AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2693-7247/091
Department of Chemistry, Panimalar Institute of Technology, Chennai, India - 600 123.
*Corresponding Author: : A. Kistan, Department of Chemistry, Panimalar Institute of Technology, Chennai, India - 600 123.
Citation: J. Sumathi, A. Kistan , B. Anna Benedict, (2022) Fluoride Adsorption From Aqueous Solution Using Natural Adsorbents Such As Centella Asiatica. J. Pharmaceutics and Pharmacology Research. 5(8); DOI: 10.31579/2693-7247/091
Copyright: © 2022, A. Kistan, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 02 May 2022 | Accepted: 20 June 2022 | Published: 01 July 2022
Keywords: adsorption of fluoride; centella asiatica; wastewater treatment; removal of fluoride; adsorption kinetics; intraparticle diffusion
In this study carried out by means of photo-colorimetry using the SPANDS indicator at 620 nm to adsorb Fluoride ion from aqueous solution. The influence of pH was studied by altering the pH from 2 to 12 using a 0.1 Molar concentration of HCl & NaOH solution using 10 ppm Fluoride concentration of as an initial point a kinetic investigation was conducted at pH 6.90 0.10 at various time intervals. The adsorption isotherm was investigated at pH of 6.90 by changing the initial fluoride concentration from 5 to 50 mg / L. The skills of 4 different isotherms used such as Langmuir, Freundlich, Temkin, and Redlich-Peterson adsorption isotherms were examined. To elucidate the mechanism of fluoride adsorption on the surface of the adsorbent, the kinetic model's pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich models are employed to examine the current adsorption data to determine the related kinetic parameters. Lastly, the effects of a number competing for ions (Cl-, NO3-, SO42-, PO43-, Ca2+ and Mg2+) were detected using, 20 - 200 mg / L solution at pH 6.90 ± 0.10 over 1 hr. SEM, XRD, and FT-IR techniques were used to investigate the surface morphology and size distribution of the natural adsorbent for fluoride adsorption (Centella Asiatica). The impact of adsorbent dosage, contact time, pH, co-ions, initial fluoride concentration, and shaking speed Centella Asiatica was determined and clearly reported
Water is an essential natural resource for sustaining life and environment that we have always thought to be available in abundance and free gift of nature. However, chemical composition of surface or subsurface is one of the prime factors on which the suitability of water for domestic, industrial or agricultural purpose depends. Freshwater occurs as surface water and groundwater. Though groundwater contributes only 0.6% of the total water resources on earth, it is the major and the preferred source of drinking water in rural as well as urban areas, particularly in the developing countries like India because treatment of the same, including disinfection is often not required. It caters to 80% of the total drinking water requirement and 50% of the agricultural requirement in rural India. But in the era of economic growth, groundwater is getting polluted due to urbanization and industrialization. Over the past few decades, the ever-growing population, urbanization, industrialization and unskilled utilization of water resources have led to degradation of water quality and reduction in per capita availability in various developing countries. Due to various ecological factors either natural or anthropogenic, the groundwater is getting polluted because of deep percolation from intensively cultivated fields, disposal of hazardous wastes, liquid and solid wastes from industries, sewage disposal, surface impoundments [1,2]etc. During its complex flow history, groundwater passes through various geological developments leading to consequent contamination in shallow aquifers. Presence of various hazardous contaminants like fluoride, arsenic, nitrate, sulphate, pesticides, other heavy metals etc. in underground water has been reported from different parts of India [3,4]. In many cases, the water sources have been rendered unsafe not only for human consumption but also for other actions such as irrigation and industrial needs. Therefore, now there is a need to focus greater attention on the upcoming influence of water resources development and development taking into consideration all the related issues. In India, fluoride is the major inorganic pollutant of natural origin found in groundwater.
Experiments on fluoride adsorption
Dissolving 0.221 NaF (analytical grade) in 1 L of distilled water obtained a stock solution (100 mg/L). All of the solutions for the fluoride removal studies were made by diluting the stock solution appropriately. Adsorption studies were carried out in a conical flask immersed in a temperature-controlled water bath and shaken by mechanical shaker (Tai Tec, Thermo Minder Mini-80, Japan) for the required time at a rate of 120 cycles / minute for each desired initial fluoride concentration solution. Photocolorimetry was used to analyze fluoride using the SPADNS indicator at 620 nm [5]. The adsorption isotherm at pH 6.90 ± 0.10 was studied by varying the initial fluoride concentration from 5 to 50 mg / L. The effect of pH was investigated by adjusting the pH from 2 to 12 using 0.1 M NaOH and HCI solutions under an initial fluoride concentration of 10 mg / L. A kinetic study at pH 6.90 ± 0.10 was carried out at different time breaks with an initial fluoride concentration of 10 mg/L. Finally, the effects of several competing ions (CI-, NO3-, SO42-, PO3-4, Ca2+, and Mg2+) were observed using, 20 - 200 mg / L solutions at pH 6.90 ± 0.10 over 1 Hr.
Physical characterization
SEM, XRD, and FTIR spectrum analyses were used to examine the surface shape and size distribution of the ayurvedic adsorbent powders. XRD and SEM were used to characterize the AUYRVETIC Adsorbent powders before and after batch adsorption tests. For the production of XRD and SEM samples, a representative experiment was carried out under the following conditions: 30 oC, initial F- concentration = 50 mg / L, shaking speed = 150 rpm, and contact time = 1 h, respectively. An X-ray diffractometer, Model (Phillips, 'X' Pert), was used to record the X-ray diffraction patterns. Two ranges of 0o to 60o were scanned on the sample. Jeol, XA-840 A, was employed for the SEM study. A Nicolet 560 FTIR spectrophotometer was used to record the FITR spectra.
Chemicals Used
A stock solution of fluoride was made by dissolving 2.21 g of sodium fluoride in 1 L distilled water. The NaF substance used of analytical grade (Merck, Germany). The necessary concentration of fluoride solution was made by sequential dilution of 1000 mg / L Fluoride solution [6].
Theory of isotherm models
The ability of four commonly used isotherms to predict adsorption equilibrium data were investigated: the theoretical Langmuir, empirical Freundlich, Temkin, and Redlich-Peterson isotherms. The pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich models are used to analyse the present adsorption data and derive the corresponding kinetic parameters to clarify the mechanism of fluoride adsorption onto the surface of the adsorbent.
Adsorption via Langmuir [7] isotherm is the most well-known of all isotherms, and it's used to describe saturated monolayer adsorption in solid/liquid systems. It can be written as:
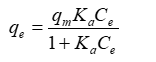
Where Ce denotes the equilibrium concentration (mg/L), qe denotes the quantity of ion adsorbed (mg / g), qm denotes the qe for a full monolayer (mg / g), and Ka is the adsorption equilibrium constant (L / mg). The aforementioned equation (Eq. (1)) can be employed as a linear form to evaluate the adsorption capacity for a specific range of adsorbate concentration.
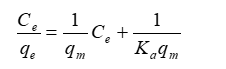
The slope of the linear plot of Ce / qe vs Ce can be used to estimate the constants qm and Ka from a linearized form of Eq. (2).
The Freundlich adsorption isotherm [8], which is based on adsorption on a heterogeneous surface, is the first known linking for characterising adsorption equilibrium and is provided by:
qe = KF Ce1/n (3)
Where qe is the quantity of ion adsorbed (mg / g); Ce denotes the equilibrium concentration (mg/L); and KF and 1/n denote the adsorption capacity and intensity, respectively. By using logarithms, Eq. (3) can be transformed to a linear form [9].
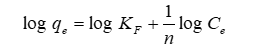
Eq. (4) should provide a straight line when plotting log qe versus log Ce. The values for n and KF can be calculated using the slope and intercept from the Fig. 6. [10] Temkin Isotherm, the most basic form of the adsorption isotherm model, was intended to account for the chemisorption of adsorbate onto the adsorbent and is denoted as
qe= a + b log Ce (5)
Where qe and Ce have the same meaning as noted previously and the other parameters are called the Temkin constants. The plot of qe versus log Ce will generate a straight line. The Temkin constants a and b can be calculated from the slope and intercept of the linear plot. Redlich-Peterson isotherm [11] contains three parameters and incorporates the features of the Langmuir and the Freundlich isotherms. It can be described as follows:
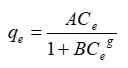
Eq. (6) can be converted to a linear form by taking natural logarithms:
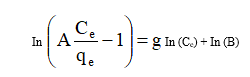
Three isotherm constants, A, B, and g (0 < g>
Effect of adsorbent dose on fluoride removal
The amount of fluoride ions removed by 20, 15, 25, 50, 100 mg / 50 mL of Centella Asiatica (Fig. 1). The increase in the amount of fluoride ions adsorbed with a decrease in the dose of the adsorbents was due to the availability of higher F-/ adsorbent ratio. Further experiments were carried out using 10 mg / 50 mL as the dose of the adsorbents as it exhibits appreciable removal capacity for optimizing of adsorption parameters [13,14].
Effect of contact time
The effect of the contact time on fluoride adsorption by Centella Asiatica was studied. As it is shown from Fig. 2. The adsorption occurred in two steps, an initial fast step which lasted for 10-20 min and a slower second phase which continued until the end of the experimental period. The equilibrium was reached within 40 min [15]. further increase in contact time did not show an increase in adsorption.
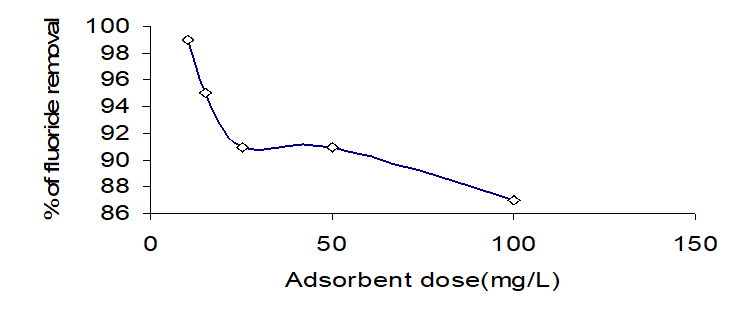
Effect of pH
The effect of pH on fluoride removal from ground water by Centella Asiatica was studied at various pH values, [Ranging from 2 to 12]. Fig.3 shows the effect of pH on fluoride adsorption onto the Centella Asiatica and shifts in pH after adsorption. The CA exhibited strong adsorption of fluoride when the pH was between 3.0 to 6.5 and 8 to 11. The removal efficiency fell sharply as pH decreased below 5 or increased above 8.0. The sharp decrease in the amount of fluoride adsorbed under alkaline pH condition is probably due to competition for adsorption sites among F- and OH- [16]. On the other hand, under acidic condition the reduction might be ascribed to the creation of weakly ionized HF acid.

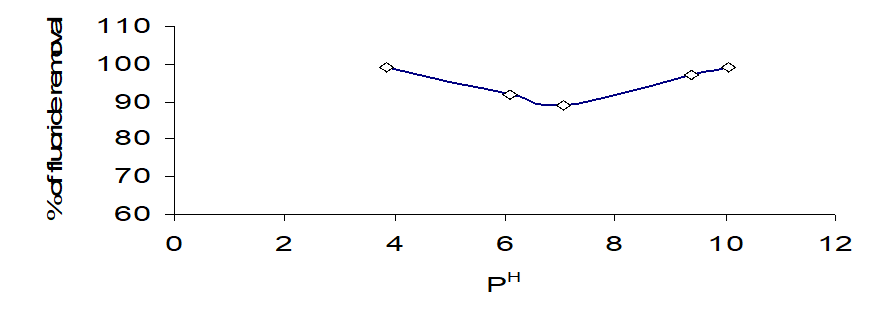
Effect of co-ions
Fluoride contaminated water contains several other ions that may influence the adsorption process. This study assessed fluoride adsorption behaviour in the presence of 0.030 mg / 50 mL (or) 0.30 g / L salt solution of chloride, sulphate, nitrate carbonate & phosphate, independently, at an initial fluoride concentration of 0.01 ppm/ 50 mL (or) 100 ppm / L. The effect of these coexisting ions on F-removal is shown in Fig. 4. It was observed that, fluoride removal decreased from 79% to 67% in the presence of Nitrate and to 67% in the presence of chloride but slightly increased in the presence of sodium carbonate (NaCO3) and Sodium Sulphate (Na2S2O3). (Fluoride removal in the presence of an ions increased in the order PO43- < NO3>
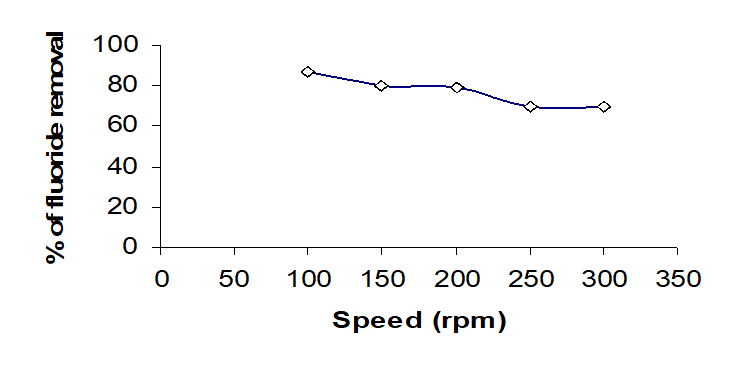
Effect of Speed
Adsorption studies were carried out with a mechanical shaker at pH 7.0 fluoride solution was 100 mg/l. The agitation speed varied from 100 to 300 rpm. The adsorption removal efficiency weakly with increasing agitation rate because on agitation rate of 120 rpm was enough to remove fluoride as seen Fig. 6.The result of adsorption of 60 min is that the fluoride removal was constant 87% [18].
Effect of concentration of fluoride
A series of experiments were carried out by changing the initial concentration of Adsorbate using the centella Asiatica (CA). It was observed that the adsorption capacity of the adsorbent increase from 53% to 83% when the initial concentration of fluoride is 100 mg / l. It was noticed that with increase in initial fluoride concentration, the percentage removal of fluoride decreases (Fig. 5). It may be because at higher adsorbate concentration the binding capacity of the adsorbent approaches saturation resulting in a decreases in overall percentage removal [19]
Mathematical Adsorption Isotherm
The ability of CA to adsorb fluoride from aqueous solution was evaluated from the general nature of the adsorption isotherm plot (Ce against qe,) The equilibrium adsorption data were fitted to the Langmuir and Freundlich isotherm models that have been used most frequently to describe equilibrium adsorption data. For a solid-liquid system, the Langmuir isotherm is expressed in linear form as
1/qe = (1/QbCe) + 1/Q
where qe is the amount of solute adsorbed (mg g-1) at equilibrium and Ce is equilibrium solute concentration (mg dm-3) in solution. The values of empirical constants Q and b, denoting monolayer capacity and energy of adsorption respectively, are evaluated (0.6552 mg g-1 and 0.3563 dm3 mg-1, respectively) from the slope and intercept of the linear plot of 1/qe against 1/Ce (Fig. 7).
The Freundlich model is expressed as
Qe = Kf (Ce)1/n
where Kf and 1/n are the characteristic isotherm constants of the system. The Freundlich equation is an empirical expression that encompasses the heterogeneity of the surface and the exponential distribution of sites and their energies. For linearization of the date, the Freundlich equation is written in logarithmic form as
ln qe = ln Kf + (1/n) ln Ce

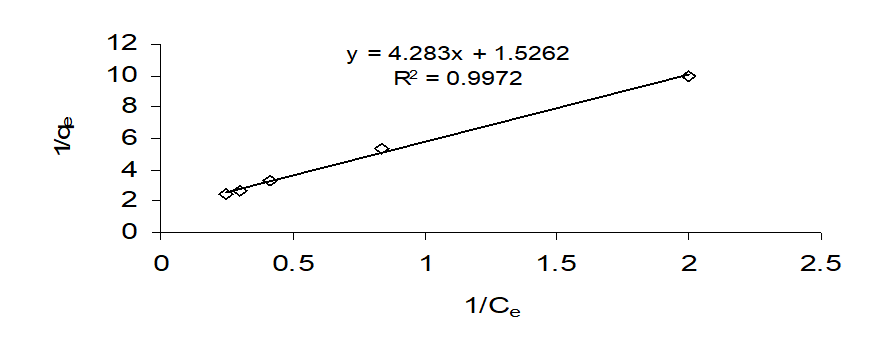

A linear plot of lnqe against lnCe yields a slope of 1/n and intercept of ln Kf (Fig. 8) The value of Kf (0.162 mg g-1) is roughly an indicator of adsorption capacity and the value of 1/n (0.6767) indicates the adsorption intensity. Evaluation of the preferred isotherm model, judged from the regression coefficient value (R2), indicates that either the Langmuir (0.9972) or Freundlich (0.9982) equation is applicable to fluoride CA interaction. The influence of isotherm shape evaluated from ‘r’, a dimensionless constant, defined as:
r = 1 / (1+bC0)
where b is the Langmuir constant and C0 is the initial fluoride concentration (100 mg / L). The parameter ‘r’ indicates the shape of the isotherm accordingly:

Favorable adsorption is predicted in the present case from the r value of 0.027.
3.9Kinetics and rate parameters
In order to understand the kinetics of the process, the data were fitted to a pseudo-first-order rate expression, the Lagergren [20] rate equation, as
ln (qe – qt) = ln qe – K1t
where qe and qt are the amounts of fluoride (mg g-1) on Centella Asiatica at equilibrium and at time ‘t’, respectively, and K1 (min-1) is the first-order rate constant. A plot of ln (qe – qt) against time (t) should yield a straight line (Fig. 9), and the rate constant K1 was evaluated from the slope and was found to be 4.4 x 10-2, 0.38 x 10-2, 0.12 x 10-2, 0.09 x 10-2 and 0.1 x 10-2 min-1 for the concentration of fluoride ion, 100, 200, 300, 400 and 500 mg / L respectively. The values of K1 were found to decrease with increase in the initial concentration of fluoride from 100 to 400 mgL-1 and then a small increase was found at F- concentration of 500 mgL-1. An examination of the effect of fluoride ion concentration on the K1 helps to describe the mechanism of removal taking place. In cases of strict surface
adsorption, a variation of rate should be proportional to the first power of concentration. However, when pore diffusion limits the adsorption process, the relationships between initial solute concentration and the rate of reaction will not be linear. Hence, it seems likely that pore diffusion limits the overall rate of fluoride adsorption [21].
The data may be fitted to the pseudo-second-order expression
t/qt = (1/K2) (1/qe2) + t/qe
Applying the boundary conditions of t=0 to t=t and qt=0 to qt = qt. A plot of t/qt against ‘t’ should yield a straight line (Fig. 10) with K2 (g mg-1 min-1), the second order rate constant, obtained from the intercept.
The applicability of a particular rate expression for the fluoride- Centella Asiatica was evaluated from the goodness of data fit and regression coefficient value (R2). In the present situation the Lagergren first-order equation gives a lower regression value (R2 > 0.8843) than the second-order equation (R2 > 0.9933), showing adherence to the pseudo-second-order rate law.


3.10 Adsorption Experiments
SEM micrographs of Centella Asiatica powder before and after adsorption is presented in Fig. 11 , 12. The SEM micrographs clearly show the surface modification. The SEM micrographs of fluoride loaded Centella Asiatica powder adsorbent shows small spherical fluoride ion adsorbed over the surface of adsorbent. According to the SEM spectra there is an electrostatic attraction between adsorbate and surface of the adsorbent particles [22]. In this experiment almost above 85% of the fluoride has been removed. The FT-IR spectra of the untreated Centella
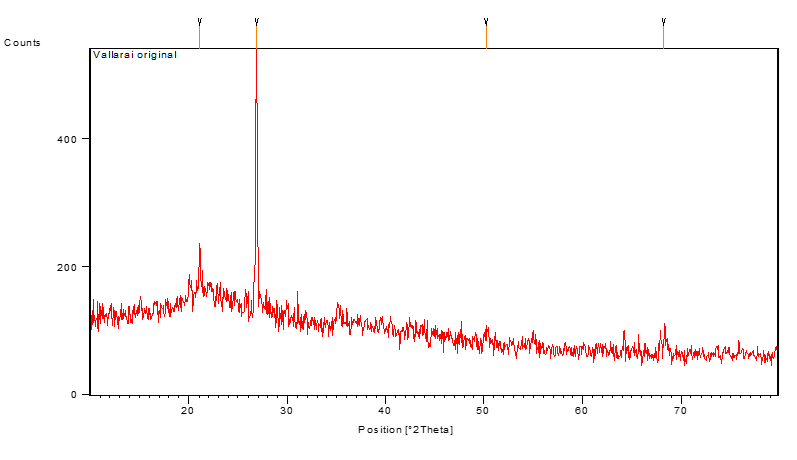
Asiatica adsorbent and that of after treated with fluoride ions are shown in Fig 13, 14. The FT-IR spectrum provides direct evidence for the adsorbing of fluoride ions onto Centella Asiatica powder. The XRD patterns of the Centella Asiatica powder adsorbent before and after doping are shown in Fig. 15, 16 and (Fig. 16) respectively. It shows that the presence of fluoride appears in the spectrum after doping Fig. 16. Its evident from the XRD studies (Fig. 15, 16) that the Centella Asiatica showed significant changes after the adsorption of fluoride ions. This also suggests that the update of fluoride ions Centella Asiatica was due to the precipitation.



For fluoride removal from aqueous solution, a novel media, ayurvedic powder adsorbent such as Centella Asiatica, has been created. The ayurvedic powder adsorbent has several features that make it a highly effective medium for eliminating fluoride at normal pH. The smaller particle size of powder adsorbent increased agitation time, speed, and temperature, indicating that fluoride removal is favored at a lower concentration. The equilibrium adsorption can be attained in as little as 60 minutes. According to the findings, the amount of fluoride removed from water is dependent on contact time, pH, and adsorbent dosage. De-fluorination of water happens via doping of fluoride ions onto the ayurvedic adsorbent powder, according to the results of equilibrium, FT-IR, XRD, and SEM tests. Centella Asiatica's experimentally measured fluoride adsorption capacity was higher than that of other adsorbents previously reported. For each adsorbent, the optimal pH for maximal fluoride removal differs. The theoretical Freundlich and Langmuir models accurately represented the findings of this study. When the initial fluoride content was low, the removal effectiveness was highest, indicating that this technology might be used to treat domestic applications. This research reveals that ayurvedic powder adsorbents can remove up to 99 percent of contaminants and that this process may be run in a simple, dependable automated manner with minimum manpower utilizing a compact modular approach. We may conclude from this research that the ayurvedic powder adsorbent Centella Asiatica could be employed as a sorbent. From this study, we can conclude that ayurvedic powder adsorbent Centella Asiatica could be used as a promising alternative adsorbent for the de-fluorination of water.
I hereby declare from all authors that manuscript is new and not submitted anywhere for the publication. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit-sectors.
Authors declares there is no conflict of interest for this research work
One of the authors J. Sumathi expresses her sincere thanks to Dr. Prakash Periakaruppan, Assistant Professor, Department of Chemistry, Thiagarajar College, Madurai, Tamil Nadu, India, for his useful discussions, tremendous care and continual support.