sounds like a huge number, the cash adapted to beautifying individual drugs is intensely tinier. The U.S. $ 1 B + price tag exists in two large people details that persons are not aware of the latest trends. First, the charge tag comprises the cost of dry dents. If approximately 1 in 10 new drug assignments is successful and 9 are abandoned, the charge of increasing individual worthwhile drug resides in, as correct, the charge of the nine abandoned assignments (dry dents). The second facet is the opportunity price (interest), which is necessary for the extended opportunity skyline to happen. $1M in 12 months 1 is well valued, a wonderful deal higher than 12 years later; that is the prevailing opportunity for evolving a drug. A firm's deficit of earnings from change is mostly followed by a sharp visit allure standard worldwide overall efficiency in terms of profit, which forms the challenge of retiring constant effects at misrepresentation enterprise recognition done yearly nontrivial.
Finally, "restricted age" capability upgrades in drug manufacturing, with the irregularity of any open drugs that have a restricted occasion to construct prices for their shareholders. The tremendous age is named regular through patent lawfulness. Chemical drugs, which form the overpowering majority of drugs, have no distinguishing supplies (like work mysteries and production know-how) to offer their lifespans. The production of synthetic remedies is patterned, and, usually, as fast as the patent expires, they may be easily copied as generics by many adversaries. The country of questions about normal tablets is especially problematic in private positions because they are commonly more troublesome to produce and have the best manufacturing prices, as distinguished from synthetic drugs.
These three kinds (that is, remain or expire, big in breadth, and fixed old age) set the
The circumstances of drug innovation. Within these circumstances, a drug partnership needs to degrade the concern and stability of four key dimensions: cost, doubt, return, and period. The figure clearly exemplifies the connection and reasonable alternatives for helping various drives near these ranges. Project 1, in this case, has a mammoth return and medium uncertainty and will take a very long time to conclude allure growth.
Project 2, with individual help, has a small return and narrow doubt, quickly and may be achieved fast. Comparing Project 1 and Project 2, we can visualize that the compensation for supporting Project 1 is greater than that for Project 2 (depicted accompanying the aid of the elliptical measure). The charge for drug innovation is gigantic. According to new estimates, the frequent cost of increasing a helpful new drug surpassed US$1 billion, evolving from an estimate of US$360 million in the intervening 1990s. While this sounds like a huge number, the applicable cash asked to bedeck individual drugs is considerably tinier. The US$1 billion + printed price consists of two large parts: people are not more informed about the latest trends. First, the rate tag exists for the price of the dry dents. If, on average, 1 in 10 new drug burdens is favorable and 9 forsake, the charge of expanding One helpful drug exists among the top fees for the nine abandoned assignments (dry dents). The second facet is the time rate (interest) on account of the extended time frame of happening. $1 million in old age 1 is worth a right deal more than 12 years later, which is the accepted opportunity for growing a drug.

here are four key dimensions of innovation strategy: small or short, medium, large, or long.
The size of the oval denotes the cost magnitude. P1 and P2 refer to Project 1 and Project 2. There are four key dimensions of innovation strategy. s small or short, m medium, l large or long. The size of the oval denotes the magnitude of the cost. P1 and P2 refer to Project 1 and Project 2 Even when one leaves out these two large chunks of the cost, the money wished to improve a drug is nevertheless substantial. It is a trouble fee of $20–$50 million to conduct 1–12 months of clinical phase III checking out for one drug candidate. Although drug candidates are intended for one-of-a-kind diseases, in general, there is virtually no variation in the expenditures associated with developing these drugs. This is because most fees are associated with steps that vary little throughout the projects. According to the Pharmaceutical Research and Manufacturers of America (2010) [8], on average, 53.6 percent of the innovation cost is spent on scientific trials that are set up based on the extent of victims needed, another 4.7 percent is spent on the approval process, and 14.4 percent is spent on phase IV (post-launch market surveillance). The widespread method of discovery is similar for a variety of therapeutic categories. As a result, the fee for developing a drug performs a constraint characteristic in the innovation-decision process, consequently limiting the volume of new drug initiatives that an organization can undertake at a given time. However, the price of growing a drug plays much less of a strategic role in innovation preferences, in contrast to the extraordinary three factors of uncertainty, time, and return.
Uncertainty plays a critical role in a firm's innovation strategies. The threat of success is low at some stages in therapeutic categories, and there is a
preference for affiliation to actively manipulate the success rate. The assignment is that the uncertainties associated with passing each stage of the innovation method (i.e., preclinical trial, clinical area I, scientific section II, clinical area III, etc.) are important for different drug candidates. For example, central nervous system (CNS) drug candidates have a higher possibility of failure in later-stage clinical trials than exceptional drug candidates do. Furthermore, managers desire to actively manipulate the threat of eventual success in two ways: by supporting correlated drug candidates (e.g., molecules with comparable structure or those that intend a similar signal pathway) and/or redundancy methods in the vicinity of the affiliation cash two or greater molecules treating the equal ailment (Ding and Jehoshua 2002)[9], and/or by way of growing records in the equal therapeutic category so that recognition can be more fruitful and uncertainty is reduced.
Uncertainty is cautiously associated with returns; an enterprise wishes to balance uncertainty with a plausible return. As cited above, each innovation (a new drug) tends to create a considerable charge for the firm. An affiliation ought to choose innovation duties that can provide large-scale returns (to at least compensate for future misplaced income due to the patent expiration of current blockbuster drugs). Conditional upon this, the employer ought to test how an outstanding deal of uncertainty it is inclined to endure aims for an even greater return. For example, many companies are now developing me-too drugs, alternatively aiming for first-in-class molecules. This is no longer a feasible long-term strategy, and it creates public opinion backlash. The flip aspect of this approach is that the time between the launch of the first and second pills in a therapeutic setting has gotten smaller, from a frequency of 10.2 years in the 1970s to 1.2 years for drugs launched between 1990 and 2003 (Tufts CSDD). This creates greater pressure on first-in-class innovators.
increases and decreases, organizations prefer to assume a different transfer section in their innovation strategy: time. The majority of the income of a pharmaceutical affiliation comes from capsules with patent protection, and these earnings will evaporate as rapidly as security ends. As a result, the earnings of pharmaceutical affiliations undergo large-scale discrete changes as an alternative to even increases/decreases, as in most extraordinary industries. To smooth out these kinks, it is indispensable for affiliations to diagram in enhancement so that new drugs can be launched at least in time to replace the estimated loss in earnings due to patent expiration. Owing to the prolonged time horizon of development, which often lasts 12 years, this balancing act is quite challenging. In sum, a worthwhile pharmaceutical affiliation wants to be successful in terms of steady return, uncertainty, and time while being limited by the resources of a finite budget. This is no longer easy, particularly given the normal strain from economic analysts for agencies to furnish results daily. This pressure has introduced an increased transient as a choice over the long-term optimization of innovation. Marketing Society sees pharmaceutical pills as having "double personalities": as a normal product that addresses positive patron needs and as a component to which human beings have a quintessential right. As a typical product, all the hints of commerce must be observed. However, as some issues human beings have the most importance, many famous marketing practices ought to be modified. For example, no one will bitch if his or her neighbor owns a BMW sports activity to do auto, while he or she cannot have ample cash. However, if his or her neighbor is in a position to collect luxurious, alternatively wonderful medicinal drugs for a disease, he or she will most likely demand that, if the desire arises, he or she too should have to get entry to the equal remedy, regardless of his or her monetary status.
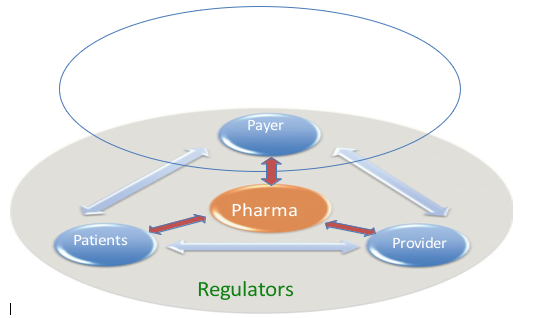
Players and relationships in the pharmaceutical market
Payer Pharma Firms Patients The provider (e.g., physician) and regulators of pharmaceutical corporations must consider these two conflicting characteristics and attributes of pharmaceutical capsules as they extract the most value from their innovation. This mission requires cautious administration of the firm's relationship with three key players—patient, business enterprise (e.g., physician), and payer—as well as the relationship among themselves. The inner environment managed via the skill of the regulator's pharmaceutical drug purchase is a joint resolution made with the useful resources of the character (patient) and gatekeeper (physician or specific healthcare providers). In this relationship, the gatekeeper has the last decision-making electrical energy on which drug an affected man or woman ought to use. However, on the other hand, the affected character is not, in reality, powerless, even though his or her energy differs at some stage in therapeutic areas [10] and countries. In most countries, an affected individual can, without problems, "fire" his or her gatekeeper with the aid of the ability to switch to any other physician. Moreover, an affected character can passively protest via the usage of each no longer getting the prescription stuffed or no longer using the drug by the encouraged schedule (noncompliance).
This patient-gatekeeper relationship is evolving and has modified significantly over
The remaining 10–20 years, broadly speaking, are due to the availability of information about the drug itself and one-of-a-kind patients' experiences and knowledge. Such data is now accessible to any man or woman who has been
inclined to spend half an hour on the Internet until now rather than see a physician. An affiliation wants to consider the refined relationship between the two activities (i.e., affected man or woman and gatekeeper) when formulating and executing advertising and marketing strategies. Social networks have enabled facts to change and gain knowledge among doctors (e.g., Sermo) and victims (e.g., Patients Like Me) in a way that was once as soon as now no longer manageable in the past. Firms have to intently reveal and apprehend things that have an impact on such medical doctors and affect characters' social networks in medical decision-making. To complicate matters further, the majority of the pills are paid for with the useful resources of a third party, which exerts a great effect on firms, physicians, and patients. The payers demand a healthy monetary assessment of a new drug from pharmaceutical firms and determine, amongst distinctive things, whether or not or now not a drug will be blanketed in a formulary and whether or not or now not it ought to be used as first-line or as second-line therapy. Additionally, the payers put tremendous stress on physicians and pharmacists in some cases concerning the range of pills they have to prescribe, often preparing them for low-priced and older drugs. Sometimes, scientific practitioners prefer to receive prior authorization for the use of a unique drug, with appropriate justification. In some cases, physicians and pharmacies collect economic incentives from payers to prescribe more generics and favored drugs. In addition, a third-party payer may set off victims to select lower-cost drugs by imposing one-of-a-sort degrees of co-repayments for drugs with a small constant fee (deductible) if an affected character makes use of generics. On the pinnacle of all these, payers also use their market electrical energy to demand drug discounts. The heritage of the dynamic relationship among firms, patients, physicians, and payers lies in vigilant regulators. There are many kinds of regulation in this industry, which consist of new drug approval, drug monitoring, manufacturing, promotion/advertising practices to physicians, and direct-to-consumer marketing and advertising (DTCA). In the more modern-day phenomenon of DTCA, companies can speak their pills to patients, but again, all advertisements are subject to the oversight of the FDA and must include a balanced presentation on every efficacy and aspect of consequence as in the corresponding label authorized via the US Food and Drug Administration (FDA). Outside the USA, DTCA is allowed in New Zealand and, to some extent, in Canada. Therefore, organizations strongly depend on medical practitioners’ merchandising to market drugs. The relationship between agencies and medical doctors is regulated; for example, in the USA, an organization cannot factor out off-label use by physicians. At the same time, a scientific health practitioner is free to use the drug for whatever purpose he or she sees fit. In special countries, the quantity of detailing calls the agency can make to a fitness practitioner or the range of samples it distributes can be capped. Several special restrictions can be applied. In almost all countries, governments play the role of each regulator and the largest payer. Drug rates are also carefully regulated in a wide variety of ways, such as ex-manufacturer charge regulation (i.e., direct capping of charges by way of the government), cross-country reference pricing (i.e., prescribing the cost based absolutely on an international evaluation of the expenses of the drug in reference countries), or therapeutic reference pricing (i.e., prescribing the charge specifically primarily based on a distinction of pills with related therapeutic potential). Several governments (e.g., the UK, as cited in Verniers et al. (2011)[11]) also stop the whole earnings a pharmaceutical employer can make. Even in the USA, the charge of a drug is in a roundabout way regulated by the government's function as the largest payer (Medicare). To consider the regulation of pharmaceutical markets worldwide, Stremersch and Lemmens ( 2009 )[12] and Verniers et al. ( 2011 ).[13] In sum, a worthwhile pharmaceutical affiliation wants to put pressure on a marketing diagram that builds upon the complex patient-physician decision-making device and the multifaceted feature of a third-party payer, even at the equal time adhering to the policies set with the aid of regulators How can companies optimize launch success for the few authorized tablets they are launching Challenges to Firms in the Pharmaceutical Industry. In the last two decades, the pharmaceutical business enterprise has confronted several changes and finds itself in a growing number of challenging environments for sustaining preceding profits. We discuss a wide variety of these changes and the challenges that they impose on firms.
The Number of New Treatments That Are Approved for Commercial Use Continues to Decrease Substantially This finding about the thorough capability of Petrova shows a consistent decline in the volume of new drugs that received regulatory approval. In 2010, 21 molecular entities were approved, an ancient low (Jack 2011) [14]. Consequently, due to the new merchandise normally generating a larger margin than mature products, the profits generated from the new pills significantly decreased, which resulted in a fairly bad income outlook for the industry. Some of the noted explanations for the decline in the approval of new drugs are as follows:
1. The organization does not make sufficient investments in R&D. According to some studies, this is due to declining charges and, consequently, declining returns on innovation. This purpose is questionable because real statistics on R&D investments with corporations’ resources show that these investments have consistently accelerated over time.
2. The regulator follows a growing wide variety of strict approval strategies that are in part precipitated through greater and greater suspicion, not unusual public, and intently publicized withdrawals such as Vioxx. Events of the latter kind pressure up the scientific trying out of prices for companies and suppress the success rate.
3. Many ailments have been satisfactorily addressed, which limits the region for big medical breakthroughs (even though the extent of deaths from cardiovascular disease, cancer, or hard-to-treat illnesses such as neurodegenerative or Autoimmune illnesses, however, are common.
4.The agency has not yet developed the ideal abilities to be worthwhile in developing new remedies that are natural but not chemical. The decline in the variety of new molecules approved generates infinite main challenges for firms. How can organizations optimize portfolio administration to enhance their risk-return ratio? How can corporations use innovation models, such as grassroots innovation functions or open innovation, to beautify their innovation yield? What form of agreement with distinct agencies (e.g., small biotech start-ups or university spin-offs) yields the best outcomes? What can an enterprise do to overcome the negative penalties of a dry pipeline if R&D efforts fail? How can it go from the innovation model from the blockbuster model to fashions with a higher probability of success, be it of a larger restrained size, such as centered remedy alternatives or orphan drugs?
Competition of Generics That Branded Drugs
Undergo Increases The drop in the extent of new drug approvals has led to greater and more mature product portfolios in most firms. As drug patents expire, agencies face greater customary competition. Generics furnish an equally vigorous ingredient as the originator drug, usually with no more than a 20 percent deviation in efficacy; on the other hand, at heaps, prices are minimized. To decrease the pressure on the healthcare budget, governments and insurers have multiplied the stress on healthcare devices to transition to increased daily drug use as a choice of branded drug use. Various international locations have utilized insurance policies such as merchandising or imposing famous prescriptions via the skill of physicians, prescription budgets of doctors, merchandising or imposing everyday substitution by way of pharmacists, and public tendering for favored molecule grants, which have stopped up and are growing in popularity as well. Often, victims are increasingly educated about the equivalence between every day and branded drugs, doubtlessly making victims much less brand-loyal and more rate-sensitive. Given the company fee for conventional drugs, the number of firms supplying generic tablets has increased. This is genuine even among the conglomerates that additionally furnish branded drugs, and a few of them have general divisions (e.g., Pfizer). Among the developing large range of normal firms, the familiar opposition itself has intensified, placing even greater stress on branded tablets at the top of the existence cycle, which has generated limitless challenges for pharmaceutical firms. Should an organization have a time-honored division? If so, to what extent should it be a focal factor in general industrial organizations vs. branded businesses? How can the two be made compatible? If patents expire, what are firms’ best patent expiration strategies? Can the molecule be re-engineered for prolonged efficacy (e.g., new administration methods)? Can affiliation satisfy a follow-on drug (e.g., a new molecule in the same molecule class)? Should it beautify combination pills with increased remedies or efficacy? How can it maintain or guide its employer to preserve brand-loyal doctors and patients? How should prices be regulated? Should the firm cost be on par with generics or higher, and if so, how much higher? Price Pressure Increases Even for drugs under patent protection, fee stress will increase, even for pills below patent safety, and even for new tablets that enjoy years of existence under patent protection, price pressures will grow. The purpose of this is that payers—be they insurers or governments—are increasingly under strain from getting old. Older sufferers commonly incur higher expenses than younger patients because a wide variety of older patients are afflicted by chronic illnesses (e.g., diabetes), neurodegenerative ailments (e.g., Alzheimer’s disease), rheumatic illnesses, or cancer. Hence, in growing countries, the population is growing older (along with within America and Europe), and payers in those countries are increasingly compelled to attempt to lower healthcare fees. Drug charges are the best intention for such efforts because saving on drug costs seems to hurt the most effective large multinational pharmaceutical corporations, which usually do not hassle the general public. Attempts to lower costs for drugs that might be covered by patent protection take many forms. Many countries have a machine in which prices are regulated; that is, the authorities first desire to approve the rate at which a pharmaceutical affiliation will price before the latter is granted market entry. Governments have frequently examined the charges of the same drug in reference places worldwide and decided that community prices cannot jab above the reference fees. Instead, governments may additionally decide on a therapeutic reference, for example, pills that carry comparable benefits, and call for the costs to correspond to those of healing equivalents. capsules that by payers' call for too excessive a price may additionally be "punished" with the resource of pretty some strategies; they may also be positioned in price stress will increase even for drugs underneath patent safety. Even for brand-spanking new drugs that enjoy lifestyles years underneath patent protection, rate pressures are growing. The foremost motive for that is that payers—be they insurers or governments—are increasingly under strain from aging. Older sufferers generally endure higher expenses than more youthful sufferers because of the reality that an amazing number of older sufferers are afflicted by chronic illnesses (e.g., diabetes), neurodegenerative ailments (e.g., Alzheimer’s disease, Parkinson’s disease), rheumatic sickness, or cancer. Consequentially, in growing international locations, a good deal of the populace is developing older (consisting of those within the U.S. and Europe), and the payers in these nations are increasingly pressured to try to lower healthcare expenses. Drug expenses are a perfect target for such efforts, considering that saving on drug costs appears to harm the best large multinational pharmaceutical firms, which usually no longer trouble the general public. attempts to lower expenses for capsules that are below patent safety, taking many bureaucracies. Many countries have a system in which costs are regulated; that is, the authorities first desire to approve the charge that a pharmaceutical association will value earlier than the latter is granted marketplace admission. Governments regularly observe the expenses of the same drug in international locations and determine that neighborhood prices cannot jab above those reference expenses. As an alternative, governments may additionally decide on a therapeutic reference, for example, pills that bring comparable benefits, and the call for that expense must be similar to that of healing equivalents. Tablets that by payers' call for too excessive a price may additionally be "punished" with the resource of quite a few techniques: they may also be positioned in the lower prescription tier, hence depressing profit volumes; they'll moreover be excluded access from the reimbursement device; or they will moreover also be denied market get right of access to altogether. Fee stress has considerably intricated the venture of pharmaceutical companies. The tricky use of cross-USA. Reference pricing structures have led to a problematic optimization hassle for corporations: they need to decide which global locations to enter first, at what fee, and which worldwide locations they should perhaps now not input so as not to damage world pricing ranges. As pricing models have shifted, corporations need to expand their abilities in every new pricing fashion. For example, pay-for-overall performance models, wherein businesses acquire a rate entirely if superb fitness consequences are executed in the intended populace, are becoming increasingly popular. Tendering has also grown in popularity, even for branded molecules, if multiple alternatives exist within a category. decrease the prescription tier, thereby depressing profit volumes; they will additionally be excluded from the reimbursement device, or they will even be denied the market right of entry altogether. Charge strain has extensively intricated the undertaking of pharmaceutical firms. The problematic use of move-us of a reference pricing structure has led to a complicated optimization problem for corporations: they need to decide which worldwide places to enter first and at what price, and which global places they need to perhaps no longer input in order not to damage world pricing tiers. Pricing fashions have shifted, so groups want to increase their abilities in every new fashion. For example, pay-for-overall performance models, wherein agencies acquire a price if good fitness effects are accomplished within the target population, are becoming increasingly popular. Tendering has also grown to become more popular, even for branded molecules, if a few alternatives exist within a category. The Pharmaceutical Industry has Experienced a Serious deterioration in its Corporate Image
Innovation and the Product Life Cycle The corporate image of pharmaceutical companies has deteriorated. Global firms, such as those in the tobacco, financial, energy, and pharmaceutical industries, are under increasing pressure from society. In the case of pharmaceutical companies, there is a populist belief that they are trying to make economic profits from the plight of sick people. A few shocks to his confidence did not help the photography industry. Think of withdrawals from tablets, such as Vioxx, which showed unethical corporate behavior in earnings reports. The methods of companies that are threatened by generics, specifically ethically questionable practices such as "permanent greening" (milking the patent life cycle by extending it through dubious "innovation") and grant suppression, have also been scrutinized by the public. live ingredients, bribing a dominant organization to stop providing generics, and suing well-known manufacturers over dubious patents. A large part of the pharmaceutical business was once investigated by the European Commission for such practices, and complaints were filed against the draft classification (e.g., they reflect on Astraneca's advertising practices in the PPI category, in which two of its drugs, (Pri)Losec and Nexium, are now being challenged by regulatory authorities and consumers). A weak corporate photo of a pharmaceutical company needs to be fixed. Rather than focusing on the short term, pharmaceutical companies want to strengthen long-term assurances to maintain the long-term beliefs of the population. In the words of Singh and Jayanti later in the book, the enterprise desires to move from a common sense of combat (with the payer, patient, or health care provider) to a good judgment of cooperation, uniting in a mutually beneficial collaboration with the entire health care cost chain. The study conducted by Petrova provides a complete overview of the drug innovation process. This study evaluated a variety of intellectual property safety mechanisms related to the pharmaceutical industry. It deals with the issues associated with me and subsequent drugs, with the basic types of businesses operating in the industry, as well as issues related to the collaborative ways that have emerged in drug innovation, with a unique focal point on alliances. Ding, Dong, Eliashberg, and Gopalakrishnan provide definitions of portfolio management, evaluate applicable information and evidence in the pharmaceutical industry, and review current portfolio management practices. Subsequently, they delved deeper into specific managerial problems in the framework of portfolio administration in the pharmaceutical industry. Betz, Camacho, Gerards, and Stremersch provide a concrete conceptual innovation or bottom-up innovation and show how it can be used in the pharmaceutical industry. Camacho et al. (2012) [15] anchor their conceptualization of the theory of self-determination. They describe the main drivers of motivation and success for employees of pharmaceutical corporations to develop progressive ideas and support them in new business lines. They share their experience-building Inspire, the core innovation software, at Merck KGaA, Darmstadt, Germany. Wuyts' study focuses on three fundamental questions: competing views on why companies benefit from portfolio diversity; how differences among corporations in their allocation of managerial assets to portfolio management and their internal research and development techniques can help explain why some companies benefit more from portfolio diversity than others; and why technological developments such as the upward pressure of nanotechnology and institutional features such as healthcare reforms are changing the nature of collaborative portfolios and alliances in the pharmaceutical industry. In their study, Chan, Narasimhan, and Xie addressed the topic of innovation through the evaluation of the efficacy and side effects that companies in the pharmaceutical business examine as their breakthrough drug goes through scientific trial data. They argue that countless fundamental problems cannot be solved by scientific records alone, and they agree that by supplementing this information with data on post-marketing prescription preferences, researchers can also gain. In the diffusion choice chain, Landsman, Verniers, and Stremersch award each sequence of choices that managers must make, as well as the analytical equipment that pharmaceutical companies can use to improve their decision-making. A thriving set of choices consists of those related to specific techniques for evaluating the industrial potential of a treatment, choices focused on making the best use of the potential of a new treatment, and choices related to the approach that will be used to exploit new treatment options in different countries. Kappe focuses on innovative techniques available for drugs that are already on the market and near patent expiration. This condition affects only one party of its kind: brand name, generic drug manufacturers, doctors, patients, insurance companies, pharmacists, or the government. Focusing on patent expiration penalties for brand-name manufacturers, this chapter discusses the regulatory landscape for prescription drugs, the determinants and impact on well-known entry, and more than a few lifestyle cycle extension strategies. The innovation part is concluded with a study by Jain and Conley. It summarizes an extensive list of patent extension and market exclusivity preferences and pre- and post-expiry prescription drug prices and analyzes how promotional activities and newer product branding steps such as advertising, marketing, and product configuration influence patient behavior. uncovered by such innovations and carefully examines two rather unique pharmaceutical cases: the markets for gastroesophageal reflux ailments and neurological medicines. The main topics of this study were pharmaceutical advertising techniques and their effectiveness. Pharmaceutical advertising and marketing techniques vary from sampling to detailing magazine advertising to DTCA and various promotional efforts. The details are covered in this section. Beginning with sampling as a promotional tool, Dong, Li, and Xie provide an overview of common practices in US pharmaceutical sampling. He discusses the relatively large number of record sources that can be used to search for drug samples, and the current literature reviews the results of drug revenue samples from the educational literature and empirical research in the field. Sridhar, Mantrala, and Albers explored the following questions: How is it for personal promotion or detailed information for doctors? What is a generalized quantitative estimate of the effectiveness of detailing? The details of effectiveness vary by product lifecycle stage and geography. They provide evidence based entirely on a meta-analysis of 373 econometric estimates of pharmaceutical detail elasticities that appeared in forty-eight papers. The authors believe that the best expense-to-sales ratios today should (1) be in the neighborhood of 6-7 p.c. over the lifestyle cycles of pharmaceutical products, (2) contain reasonable shifts from greater attention to detail to less as the item ages, and (3) be greater in Europe than in the US. Fischer explored a variety of advertising spending models: physician-oriented, patient-oriented, and multi-stakeholder-oriented. It summarizes findings from management surveys and econometric models, analyzes drug demand, and concludes with guidelines for determining the best advertising budgets. With a comparable focus on more than one strategic advertising variable, Wieringa, Osinga, Ruiz-Conde, Leeflang, and Stern address the following questions: What effect do advertising variables have on the diffusion pattern of newly introduced pharmaceutical innovations? What effect does momentum have on the effectiveness of pharmaceutical advertising? Focusing on compound demand for prescription drugs, they review articles that address the effectiveness of pharmaceutical advertising and discuss the value and relevance of pharmaceutical promotional effects, distinguishing between product-class demand results and manufacturer-level demand results. It evaluates the goals and findings of research that examines how advertising efforts influence the diffusion of new pharmaceutical improvements and provides an overview of research that studies how dynamics affect the effectiveness of drug promotions. Liu and Gupta evaluate DTCA records and argue that spending on prescription capsules in the US is growing explosively. They examine the subsequent fairly large number of methodologies designed to examine the effectiveness of such spending, thinking about patients, doctors, and governments as audiences. They conclude with precise findings associated with the short-run and long-run elasticities of these advertising efforts, suggesting that they are one-half of the distribution of advertising and marketing elasticities. In addition, direct-to-consumer marketing and direct-to-physician marketing are prevalent matters addressed with the help of Vakratsas and Kolsarici. They provide a research evaluation of marketing mix efforts aimed at patients and physicians and discuss the relative results of these advertising and marketing efforts. Based on the evidence they review, they conclude that DTCA elasticities are smaller than physician-direct elasticities, making the physician the primary decision-maker in the prescribing process. Desiraju and Tran's study presents spillovers and related externalities in the industry. Spillover can also occur in the Canadian market, for example, because a lot of the Canadian population lives particularly close to the US border and has access to American television broadcasts. Examining the existing literature on spillover effects, they address specific questions such as: Does the US DTCA affect earnings in Canada due to spillovers from the regulatory version? If so, what is the size of the return from such spillover? The section concludes with Singh and Jayanti, who address the institutional principle, looking at the dominant common sense underlying pharmaceutical advertising and marketing strategies and contrasting it with the organizing common sense of fee chain partners. Two key questions are mentioned in this chapter: What specific advertising and marketing techniques do pharmaceutical companies use to acquire interactive scientists, and how do these techniques relate to precise tactics? And under what conditions and why do pharmaceutical advertising and marketing techniques increase (or decrease) the aversive (approving) response of cost chain partners? The assessment suggests that the pharmaceutical cost chain exhibits dynamics that are stable with different components of institutional theory: (1) device warfare due to the coexistence of competing logics; (2) institutional failure to address the hostility of logics that are amplified through pharmaceutical advertising and marketing practices; and (3) the escalation of conflicting logics that call for a regulatory intervention that restricts and limits advertising and marketing efforts.
Research method
For this take a look at, a combined methods study was conducted to research the relationship between innovation and advertising and marketing within the pharmaceutical industry. The research used both qualitative and quantitative information series techniques to gain complete expertise on the subject.
Quantitative data: A survey questionnaire evolved and was distributed to pharmaceutical enterprise professionals, together with executives, marketers, and product managers. The survey protected closed-ended questions to acquire quantitative records on diverse components of innovation and marketing techniques within the industry. The questionnaire focused on topics including investment in research and improvement, adoption of progressive technology, advertising budgets, promotional techniques, and customer perceptions of pharmaceutical products.
Qualitative information: In-depth interviews were performed with key enterprise experts, which included executives from pharmaceutical organizations, advertising professionals, and industry analysts. The interviews were semi-structured, taking into consideration open-ended questions and certain discussions. The qualitative records accrued via interviews furnished insights into the demanding situations, opportunities, and techniques related to innovation and advertising in the pharmaceutical industry.
facts evaluation:
Quantitative records received from the survey questionnaire were analyzed with the use of a statistical software program. Descriptive information, including the mean, median, and preferred deviation, has been calculated to summarize the facts. Inferential statistics, which include correlation evaluation, regression evaluation, and t-checks, were conducted to perceive relationships and extensive variations among variables.
Qualitative records from the interviews were transcribed and analyzed thematically. The transcripts were coded to become aware of habitual subject matters and patterns associated with innovation and advertising strategies. The thematic evaluation involved organizing the records into significant categories and deciphering the findings based totally on the study's objectives.
The evaluation of the quantitative facts found several key findings associated with innovation and marketing inside the pharmaceutical enterprise. those blanketed: tremendous correlation between research and improvement investment and progressive product improvement.
The adoption of digital technologies and information analytics for targeted marketing campaigns.
increasing advertising and marketing budgets to sell new pharmaceutical merchandise.
The significance of customer belief and trust in pharmaceutical branding and advertising efforts.
The qualitative analysis of the interview data furnished extra insights into the challenges and opportunities confronted by way of pharmaceutical companies in phrases of innovation and advertising. those blanketed regulatory hurdles, marketplace opposition, changing purchaser expectations, and the want for effective communique and collaboration within the industry.
The findings of this study spotlight the critical role of innovation and advertising in the pharmaceutical enterprise. Pharmaceutical corporations need to invest in studies and improvements to broaden progressive merchandise that addresses unmet medical wishes. simultaneously, they have to appoint effective advertising strategies to promote those merchandise and construct acceptance as true with healthcare experts and clients.
The look emphasizes the developing importance of digital technologies and data analytics in pharmaceutical marketing. Using targeted advertising campaigns primarily based on client alternatives and behavior can improve logo visibility and enhance product adoption costs.
Moreover, the look underscores the importance of the purchaser's notion and belief in pharmaceutical branding. Organizing a positive logo picture and maintaining obvious communication with customers is vital for long-term achievement in the industry.
On average, this study contributes to deeper information on the connection between innovation and marketing in the pharmaceutical enterprise. The findings can help pharmaceutical companies formulate powerful techniques to navigate the complex panorama of innovation and advertising, ultimately leading to advanced product development, market penetration, and purchaser pride.
The pharmaceutical business is one of the most dynamic and complex industries today, involving the commercialization of the latest scientific research, a large internet of stakeholders (from marketers to doctors), multi-level grant chains, fierce opposition in the marketplace, and a difficult regulatory environment. The stakes are high, and each new product raises the prospect of spectacular success—or failure. Worldwide sales approach $1 trillion; in the US alone, pharmaceutical advertising is a multi-billion dollar industry by itself. This quantity shows contributions from worldwide professionals to seize the kingdom of the artwork in research, analysis, and exercise and covers the full spectrum of innovation and marketing subject matter that includes research and development, promotion, pricing, branding, aggressive strategy, and portfolio management.
The completion of this research project would not have been possible without the contributions and support of many individuals and organizations. We are deeply grateful to all those who played a role in the success of this project I would like to thank my mentor, Dr. Naweed Imam Syed, Prof. Department of Cell Biology at the University of Calgary, and Dr. Sadaf Ahmed, Head of the Psychophysiology Research Lab at the University of Karachi, for their invaluable input and support throughout the research process. Their insights and expertise were instrumental in shaping the direction of this project.
I, at this moment, declare that:
I have no pecuniary or other personal interest, direct or indirect, in any matter that raises or may raise a conflict with my duties as a manager of my office.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Financial support and sponsorship
No Funding was received to assist with the preparation of this manuscript.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.

Dear Editorial Team, Journal-Clinical Cardiology and Cardiovascular Interventions, “Publishing my article with Clinical Cardiology and Cardiovascular Interventions has been a highly positive experience. The peer-review process was rigorous yet supportive, offering valuable feedback that strengthened my work. The editorial team demonstrated exceptional professionalism, prompt communication, and a genuine commitment to maintaining the highest scientific standards. I am very pleased with the publication quality and proud to be associated with such a reputable journal.” Warm regards, Dr. Mahmoud Kamal Moustafa Ahmed

Dear Maria Emerson, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews’, I appreciate the opportunity to publish my article with your journal. The editorial office provided clear communication during the submission and review process, and I found the overall experience professional and constructive. Best regards, Elena Salvatore.

Dear Mayra Duenas, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews Herewith I confirm an optimal peer review process and a great support of the editorial office of the present journal
Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. I am really grateful for the peers review; their feedback gave me the opportunity to reflect on the message and impact of my work and to ameliorate the article. The editors did a great job in addition by encouraging me to continue with the process of publishing.

Dear Cecilia Lilly, Editorial Coordinator, Endocrinology and Disorders, Thank you so much for your quick response regarding reviewing and all process till publishing our manuscript entitled: Prevalence of Pre-Diabetes and its Associated Risk Factors Among Nile College Students, Sudan. Best regards, Dr Mamoun Magzoub.

International Journal of Clinical Case Reports and Reviews is a high quality journal that has a clear and concise submission process. The peer review process was comprehensive and constructive. Support from the editorial office was excellent, since the administrative staff were responsive. The journal provides a fast and timely publication timeline.

Dear Maria Emerson, Editorial Coordinator of International Journal of Clinical Case Reports and Reviews, What distinguishes International Journal of Clinical Case Report and Review is not only the scientific rigor of its publications, but the intellectual climate in which research is evaluated. The submission process is refreshingly free of unnecessary formal barriers and bureaucratic rituals that often complicate academic publishing without adding real value. The peer-review system is demanding yet constructive, guided by genuine scientific dialogue rather than hierarchical or authoritarian attitudes. Reviewers act as collaborators in improving the manuscript, not as gatekeepers imposing arbitrary standards. This journal offers a rare balance: high methodological standards combined with a respectful, transparent, and supportive editorial approach. In an era where publishing can feel more burdensome than research itself, this platform restores the original purpose of peer review — to refine ideas, not to obstruct them Prof. Perlat Kapisyzi, FCCP PULMONOLOGIST AND THORACIC IMAGING.

Dear Grace Pierce, International Journal of Clinical Case Reports and Reviews I appreciate the opportunity to review for Auctore Journal, as the overall editorial process was smooth, transparent and professionally managed. This journal maintains high scientific standards and ensures timely communications with authors, which is truly commendable. I would like to express my special thanks to editor Grace Pierce for his constant guidance, promt responses, and supportive coordination throughout the review process. I am also greatful to Eleanor Bailey from the finance department for her clear communication and efficient handling of all administrative matters. Overall, my experience with Auctore Journal has been highly positive and rewarding. Best regards, Sabita sinha

Dear Mayra Duenas, Editorial Coordinator of the journal IJCCR, I write here a little on my experience as an author submitting to the International Journal of Clinical Case Reports and Reviews (IJCCR). This was my first submission to IJCCR and my manuscript was inherently an outsider’s effort. It attempted to broadly identify and then make some sense of life’s under-appreciated mysteries. I initially had responded to a request for possible submissions. I then contacted IJCCR with a tentative topic for a manuscript. They quickly got back with an approval for the submission, but with a particular requirement that it be medically relevant. I then put together a manuscript and submitted it. After the usual back-and-forth over forms and formality, the manuscript was sent off for reviews. Within 2 weeks I got back 4 reviews which were both helpful and also surprising. Surprising in that the topic was somewhat foreign to medical literature. My subsequent updates in response to the reviewer comments went smoothly and in short order I had a series of proofs to evaluate. All in all, the whole publication process seemed outstanding. It was both helpful in terms of the paper’s content and also in terms of its efficient and friendly communications. Thank you all very much. Sincerely, Ted Christopher, Rochester, NY.
