AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2640-1053/214
1Riggs Pharmaceuticals, Department of Pharmacy, University of Karachi.
2Pharmaceutical Inc OPJS University Rajasthan.
3Assistant Professor, Dow University of Health Sciences, Karachi Pakistan.
4Associate Professor, Department of Pathology, Dow University of Health Sciences, Karachi, Pakistan.
*Corresponding Author: Rehan Haider, Riggs Pharmaceuticals, Department of Pharmacy, University of Karachi.
Citation: Rehan Haider, (2024), Genetically Engineered Mouse Model in Preclinical Anti-cancer Drug improvement, J Cancer Research and Cellular Therapeutics, 8(7); DOI:10.31579/2640-1053/214
Copyright: © 2024, Rehan Haider. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 October 2024 | Accepted: 22 October 2024 | Published: 04 November 2024
Keywords: genetically engineered mouse model (gemms), cancers drug development, preclinical studies, tumor microenvironment, pharmacokinetics, pharmacodynamics, drug resistance, personalized medicinal drug
Genetically engineered mouse fashions (GEMMs) have grown to be essential equipment in preclinical anti-most cancers drug development. Those fashions are designed to duplicate precise genetic alterations discovered in human cancers, providing a more accurate illustration of tumor biology and therapeutic responses. GEMMs allow for the look at tumor initiation, development, and metastasis inside a physiologically relevant microenvironment, offering insights into mechanisms of drug resistance and cancer evolution. Importantly, GEMMs assist in evaluating novel therapeutics' efficacy and protection, mimicking human pharmacokinetics and pharmacodynamics more intently than traditional xenograft fashions. Their use helps the identity of predictive biomarkers, permitting a more personalized method for cancer remedy. Moreover, GEMMs are treasured for testing combination treatment plans, assessing capability synergistic consequences, and understanding the tumor's immune panorama in immunotherapy research. No matter their blessings, GEMMs face barriers, consisting of high costs and time-extensive improvement. Additionally, no longer all cancer mutations are difficult to replicate in mice. Nonetheless, persistent advances in genetic engineering techniques, including CRISPR/Cas9, are increasing the application of GEMMs in oncology studies. Via improving the translational relevance of preclinical research, GEMMs play a pivotal function in accelerating the invention and development of more powerful anti-most cancer treatment plans, in the end improving patient results.
In step with the maximum recent American Most Cancers Society records, an envisioned 569,490 people died from cancer in 2010 (American Cancer Society [ACS], 2010[1]. The wide variety of most cancers-associated deaths currently handed the ones from coronary heart disorder in Americans <85 years vintage (Kung et al., 2008)[2]. Developing new and greater efficacious anti-most cancer compounds is a paramount health care priority. At the Developmental Therapeutics program of the U.S. Countrywide Cancer Institute, ability therapeutic marketers are commonly tested for interest in an in vitro 60-tumor cell line screen and sooner or later using in vivo xenograft research in rodents (Fig 1). Once decided on for additional trying out the use of mounted standards (drugability, the novelty of structure and/or mechanism of movement, efficiency, mobile panel selectivity, and many others.), extra studies (pharmacokinetics, pharmacodynamics, variety finding toxicity, method, mechanism of motion analysis, IND-directed toxicology, etc.) are initiated observed by way of the development of a specific few candidate dealers into human clinical trials. Efficacious capsules with tolerable toxicity are ushered into early-phase scientific trials. However, most of the promising compounds identified in the multi-layered preclinical screenings aren't as successful in human patients. Consequently, preclinical models that better predict drug efficacy and toxicity in humans are wished. Differences in drug absorption, distribution, metabolism, and elimination (ADME), immune responses, and over-interpretation of the preclinical efficacy records may additionally contribute to scientific disasters. But, a widespread hassle is producing mouse models that histologically, genetically, and behaviorally recapitulate human disease Over the last two decades, researchers have identified a number of the underlying genetic abnormalities that cause positive cancers and have genetically engineered them into mouse fashions to explore the oncogenic nature of these genes and related pathways. The resulting mouse fashions generate tumors that could highly mimic those seen in people. It's far expected that healing compounds that show efficacy and coffee toxicity in these more human-like genetically engineered mouse (GEM) models can be more of a hit in scientific trials and cause expanded cancer survivorship. Hundreds of GEM models currently exist that can be related to some form of human tumor development (see the eMICE website). Its miles properly past the scope of this manuscript to adequately describe each of them. We rather in a short overview of some of the floor-breaking GEM models advanced within the Eighties and 90s, describe more recent and complex models, spotlight a number of the advantages and barriers for every, and discover the potential roles of molecular-centered treatment plans and GEM models in preclinical drug trying out. For added info on each version, we refer to the reader to the original articles or previous indepth opinions. Whilst mice that increase genetically engineered tumors preserve a whole lot of promise, the tremendously gradual increase in the price of those tumors and their low penetrance (no matter genetics, a few mice will no longer broaden tumors all through the time constraints of the experiment) makes them sick-suited to update xenograft fashions as the primary in vivo screening device. Alternatively, we envision GEM fashions as a further screening the mechanism that narrows and optimizes the sphere of therapeutic compounds earlier than luxurious and timeconsuming human clinical trials are initiated.
Figure 1: Wherein gemstones may be maximum useful inside the Preclinical Drug development.
Simplistic assessment of preclinical drug development manner within the NCI/DTP. Novel dealers are brought via the 60 cell Line screen (reviewed in Priests et al., 1991), which incorporates 60 human-cultured tumor cellular lines in a 96-well plate array. If anti-proliferative activity is observed in several mobile lines, the agent is examined in a hollow Fiber assay, which measures the anti-proliferative hobby in cultured cells grown in hole polyvinylidene fluoride (PVDF) fibers implanted intraperitoneal and subcutaneously in mice (reviewed in Decker et al., 2004). If lively in hollow Fiber assays, dealers are administered to immunodeficient animals that possess human tumors generated from serially passaged human tumor mobile lines (xenografts). Tolerable toxicity and interest are measurable in xenograft models leading to simultaneous investigations into the agent’s/PD, mechanism of movement, and toxicology. Based totally on this information, wherein applicable an IND utility is generated for FDA approval to initiate scientific trials. The sellers may also require further preclinical studies or can be permitted for a small-scale segment 0 trial (reviewed in Murgo et al., 2008), or preliminary segment I human scientific trials. Assay Time is a satisfactory case state of affairs for a single agent and no longer consists of time for optimization and validation repetitions. PD = pharmacodynamics, PK = pharmacokinetics, GEM = genetically engineered mouse, IND = Investigational New Drug.
After a potential healing agent suggests a few interests in cultured tumor cell growth Assays, its activity and toxicity are explored through in vivo xenograft models. Fragments of human tumors or cultured human tumor cells are implanted (often subcutaneously) into immunodeficient rodents. As soon as the tumors develop to a predetermined length, the capability antimost cancer agents are administered and the tumor response, generally assessed by way of monitoring the tumor size, is measured through the years. Drug doses and management schedules are adjusted to optimize efficacy and decrease toxicity over numerous experimental cycles. This system generates valuable records and fast returns a prediction on an agent’s pastime, regularly for numerous months. Lamentably, promising anti-cancer compounds located through this route often fail in human scientific trials, typically due to low efficacy [3,4]. The low predictive cost of these xenograft models exemplifies the want for better in vivo mouse models for preclinical drug checking out.
The transgenic mouse era has advanced from the early 1980s [5] such that researchers can now conditionally and reversibly alter single gene expression. Thousands of publications have supported the speculation that oncogene expression or tumor suppressor gene ablation in everyday mammalian cells is sufficient to pressure tumor development. The first file of a heritable tumor-susceptible transgenic mouse came here in June 1984 (Brinster et al., 1984[6]. Those mice used the SV40 enhancer location to assist force expression of a construct including the mouse metallothionein-1 gene promoter fused to the thymidine kinase gene from the herpes simplex virus (HSV). They blanketed the complete SV40 upstream location, along with enhancer, promoter, and two T-antigen genes transcribed inside the contrary direction. It became the notion that T-antigen genes would be inactive in mice. , they observed that the transgenic mice constantly advanced brain tumors, in addition to sporadic tumors in other tissues. Comply with-up experiments confirmed that the use of only the SV40 enhancer/promoter place and the massive T-antigen gene becomes sufficient to pressure tumorigenesis [7]. Next reports have shown that expressing the large T-antigen in a particular cellular type can sell tumor development. For example, Ornitz et al., (1985) established expression of the massive T-antigen in acinar cells generated exocrine pancreatic tumors, whereas Hanahan (1985) used the insulin promoter using the large T-antigen particularly in pancreatic beta-cells to produce endocrine pancreatic tumors. Following the sudden oncogenic capability of SV40, researchers attempted to rationally design a tumor-generating transgenic mouse (“oncomouse”). So that you can create a mouse version of a chromosomal translocation visible in some human B-cellular lymphomas, [8] generated a transgene consisting of an immunoglobulin enhancer (E) driving expression of the Myc gene. Those mice heritably expand pre-B cellular and mature B-cellular lymphomas. Further research through Strasser et al., (1990) showed that Myc required extended expression of the anti-apoptotic thing Bcl-2 to force tumorigenesis. Suppression of apoptosis is now understood to be a hallmark of many cancer cells.
3.1 MMTV precipitated breast cancer
the use of reciprocal matings between excessive tumor and occasional tumor mouse traces, Bittner (1936) suggested tumor prevalence in F1 girls became dependent on the stress of the mom. Virologists validated a plague (dubbed Murine Mammary Tumor Virus, MMTV) was chargeable for inducing tumors in mammary tissue and becoming handed from mom to offspring through her milk. Next studies showed some mouse strains additionally had MMTV virus in their eggs and sperm (reviewed in Heston & Parks, 1977).[9] The application of MMTV was expanded when it became proven that a short regulatory region (known as long terminal repeat, LTR) became enough to confer hormone-responsive and cellular-specific expression in vitro [10]. Stewart (1984)[11] used the MMTV LTR to force Myc expression in his transgenic mice that advanced breast adenocarcinomas in mammary epithelial tissue. When you consider that then the MMTV LTR has been fused with an expansion of purported oncogenes to expand tumors in murine mammary tissue which are comparable in morphology and gene expression profile to certain varieties of human breast cancers [12]. For example, the MMTV-driven Polyoma middle-T antigen (PyMT) the model develops tumors much like a human breast most cancers with luminal-type morphology approximately 2-3 months after beginning (Man et al. 1992). A version expressing MMTV-pushed Wnt1 (wingless-kind MMTV integration website own family, member 1) generates mouse mammary tumors with characteristics similar to those of human basal-kind breast cancers [13] several members of the Wnt gene circle of relatives (encoding secretory glycoproteins that generally stimulate cellular proliferation and differentiation) are expressed inside the mouse mammary tissue all through diverse tiers of development. Wnt-1 isn't usually expressed in the mammary tissue; however, while driven ectopically by using MMTV, it develops oncogenic houses. Like most of the opposite “first generation” transgenic oncomice, many of these models have a low tumor penetrance and broadly variable latency length, making them tough to apply directly in large-scale preclinical drug screenings. These obstacles are partially conquered by resecting and transplanting transgenically brought tumors into many syngeneic recipient animals, generating a large cohort of tumor-bearing animals for drugscreening functions [14,15]
3.2 Activated Kras
The KRAS2 gene encodes a G-protein that is a mammalian cellular homolog of a transforming gene isolated from the Kirsten RAt Sarcoma virus. This membrane-associated intracellular signal transducer performs a vital position in ordinary tissue signaling, proliferation, and differentiation [16]. Numerous oncogenic point mutations intervene with the intrinsic GTPase interest of Kras, causing accumulation in a constitutively lively GTP-sure nation.[17] Expressing an activated Kras mutant transgene in acinar cells induces neoplasia in the fetal pancreas with massive tumors developing only days after pancreatic differentiation. Certainly, activating factor mutations within the Kras gene has finally been proven to arise in 75 to 95% of spontaneous human pancreatic cancers [18] in addition to > 90% of spontaneous and chemically caused mouse lung tumors [19]. Activated Kras expression inside the mouse lung generates more than one tumor at an early age, so much so that the mice succumb fast due to respiration failure [20] The numerous penetrance and multi-focal primary tumor formation further to the fast lifestyle span limits the usage of this version in addition to studies of tumor development.
3.3 Knockout oncomice
The examples described thus far depend on the over-expression of a nucleic acid sequence with purported oncogenic houses or mutations to pressure tumorigenesis in mice. at some point in the late 1980’s using homologous recombination in mouse embryonic stem cells enabled researchers to inactivate (“knockout”) unmarried genes. This era created a new wave of transgenic oncomice starting with the heterozygous null retinoblastoma (Rb) mouse [21]. Rb inhibits the cell cycle by way of repressing the expression of genes required for S phase progression (reviewed in Hanahan & Weinberg 2000). Mice missing one Rb allele increase pituitary adenomas, while RB null offspring fail to increase past embryonic day 14 or 15, in all likelihood due to immoderate neuronal cellular loss of life. The significance of tumor suppressor expression has become obvious from this and different knockout mouse models. Diverse mobile stresses prompt p53 to modulate the expression of its target genes, lots of which adjust mobile cycle arrest, apoptosis, DNA repair, or cellular metabolism. Reduced or null expression of p53 has been found in several human cancers (reviewed in Harris & Hollstein 1993). [22] Mice missing one or each p53 alleles are born every day however are predisposed to growing spontaneous lymphomas and sarcomas later in life [23]. missing a chief tumor suppressor pathway, these p53 null mice have become a useful heritage with which to clarify the oncogenic capability of other genes. The Rb/p53 double knockout mice expand relatively competitive tumors inside the cerebellum seen as early as 7 weeks of age.[24] Mammary and skin tumors increase frequently in woman mice sporting conditional null Brca2 and p53 alleles, [25] suggesting synergistic inactivation of Brca2 and p53 can mediate mammary tumorigenesis. When you consider that these early transgenic and knockout mouse models have genetic changes that are expressed within the germline and maximum, if no longer all, somatic cells, the models are more consultant of human cancer predisposition syndromes. This isn't always the case for most human cancers, which broaden spontaneously in a small number of cells inside the grownup. Many genes have distinct capabilities and expression styles in the course of embryonic improvement that are nevertheless poorly understood and extraordinary from those inside the adult. Furthermore, those early transgenic fashions are notorious for their variable penetrance and tumor latency, making it almost possible to accumulate the variety of animals with synchronous tumor improvement wished for large research. While large-scale manufacturing of transgenic mice is now viable with IVF and other excessive manufacturing methods (JAX® pace enlargement provider; Charles River Laboratories' fast growth offerings), no longer obviate the problem of variable penetrance and tumor latency; it truly offers a massive range of animals that can be held concurrently to have a look at for tumor development. Early GEM models generated a couple of primary lesions which a long way exceeded that found in human beings, limiting their predictive value and usefulness for preclinical studies. To better model maximum human cancers, genomic alterations have to arise in a small variety of cells in the grownup mouse tissue similar to the microenvironment wherein human cancer develops
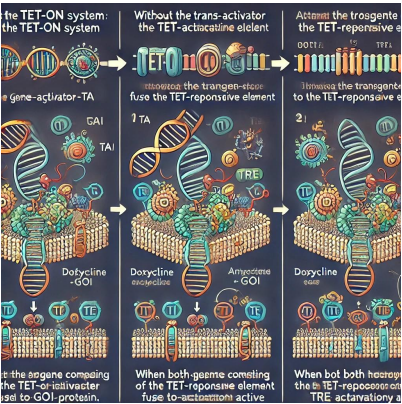
Figure 2: Schematic representation of the Tet-ON system; 1) Without the Tet-Activator (TA), the transgene such as the Gene-of-interest (GOI) fused to the Tet-Responsive element (TRE) will no longer be expressed. 2) Doxycycline can bind to the Tet-Activator protein, altering its protein shape such that TA can now bind to a TRE. 3) If both the Tet Activator and the TRE-GOI transgenes are present inside the identical cellular, upon doxycycline management and TA activation, the GOI can be transcriptionally active.
Taking advantage of the Lox-forestall-Lox conditional transgene expression system, Jackson et al., (2001) created a transgenic mouse version that replaces one wild-type Kras allele with a transcriptionally silent oncogenic Kras-G12D allele. Intranasally introduced adenovirus containing the Cre-recombinase (adeno-Cre) splices out the LSL and allows expression of the activated Kras-G12D transgene inside the lung. Small lesions may be seen 2 weeks after induction; by 12 weeks after induction, adenomas are found, a few with cytogenic characteristics of malignancy. With the aid of 16 weeks of submit induction, huge adenomas and adenocarcinomas are found in many animals. Virus titrations in some of animals demonstrate that the quantity of virus particle equivalents used for induction is at once associated with the quantity of tumors that broaden. Across the equal time, Fisher et al., (2001) created an activated Kras Tet-ON/OFF inducible version. Doxycycline (DOX) administration induces expression of the murine oncogenic Kras G12D allele in alveolar type II pneumocytes. Hyperplasia is determined after just seven days of DOX management; at 8 weeks submit induction, adenomas, and adenocarcinomas are discovered inside the lungs. Removal of DOX from their weight loss program reasons a speedy decrease in activated Kras [removed]inside seven days) and apoptotic regression of the tumors. One month after DOX withdrawal, lesions, and tumors were not found in the lungs, implying expression of the mutant Kras gene product became required to hold the viability of tumor cells. The requirement of persisted oncogene [removed]or regular inhibition of tumor suppressor) to maintain the brought about tumors has given upward thrust to the theory of “oncogene addiction” (reviewed in Weinstein, 2002).
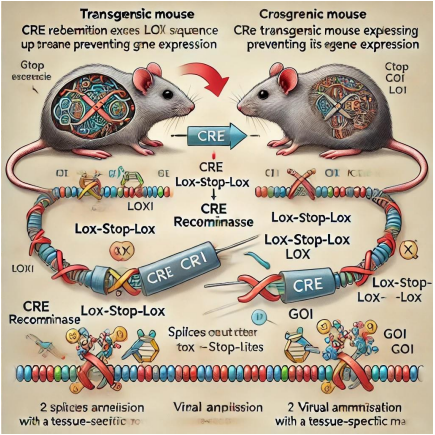
Figure 3: CRE Recombination Mediates Lox-prevent-Lox Gene Activation. One popular approach of inducing gene expression in person mice is to use CRE recombination. 1) The transgenic mouse initially has the Lox-stop-Lox collection inserted upstream of the transcription start website online for the gene of interest (GOI). This prevents GOI expression until acted upon via CRE.
CRE recombinase is added to the correct cell population either with the aid of crossing with a 2d transgenic mouse expressing CRE recombinase the usage of a tissue precise promoter, or using administering a virulent disease expressing CRE recombinase in a tissue-specific manner. 2) CRE recombinase splices out the prevent collection between the 2 loxP sites from the transgenic DNA sequence, allowing GOI expression Sos et al., (2009) currently used computational genomic analysis to become aware of molecular and genetic predictors of therapeutic reaction to clinically relevant compounds in various NSCLC most cancers cell lines which can be highly consultant of primary tumors. Certainly, one of their findings was that cells expressing activated Kras also had more suitable Hsp90 dependency. They used the LSL-KrasG12D model to verify this remark. Mice have been, to begin imaged by MRI for 12-20 weeks put up adeno-Cre administration, then broken up into placebo and 17(Dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG a well-known Hsp90 inhibitor) organizations. Mice were imaged via MRI after 1 week of drug remedy; tumor extent was reduced through 50% in 17-DMAG treated mice even as tumor volume accelerated barely in placebo dealt with animals. Before this look, there were no molecular-centered treatments for activated oncogenic Kras-driven lung tumors. However, by combining the strength of computational genomics and GEM models, researchers were able to rationally perceive a viable healing modality and compare its efficacy in vivo
4.2 Inducible oncogenic mutations within the EGF receptor
The epidermal growth component receptor (EGFr) is a transmembrane mobile surface receptor which is mutated or instead expressed in a sizable proportion of human gliomas and adenocarcinomas (in particular of the head and neck, breast, colon, lung, and pancreas). EGFr is the human homolog of neu, a rat gene first identified in ethyl nitrosourea that brought about tumors (reviewed in Maguire & Greene, 1989), and is a member of the erythroblastic leukemia viral oncogene/human epidermal increase aspect receptor (ERBB/HER) receptor tyrosine kinase own family. Of the most generally found EGFr mutations visible in lung adenocarcinomas encompass an in-body deletion in exon 19 (removing an L-R-E-A [leucine-arginine-glutamic acid-alanine) amino acid motif which is conserved in all regarded vertebrate EGFr sequences) and a factor mutation which results in a leucine to arginine substitution at position 858. these and different much less not unusual mutations inside the kinase domain of EGFr are associated with multiplied therapeutic sensitivity to tyrosine kinase inhibitors along with gefitinib and erlotinib. numerous research tested appreciably prolonged survival and time to development (TTP) in non-small-cell lung, most cancer (NSCLC) patients are treated with gefitinib as their first-line therapeutic (Han et al., 2005; Sequist et al., 2008; Yang et al., 2008). Erlotinib administration in patients who've had previous chemotherapy has a fair more survival gain and is presently recommended by the FDA for 2d line remedy in new NSCLC patients (Shepherd et al., 2005). Inducible transgenic mouse models help to clarify this statement and offer a system to test other therapeutics that function via the EGF receptor. Politi et al., (2006) generated several Tet-ON/OFF mouse systems that overexpress diverse mutant EGFr alleles upon DOX management. Lung adenocarcinomas have been found after months post transgene induction within the exon 19 deletion and L858R transgenic EGFr mouse fashions. Much like the Tet-On/OFF activated Kras gadget, after one month of DOX withdrawal the located tumor load within the EGFr transgene inducible fashions regressed to near pre-induction histology. To check the anti-tumor efficacy of an already clinically accredited healing on this version, the tyrosine kinase inhibitor erlotinib was administered to corporations of transgenic EGFr mice maintained on DOX. After much less than a week of erlotinib remedy, all of the animals that obtained a minimum of 12 mg/kg/d confirmed partial or entire responses by magnetic resonance imaging (MRI). Longer lengths of treatment correlated with extra tumor regression. Ji et al., (2006) generated similar inducible EGFr transgenic mice (exon 19 in-body deletion or L858R) but also brought a luciferase fusion gene to the assembly. This permits the monitoring of lung tumor increase and regression in vivo with the usage of a non-invasive luciferase imaging device. The potential to non-invasively image tumor load again and again in vivo enables the effects of anti-most cancer drug management to be observed long term because the authors validated with cetuximab..
4.3 Gemstones possessing multiple genetic alterations
Patients with lung adenocarcinomas containing one of the not-unusual EGFr oncogenic mutations (deletion in exon 19 of the kinase area [Del.ex19] and leucine 858 to arginine[L858R]) are touchy to the EGFr-tyrosine kinase inhibitor (TKI) treatment options (gefitinib and erlotinib). But, the TKI dealt with tumors always relapse within 12 months through developing resistance to the TKI therapy (reviewed in Morita et al., 2009), most commonly through one of the following mechanisms: EGFr-T790M point mutation (Kobayashi et al., 2005; Pao et al., 2005), MET proto-oncogene amplification (Engelman et al., 2007), or hyperphosphorylation and activation of insulin-like growth issue-I receptor (Guix et al., 2008). The EGFr-T790M mutation (observed in 50% of relapsed EGFr-mutant tumors) is the notion to increase the ATP-binding affinity using an order of magnitude (Yun et al., 2008), thereby reducing the healing ability of TKIs to out-compete ATP for receptor binding. MET amplification is a concept to induce gefitinib resistance generally by using riding ERBB3 (HER3)-dependent activation of the PI3K and Erk pathways, which adjust cellular survival and mitogenesis, respectively (reviewed in Kim & Salgia, 2009). To triumph over the obtained resistance of first technology TKIs, researchers have currently developed TKIs that bind EGFr irreversibly and are presently in or have completed segment II clinical trials (reviewed in Riely, 2008). The pan-ERBB inhibitor HKI-272 covalently binds to a cysteine inside the EGFr kinase area and regressed tumors at nanomolar concentrations in EGFR-L858R transgenic mice (Ji et al., 2006). The capability to inhibit ERBB2 (HER2) has led HKI-272 is to be investigated basically in breast cancer scientific trials (clinicaltrials.gov, 2011). But, different cancers pushed with the aid of ErbB mutations may also be dealt with using HKI-272. Similarly, the irreversibly binding EGFr and HER2 inhibitor BIBW2992 is being tested in over 20 clinical trials of patients with superior NSCLC, breast cancers, prostate cancer, malignant gliomas, and different stable tumors (clinicaltrials.gov, 2011). In addition to ERBB inhibitors, researchers are investigating different druggable targets associated with the EGFr/HER2/ERK/AKT pathways. Shimamura et al., (2008) used an EGFR-L858R-T790M double transgenic GEM model to test the efficacy of an Hsp90 inhibitor, CL-387,785. Hsp90 is a chaperone notion to resource in the proper folding of EGFr; CL-387,785 binds irreversibly to Hsp90 thereby interfering with its chaperone characteristic. Cancer-related lethality in people is regularly because of the invasion of metastatic tumors in tissues a long way from the number one tumor. However, few unmarried transgene GEM models reliably develop metastatic lesions. By crossbreeding the pancreas-precise Cre-mediated activated Kras GEM (which alone generates only pre-malignant neoplasia) and the Ink4a/ARF conditional knockout fashions (no longer result in neoplasia), Aguirre et al., (2003) generated mice that swiftly increase invasive, metastatic ductal pancreatic tumors that are lethal using 11 weeks. This tumor improvement is just like the notably lethal human form of pancreatic ductal adenocarcinoma. Even though the rapid murine mortality prevents long-term research, it may be a beneficial model of deadly human malignant cancers in a brief period of cancer prevention studies. In addition, Ji et al., (2007) crossed a lung-particular activated-Kras GEM with the following tumor suppressor knockout gemstones: Ink4a, p53, or the serine/threonine kinase 11 (Lkb1) knockout. In a number of the twin-knockout mice analyzed, the activated Kras x Lkb1 knockout had the most profound phenotype. Those mice display a significantly improved lung tumor burden, rapid tumor onset, and <50> Winslow et al., (2011) improved on this idea by administering a lentivirus expressing Cre recombinase to a double-floxed GEM model. Upon CRE recombination, activated Kras might be expressed and p53 (flox/flox) would be silenced in certain cells of the grownup lung. Because the lentivirus integrates randomly into the host cellular’s genome, the authors had been in a position to use linkage-mediated PCR (LM-PCR) to generate a unique “fingerprint” for every number one tumor and reveal that “fingerprint” in any next metastases. Even though the virus changed into added at a set time factor and plenty of number one foci appeared to effectively undergo the oncogenic double recombination occasion, only a few number-one foci had been accountable for producing macroscopic metastases outdoor the lung, suggesting different genetic or micro environmental elements manipulate a tumor’s metastatic capacity. This is a great instance of using an inducible, double GEM to model genetic changes visible in lots of human lung cancers and discover the genetics of malignant and non-malignant tumors. Tp53 is not the most effective tumor suppressor being actively investigated in the usage of GEM fashions. The tumor suppressor phosphatase and tensin homolog (PTEN) is a lipid phosphatase that negatively regulates the PI3/AKT pathway, which is often upregulated in human malignancies. Sixty percent of primary human prostate cancers show a decrease/lack of PTEN expression; this gene also is deleteriously mutated in 70% of gliomas and in some varieties of breast carcinomas and melanomas (grey et al., 1998). To research the role of PTEN in prostate cancer, Trotman et al., (2003) created numerous PTEN GEM models, every with an exceptional stage of PTEN expression. Simplest the homozygous PTEN knockout displays an enormous phenotype (invasive prostate cancers develop after six months in all mice). However, not one of the models generates metastases and the mice no longer have reduced survival compared to wild-type controls. A prostate-specific PTEN conditional knockout was created which also generated invasive prostate cancer; but in this case, metastatic prostate tumors evolved and were discovered in the lymph and lung (Wang et al., 2003). Concomitant inactivation of one or each Cdkn1b (encodes the tumor suppressor p27) alleles in a heterozygous PTEN knockout mouse accelerates spontaneous neoplasia formation which develops into prostate carcinoma at complete penetrance within 3 months from birth (Di Cristofano et al., 2001). While a conditional PTEN knockout mouse is crossed with a p53 knockout mouse the resulting offspring is a conditional double knockout GEM that develops aggressive, deadly prostate most cancers with whole penetrance in seven months (Chen et al., 2005). The cancers discovered in this new era of GEM models recapitulate the progression and histopathologic capabilities of some forms of prostate cancer determined in people. This research spotlights the function of PTEN in tumorigenesis and the want for additional cooperative tumor suppressor inactivation to generate malignancies and deadly cancers. The capacity for drug discovery and validation of the usage of conditional GEM fashions is confirmed with the Brca1/p53 mouse version. Most people with human BRCA1-associated tumors harbor mutations in both p53 and BRCA1. Poole et al., (2006) created any such CreLoxP conditional GEM model via inactivating both p53 and Brca1, especially inside the mouse mammary gland. The authors found that progesterone receptors are overexpressed in mutant mammary epithelial cells and provided a possible road of treatment. Administration of a progesterone agonist (mifepristone, RU 486) prevented mammary tumorigenesis in Brca1/p53-poor mice. as the potential to genotype a patient’s tumor to determine their status for EGFr, MET, p53, IGF, and different essential neoplasia-associated objectives turn into realistic, it is going to be feasible to higher pick the finest mixture therapy to impact the applicable oncogenic pathways.
The ability to predict the energy of transgenic mice can be illustrated in the instance of thiazolidinedione’s (TZDs). As an agonist of peroxisome proliferator-activated receptor gamma (PPAR), the TZD troglitazone was an FDA-accredited therapy for type-2 diabetes. TZD anti-tumor activity in cultured and xenografted human colon cancer cells brought about exhilaration inside the scientific community that initiated its use in phase II clinical trials in sufferers of colon cancer (Sarraf et al., 1998). However, in a look posted a few pages after the xenograft look, troglitazone showed no anti-tumor pastime in Min+/- mice; polyp formation extended with troglitazone administration in this model (Saez et al., 1998). This heterozygous knockout mouse model lacks one purposeful replica of the APC tumor suppressor gene, for this reason, predisposing them to colon cancer. Inside the section II medical trial patients who dealt with troglitazone honestly confirmed disorder development within months of remedy initiation, correlating with the outcomes anticipated from the transgenic mouse model (Kulke et al., 2002).
5.1 Cautionary Tale
Even though many regard mice as “little fuzzy people” in the evaluation of preclinical statistics, there are tremendous and often unknown differences in drug metabolism, gene expression, and ailment development among the murine version and people. One example of transgenic mouse models failing to as it should be predicted human scientific final results is the Farnesyltransferase (FTase) tale. FTase catalyzes the publish-translational farnesylation of proteins containing a C-terminal CAAX motif, wherein C is the cysteine residue to be farnesylated, A represents an aliphatic amino acid, and X is any amino acid. Whilst X is leucine, the protein is a preferred substrate for the same enzyme named geranylgeranyltransferase I (GGTase).
Mendola & Backer, (1990) showed farnesylation of Ras proteins is important for their Transformation into amazing oncogenes. This discovery ignited the speculation that interfering with crenulation the use of inhibitors of FTase (FTIs) or GGTase (GGTIs) should cause tumor growth inhibition and a feasible anti-cancer therapy. Even though some facts recommend an FTI and a GGTI wants to be applied simultaneously to obtain whole inhibition of Kras prenylation (Rowell et al., 1997), FTIs on my own have been shown to inhibit the growth of human tumor xenografts in nude mice (sun et al., 1998). FTI monotherapy also demonstrates beneficial results in transgenic mouse fashions: tumor regression in H-Ras mice (Kohl et al., 1995), tumor stasis in N-Ras mice (Mangues et al., 1998), and tumor growth inhibition in Ki RasB mice (Omer et al., 2000). Those consequences and others spurred pleasure for FTI monotherapy in scientific trials. Excluding some trials in breast cancer and leukemia patients, FTIs used as single sellers have no longer proven true efficacy towards strong tumors (reviewed in Zhu et al., 2003). Therefore, not one of the numerous mouse fashions evaluated has been capable of predicting drug efficacy in people. However, many scientific trials are currently underway combining FTIs with different tablets such as “classical” anticancer remedies (chemotherapy or radiotherapy) and molecular-centered therapeutics
5.2 Targeted Molecular Therapeutics
For decades, cancer therapy has been one or an aggregate of the subsequent three treatments surgical operation, radiotherapy, and cytotoxic chemotherapy. However, all have their obstacles and considerable facet results. Ideally, anti-most cancer treatments could “goal” cancer cells and decrease detrimental effects on non-cancerous cells. Tumors frequently incorporate gene expression profiles and mutations that make them more or less sensitive to some focused therapies than others. Identifying the tumor’s gene expression profile and correlating it to the maximum successful remedies for that particular profile will hold healing damaging side consequences to a minimum and yield a great diagnosis for the patient.
The 2 major forms of centered molecular treatment options are monoclonal antibodies, which target the receptor’s extracellular domain, and small molecule inhibitors, which goal the intracellular signaling and kinase domains. Table 1 outlines a number of the FDA-approved focused therapeutics, their molecular targets, and the varieties of cancer wherein they have been demonstrated to have a scientific advantage. Monoclonal antibodies (MAbs) are normally administered no more than as soon as weekly through intravenous injection, whereas small molecules (nibs) can normally be taken orally every day. Currently, targeted healing procedures can be administered as unmarried marketers, however, many display greater blessings in mixture with other sellers or similarly to traditional treatment options in those patient populations with tumors of susceptible genetic profiles. Tumors can be generated in genetically engineered mice that have these inclined mutations and expression profiles, supplying researchers with precious preclinical drug screening opportunities. Herceptin (HER2) is a transmembrane receptor that is over-expressed in 20-25% of breast cancers and is related to aggressive tumor conduct and poor analysis (reviewed in Nanda, 2007). Two early research have proven the anti-HER2 monoclonal antibody trastuzumab plus chemotherapy considerably improves average survival in HER2+ patients with metastatic breast cancer (Slamon et al., 2005). Antibody binding is thought to inhibit HER2 signaling, which disrupts DNA restore mechanisms and induces cytotoxicity. Resistance to trastuzumab typically occurs and may render this treatment useless in subsequent relapses. A current strength has been to conjugate the antibody with a toxin/drug, thereby developing a “guided missile” that goals a specific epitope over-expressed on most cancer cells and delivers the poisonous agent to the one's particular cells. Researchers at Genetech harvested mammary tumors from MMTV-HER2 transgenic mice, implanted them orthotopically into a big cohort of nude mice, staged the mice when suggested tumor volumes have been ~a hundred-two hundred mm3, and administered their trastuzumab-DM1 (T-DM1) conjugate at diverse time factors and doses (Jumbe et al., 2010). DM1, also known as maytansine and derived from participants of several tropical plant households, is a robust cytotoxin that irreversibly inhibits tubulin polymerization and arrests cells in the M or G2 phase (Remillard et al., 1975; Rao et al., 1979). because the toxin is especially focused on tumor cells, the
Authors saw no detrimental activities within the mice, with ~50% of the tumors displaying whole regression at doses of 15 mg/kg given once every three weeks. PD/PK measures had been received from the mice and this immunoconjugate remedy is now being evaluated in several scientific trials in sufferers with metastatic breast cancer. several therapeutics have been developed that focus on the EGFr receptor to deal with non-small cells lung most cancers and colorectal cancer, which include cetuximab, panitumumab, erlotinib, and gefitinib. Tumors expressing EGFr with a deletion in exon 19 or sensitizing factor mutation (L858R) in the ATP-binding pocket reply significantly higher to gefitinib than patients with wild-type EGFr (Lynch et al., 2008). Continually, even the tumors with increased drug sensitivity relapse when resistance to gefitinib develops. Extra therapeutics need to be generated to triumph over the inevitable drug resistance.
Table 1: Overview of FDA-Approved Targeted Therapy Compounds
| Drug Name | Molecular Target | Approved Use |
|---|---|---|
| Cetuximab (Erbitux®) | EGFR | Colorectal Cancer |
| Erlotinib (Tarceva®) | EGFR | Non-Small Cell Lung Cancer, Metastatic Pancreatic Cancer |
| Gefitinib (Iressa®) | EGFR | Non-Small Cell Lung Cancer |
| Panitumumab (Vectibix®) | EGFR | Colorectal Cancer |
| Trastuzumab (Herceptin®) | HER2 | Early and Metastatic Breast Cancer |
| Lapatinib (Tykerb®) | HER2 and EGFR | Breast Cancer |
| Bevacizumab (Avastin®) | VEGF | Metastatic Colorectal Cancer, Metastatic Melanoma, Non-Small Cell Lung Cancer |
| Sunitinib (Sutent®) | VEGFR and PDGFR | Metastatic Renal Cell Carcinoma |
| Sorafenib (Nexavar®) | Multi-Targeted Kinase Inhibitor | Metastatic Renal Cell Carcinoma |
| Toceranib (Palladia®) | Multi-Targeted Kinase Inhibitor | Canine Specific Mastocytoma |
| Pazopanib (Votrient®) | VEGFR, PDGFR, cKIT | Advanced Renal Cell Carcinoma |
| Imatinib (Gleevec®) | cKIT, ABL, PDGFR | Chronic Myeloid Leukemia, GIST |
| Dasatinib (Sprycel®) | BCR/ABL, Src Family | Chronic Myeloid Leukemia resistant to prior therapy |
| Alemtuzumab (Campath®) | CD52 on Mature Lymphocytes | Chronic Lymphocytic Leukemia, Cutaneous T-cell Lymphoma |
| Ofatumumab (Arzerra®) | CD20 on B-cells | Chronic Lymphocytic Leukemia |
| Rituximab (MabThera®) | CD20 on B-cells | B-cell Non-Hodgkin's Lymphoma |
| Nilotinib (Tasigna®) | BCR/ABL kinase inhibitor | Philadelphia chromosome positive chronic myeloid leukemia |
| Vandetanib (Zactima®) | VEGFR, EGFR, RET | Metastatic Medullary Thyroid Cancer |
Since vascularization is needed for the increase and health of a tumor, the angiogenesis-associated component VEGF has become a desired goal for therapy. Bevacizumab is an anti-VEGF antibody that has been shown to bind and inhibit VEGF, slowing angiogenesis and helping in stable tumor starvation and elimination (Brekken et al., 2000). Whilst used as unmarried retailers in unsorted cancer sufferers, anti-angiogenic therapeutics do not show a sizeable pastime. However, while used in combination with different treatment options (in particular cytotoxic chemotherapy), and subgroups of sufferers display promise (reviewed in Cabebe, 2007). Bevacizumab, similar to paclitaxel and a platinum cytotoxic agent, is now part of a first-line remedy for newly identified non-small cellular lung cancer. Loads of section II and section III trials using bevacizumab in a mixture with other healing procedures in lots of other cancers are currently ongoing (clinicaltrials.gov, 2011).
5.3 GEM oncomice in most cancer prevention
GEM fashions are starting to recognize their capacity as preclinical models of cancer prevention (briefly reviewed in abate-Shen et al., 2008). In a recent example, Ohashi et al., (2009) used the EGFR-L858R-FLAG transgenic mouse to check the cancer-preventative the ability of gefitinib administration. 20-5 3-week antique transgenic mice have been administered gefitinib, with 5, 5, and 15 mice euthanized at weeks eight, thirteen, and 18, respectively. At termination, the lung tumors have been counted and measured macroscopically and histologically. The ones receiving gefitinib failed to increase adenocarcinomas, while the businesses given automobile manipulation developed hyperplastic regions at 5 weeks and big adenocarcinomas with the aid of 15 weeks. One week after cessation of the 15-week gefitinib administration duration, the transgenic mice showed signs and symptoms of hyperplastic mobile growth in the lung (via PCNA staining), suggesting gefitinib was the lone component preventing EGFrL858R tumor improvement in those mice. long-run research is had to see if the EGFrL858R transgenic mice finally increase gefitinib resistance, as is seen in human NCSLC patients with EGFr-L858R-driven lung tumors. Comparable studies with erlotinib, different tyrosine kinase inhibitors, and novel anti-cancer-causing agents ought to be accomplished in this and other informative GEM fashions to further investigate the tumor-preventative factors of those dealers.
5.4 Cancer vaccine
it is possible that sufferers with an excessive threat (own family history, environmental publicity, or heritable mutations) related to a specific cancer subtype would possibly gain from being
immunized at a younger age. Vaccine development relies upon the identification of antigens precise for a given cancer subtype or tumor-inducing biological agent. The heterogeneity and volatile genome in maximum cancers shows resistant mutant cells might gather speedy and triumph over the immunotherapy. However, vaccines against oncogenic strains of viruses have been validated to be very beneficial and often lean at the preclinical use of GEM models to expose the oncogenic potential of viral genes.
In 1993, studies of the use of transgenic mice emerged surely showing the oncogenic ability of the human papillomavirus (HPV) early genes E6 and E7 (Lambert et al., 1993; Arbeit et al., 1993). HPV DNA has also been discovered and hypothesized using a few to induce a subset of tongue and different oropharyngeal carcinomas (reviewed in Syrjänen, 2005). Transgenic mice expressing HPV early genes were used to illustrate the oncogenic potential of HPV in certain pores and skin cancers [HPV 8 (Schaper et al., 2005) and HPV 20 (Michel et al., 2006)].
Spurred by using these and many studies elucidating the connection between HPV and cervical most cancers, the FDA accredited the HPV vaccine Gardasil (Merck) for stopping the maximum commonplace sorts of human papillomavirus (HPV)-triggered cervical cancer (reviewed in McLemore, 2006). Feng et al., (2008) currently discovered a unique polyomavirus (similar to SV40) that included itself in the genome of 80% of the Merkel cellular carcinomas they tested. Although an extraordinary cancer, this is every other instance of a virus-related cancer that could be a target for most cancer-stopping vaccines and could be tested preclinical in GEM models
5.5 Legal Obstacle of preclinical testing in GEM models
In 1988 Harvard College filed the first of three exceptionally broad U.S. patents regarding the development and use of transgenic animals. Dupont (a sponsor of one of the Harvard investigators developing transgenic mice) became the extraordinary licensee of transgenic patents and merely sublicensed the patent rights (imposing huge charges and regulations) to enterprises and academia. This arrangement significantly confined the use of transgenic mice past primary discovery programs (reviewed in Sharpless & Depinho, 2006). Happily, For the greater medical network, the primary of those patents expired in 2005 and the second expired in February 2009; the 0.33 patent protecting trying out methods of the usage of transgenic oncomice is still under pressure through 2016. Hundreds of oncomice have been developed (normally in academia), but have not begun to be thoroughly studied and used on a big scale to test the growing quantity of feasible anti-cancer treatment plans. Once the very last restriction is lifted, researchers in industry, academia, and government alike will surely make bigger their use of transgenic models for preclinical drug research.
In preclinical anti-cancer drug development, Genetically Engineered Mouse models (gemstones) play a critical role in simulating human cancer biology. Gemstones are created with the aid of manipulating specific genes to either be overexpressed or knocked out, mainly due to the formation of tumors that resemble the ones discovered in human beings.
Commonplace methods consist of:
CRISPR-Cas9 technology introduces particular mutations that mimic human oncogenes or tumor suppressor gene loss.
Cre-Lox recombination for tissue-particular or time-specific gene alteration.
Tet-ON/OFF machine to manipulate gene expression dynamically using inducible elements inclusive of doxycycline.
Xenografts of human cancer cells into immunodeficient gemstones to observe tumor development and drug efficacy.
Gems are used to assess drug efficacy, recognize mechanisms of resistance, and expect drug toxicity through modeling one-of-a-kind cancer kinds, tiers, and genetic profiles.
Gems have confirmed achievement in diverse degrees of preclinical drug improvement: Identity of Drug Objectives: GEM fashions, inclusive of the ones harboring BRAF or EGFR mutations, have helped pick out powerful centered healing procedures (e.g., Vemurafenib for BRAF mutations in melanoma). Predicting Drug Efficacy: Tumor increase inhibition has been observed with novel inhibitors focused on oncogenic pathways (e.g., PI3K, HER2), offering sturdy predictive value for medical trials. Understanding Resistance Mechanisms: gemstones monitor how tumors evolve below drug strain, uncovering secondary mutations (e.g., inside the EGFR pathway) that lead to drug resistance. Toxicity Profiling: gems expressing humanized variations of drug targets permit for the prediction of potential off-target effects in everyday tissues, enhancing the protection profiles of candidate capsules.
Gemstones constitute a transformative tool in the preclinical segment of anti-cancer drug improvement. Their capacity to faithfully reflect cancer genetics and progression gives worthwhile insights into healing responses and resistance mechanisms. But, a few limitations include: Incomplete Human representation: gemstones, despite their utility, may not mimic the complexity of human cancers, particularly in regards to immune gadget interactions or tumor microenvironments. Inter-species Variability: Drug responses in gemstones won't always translate to humans due to variations in metabolism or genetic pathways. Cost and Time: The improvement and protection of gemstones are aid-in-depth, requiring sophisticated centers and know-how. However, gems keep playing a pivotal role in narrowing down drug candidates for clinical trials, providing greater particular, customized methods for cancer treatment.
To process the thousands of novel herbal products and artificial purported anti-most cancer compounds that arise each year, preclinical drug screens need to be as brief and complete as resources permit. This can be accomplished by way of in vitro displays the use of cultured cellular lines that have oncogenic gene expression profiles similar to the ones visible in human patients. Compounds that display activity can then be moved to xenograft studies, which check toxicity and tumor regression ability. even though we've reviewed the energy of GEM models in verifying drug efficacy and pathway mechanism analysis, GEM fashions as they exist nowadays have fundamental flaws that prevent their use as high-throughput drug-screening tools. Gemstones require numerous months to develop tumors, and the penetrance is regularly far less than a hundred%. Tumors rise and grow at distinct costs in every man or woman mouse in a given observation. Besides melanoma, breast cancer, and some prostate cancer models where palpable tumors broaden, figuring out which animals have developed tumors at any given moment turns into a nearly insurmountable assignment. Many published studies use MRI pics to comply with tumor progression. Considering imaging the thorax of one mouse in an MRI can soak up to one hour, this method is incompatible with huge, excessive throughput studies. Small animal in vivo imaging (SAIVI) the usage of fluorescent or bioluminescent tagged sellers concentrated on tumors is a promising opportunity, however, the technology continues to be in its infancy, lacks suitably inducible GEM models for most varieties of cancers, and often require buy of luxurious, photosensitive probes and detection systems. Computed Tomography (CT) Imaging with medical-length instruments cannot be used due to the fact the dose of radiation used at some stage in imaging may additionally have healing effects on the tumors, especially when GEM models will require multiple imaging sessions over their lifetime to first pick out a tumor and then to monitor therapeutic drug consequences. But as smaller, less powerful, rodent-particular CT imagers become to be had by researchers, this can prove to be a beneficial method to degree tumor length in deep tissue. To make use of cutting-edge GEM models in preclinical drug trials, conventional tumor staging techniques have to be revised for every mouse to be treated as an affected person in a medical trial. Healing agents have to now not be administered en mass to all individuals in a treatment institution at a given time factor, but tailor-made to each mouse reflecting the date the tumor was recognized by using something modality the researcher has evolved. This can create a logistical nightmare, particularly if big combination treatment trials trying out multiple cars, routes of management, dosing schedules, and a couple of dose concentrations are attempted. Day zero has to be marked at the time an animal’s measured tumor length or general “tumor burden” (if the GEM generates a couple of foci concurrently) crosses a pre-determined threshold amount. Therefore, we cannot envision GEM fashions changing in vitro and xenograft models for the high throughput efficacy displays of novel dealers. But, we do trust GEM fashions can be of amazing value in the course of 3 steps within the preclinical process (discern 1). First, if GEM tumors are harvested and fragments efficaciously implanted subcutaneously (SC) or orthotopically into syngeneic, immune-ready mice, sufficient animals can be accrued to perform conventional xenograft-like monitors with genetically suitable murine tumors (allografts). Varticovski et al., (2007) offer an instance of this method, harvesting MMTV-PyMT breast tumors from some mice and passing them as fragments or cells suspensions into numerous host animals for subsequent drug studies. DNA microarrays have been used to affirm the handiest slight adjustments in gene expression after serial passages from the unique tumor. 2nd passage tumors exhibited similar sensitivity to paclitaxel and cyclophosphamide in comparison to the original tumor material. Once an agent suggests activity in vitro and tumor transplants, GEM models can be used to explore the drug mechanism and further optimize in vivo dosing before an agent head into an awful lot of extra expensive and onerous scientific trials (discern 1). Those optimization studies want now not to have excessive throughput and can take a year or more to finish. As a result, fewer anti-cancer-causing agents will efficaciously bypass those additional preclinical reviews than do presently develop to clinical trials. Currently, the handiest ~5% of novel anti-carcinogens entering scientific trials gain FDA approval (Kola & Landis, 2004); the maximum value and attrition takes place during section II and section III clinical trials. Consequently, any more time GEM-based total drug screening would upload to the preclinical drug development pipeline should be financially worthwhile to the drug sponsors and ethically useful for the volunteers participating in clinical trials if more efficacious cures are determined. GEM models might also in the long run have a fourth function in preclinical drug testing if researchers can effectively “humanize” them to be used in toxicology and pharmacology research. The cytochrome P450 (CYP) family of enzymes is expressed ordinarily in the liver and is worried inside the metabolism of a numerous range of healing compounds, pollutants, carcinogens, hormones, and xenobiotic dealers we may also come across. The seven most important CYP gene clusters found in humans are gifted and increased in mice (57 putative human CYP genes versus 102 putative useful genes in mice) (Nelson et al., 2004). But studies have shown that many character CYPs are differentially expressed or differentially active among the mouse and humans (Bogaards et al., 2000). This explains why drug metabolism inside the mouse does now not continually mirror and is expecting drug metabolism and toxicity within the human medical trials. Numerous GEM fashions have been generated in which the endogenous mouse CYPs or different xenobiotic-related metabolism genes are deleted and changed with their human orthologues, or without a doubt over explicitly the human orthologue in addition to the mouse CYP (reviewed in Cheung & Gonzalez, 2008). The expression of one or two human genes certainly is now not sufficient to declare a capacity GEM model as functionally “humanized” and geared up for excessive throughput toxicity and pharmacology research. But as transgenic technology evolves and these mice specifically several human orthologues, will indeed be able to higher predict human drug hobby and toxicity, and be of crucial importance in preclinical healing drug improvement.
The authors would like to acknowledge the contributions and support of individuals and organizations who aided in the completion of this research project. Special thanks to My Mentor Naweed Imam Syed Prof. Department of Cell Biology at the University of Calgary and Dr. Sadaf Ahmed from the Psychophysiology Lab at the University of Karachi for their invaluable input and support throughout the research.
Declaration of Interest:
The authors declare no conflicts of interest or financial disclosures related to this research.
Financial Support and Sponsorship:
No funding was received for the preparation of this manuscript.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.

Dear Editorial Team, Journal-Clinical Cardiology and Cardiovascular Interventions, “Publishing my article with Clinical Cardiology and Cardiovascular Interventions has been a highly positive experience. The peer-review process was rigorous yet supportive, offering valuable feedback that strengthened my work. The editorial team demonstrated exceptional professionalism, prompt communication, and a genuine commitment to maintaining the highest scientific standards. I am very pleased with the publication quality and proud to be associated with such a reputable journal.” Warm regards, Dr. Mahmoud Kamal Moustafa Ahmed

Dear Maria Emerson, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews’, I appreciate the opportunity to publish my article with your journal. The editorial office provided clear communication during the submission and review process, and I found the overall experience professional and constructive. Best regards, Elena Salvatore.

Dear Mayra Duenas, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews Herewith I confirm an optimal peer review process and a great support of the editorial office of the present journal
Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. I am really grateful for the peers review; their feedback gave me the opportunity to reflect on the message and impact of my work and to ameliorate the article. The editors did a great job in addition by encouraging me to continue with the process of publishing.

Dear Cecilia Lilly, Editorial Coordinator, Endocrinology and Disorders, Thank you so much for your quick response regarding reviewing and all process till publishing our manuscript entitled: Prevalence of Pre-Diabetes and its Associated Risk Factors Among Nile College Students, Sudan. Best regards, Dr Mamoun Magzoub.

International Journal of Clinical Case Reports and Reviews is a high quality journal that has a clear and concise submission process. The peer review process was comprehensive and constructive. Support from the editorial office was excellent, since the administrative staff were responsive. The journal provides a fast and timely publication timeline.

Dear Maria Emerson, Editorial Coordinator of International Journal of Clinical Case Reports and Reviews, What distinguishes International Journal of Clinical Case Report and Review is not only the scientific rigor of its publications, but the intellectual climate in which research is evaluated. The submission process is refreshingly free of unnecessary formal barriers and bureaucratic rituals that often complicate academic publishing without adding real value. The peer-review system is demanding yet constructive, guided by genuine scientific dialogue rather than hierarchical or authoritarian attitudes. Reviewers act as collaborators in improving the manuscript, not as gatekeepers imposing arbitrary standards. This journal offers a rare balance: high methodological standards combined with a respectful, transparent, and supportive editorial approach. In an era where publishing can feel more burdensome than research itself, this platform restores the original purpose of peer review — to refine ideas, not to obstruct them Prof. Perlat Kapisyzi, FCCP PULMONOLOGIST AND THORACIC IMAGING.

Dear Grace Pierce, International Journal of Clinical Case Reports and Reviews I appreciate the opportunity to review for Auctore Journal, as the overall editorial process was smooth, transparent and professionally managed. This journal maintains high scientific standards and ensures timely communications with authors, which is truly commendable. I would like to express my special thanks to editor Grace Pierce for his constant guidance, promt responses, and supportive coordination throughout the review process. I am also greatful to Eleanor Bailey from the finance department for her clear communication and efficient handling of all administrative matters. Overall, my experience with Auctore Journal has been highly positive and rewarding. Best regards, Sabita sinha

Dear Mayra Duenas, Editorial Coordinator of the journal IJCCR, I write here a little on my experience as an author submitting to the International Journal of Clinical Case Reports and Reviews (IJCCR). This was my first submission to IJCCR and my manuscript was inherently an outsider’s effort. It attempted to broadly identify and then make some sense of life’s under-appreciated mysteries. I initially had responded to a request for possible submissions. I then contacted IJCCR with a tentative topic for a manuscript. They quickly got back with an approval for the submission, but with a particular requirement that it be medically relevant. I then put together a manuscript and submitted it. After the usual back-and-forth over forms and formality, the manuscript was sent off for reviews. Within 2 weeks I got back 4 reviews which were both helpful and also surprising. Surprising in that the topic was somewhat foreign to medical literature. My subsequent updates in response to the reviewer comments went smoothly and in short order I had a series of proofs to evaluate. All in all, the whole publication process seemed outstanding. It was both helpful in terms of the paper’s content and also in terms of its efficient and friendly communications. Thank you all very much. Sincerely, Ted Christopher, Rochester, NY.
