AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2766-2314/119
Department of Food Science and Technology, Federal University of Technology, P.M.B 704, Akure, Ondo State, Nigeria.
*Corresponding Author: Omolara R. Adegbanke, Department of Food Science and Technology, Federal University of Technology, P.M.B 704, Akure, Ondo State, Nigeria.
Citation: Omolara R. Adegbanke, Victor N Enujiugha (2023), Evaluation of Amino and Fatty Acids Profiles and Sterols of Raw and Processed Conophor Nut (Tetracarpidium Conophorum), J, Biotechnology and Bioprocessing, 4(6); DOI:10.31579/2766-2314/119
Copyright: © 2023, Omolara R. Adegbanke. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 September 2023 | Accepted: 18 September 2023 | Published: 28 September 2023
Keywords: conophor nut, amino acids, fatty acids, sterols, seed oil
Conophor nut oil is reported to be rich in sn-3 fatty acid (linolenic acid) with a protein- rich defatted residue. This study investigated the effect of toasting and cooking on the amino acid profile, the fatty acid profile and the content of sterols of conophor nut (Tetracarpidium conophorum). Freshly harvested conophor nuts were processed by cooking (at 100 oC for 90 min) and toasting (at 145 oC for 50 min). Raw nuts served as the control. The raw and cooked seeds were dried (60 oC for 8 h) and all samples were pulverized; oils were also obtained from the samples. Oils extracted from the seed flours were analyzed for physicochemical properties, amino and fatty acids profiles, and sterol composition. Glutamic acid was the most abundant ranging from 2.80 for raw to 3.31 g/100 g crude protein for processed flour. However, the highest value (3.31 g/100 g crude protein) was recorded in the cooked sample. Glutamic and aspartic acids in the samples made up to 6.60-6.77 g/100 g crude protein on an average basis with a percentage of 22.76-26.49. Leucine was the most concentrated essential amino acid with values of 1.93 g/100g in the raw and 1.88 g/100 g and 1.65 g/100g in the cooked and toasted flour samples respectively. The predominant fatty acid group in the oils was the polyunsaturated fatty acids (PUFAs) with values ranging from 77.11 to 79.65 %. Alpha-linolenic acid was found to be the most abundant fatty acid in the oils (61.96-62.64 %) followed by linoleic acid (14.37-16.12 %) and oleic (13.69-16.00 %). Other fatty acids occurred in trace amounts. Campesterol and stigmasterol were the two most abundant of the sterols identified. Cholesterol was not detected in the oil obtained from the toasted conophor nut but its precursor, desmosterol was present.
The conophor plant (Tetracarpidium conophorum (Müll. Arg.) Hutch. And Dalz. (Euphorbiaceae), commonly called the African walnut is a perennial climbing shrub, open-branched and mainly tropical (Enujiugha, 2008). The nut is known as ‘ukpa’ in the Igbo and ‘awusa’ or ‘asala’ in the Yoruba speaking tribes of Nigeria. In Cameroon, it is known as ‘kaso’ or ‘ngak’ (Ajaiyeoba and Fadare, 2006). The plant is cultivated principally for the nuts which are cooked or toasted and consumed as snacks or along with boiled corn (Enujiugha, 2003). The freshly harvested mature fruits are yellowish green in colour and turn dark brown when left to age. The nuts are encased in carps which contain four (4), three (3), or two (2) nuts per pod. The seed is made up of two cotyledons enclosed in a hard brown shell-like case within the pods (Nkwonta et al.,2010). The leaves and young shoots of the plant are known to be edible (FAO, 2006). However, the nuts attract the most attention because of the high nutrient contents (Enujiugha and Ayodele-Oni, 2003).
A bitter after taste is usually observed upon drinking water immediately after eating conophor nut and this could be attributed to the presence of alkaloids and other antinutritional factors (Enujiugha, 2003). Significant concentrations of oxalates, phytates and tannins have been reported in raw conophor nut (Enujiugha and Ayodele – Oni, 2003), but the effect of processing on these factors has not been investigated. Sato et al., (1994) isolated isolectins from the nut cotyledons and evaluated the carbohydrate-binding specificity. Olabinri et al., (2010) reported on the chelating ability of Tetracarpidium conophorum nut in vitro and stated that the extract may be explored in the industrial production of iron chelators due to its high chelating ability in vitro at low doses, which would be of clinical relevance in the treatment of iron-overload disorders such as thalassemia (a group of genetically inherited blood disorders characterized by defective globin chain of hemoglobin and iron overload).
Oladiji et al., (2010) reported that Tetracarpidium conophorum oil did not adversely affect growth performance and the feeding appetite of experimental rats. Ndie et al., (2010) observed that defatted flour derived from conophor nut with high protein content, high water absorption capacity, high solubility and good pasting characteristics could be used as composite flour in the preparation of bread and confectionaries.
Sample Collection
One hundred kilograms (100 kg) of nuts were extracted from the T. conophorum fruits and transported from the plantation to laboratory within few hours of harvest. The fresh T. conophorum nuts were sorted to remove defectives and later washed using potable water. They were then divided into three potions (Raw, Cooked and Toasted). Raw was shelled and the seeds obtained were chopped and dried at 60 oC to a constant weight in a hot air oven (model HS60 manufacturer) for 8-10 h. A portion was cooked (at 100 oC for 90 min) and another toasted (at 145 oC for 50 min) respectively. The kernels obtained from cooked nuts were dried to a constant weight as for raw. The samples were dry-milled separately using a local attrition mill and the flour obtained in each case was packed in cellophane bags and kept frozen at -4 oC prior to further analysis.
Extraction of Conophor Oil
Samples of oils and defatted samples of the seed flours were obtained by continuous solvent extraction method using n-hexane for 8 h. Defatted meal was later dried at 60 oC in a hot air oven (model HS60) to remove residual solvent and later pulverized into powder, sieved and packaged in air tight container for further analysis. The oil extracted was dried to remove residual n-hexane and later stored in air-tight and amber containers and kept in the freezer for further analysis.
Amino Acid Analysis of Raw and Processed Conophor Seeds
Amino Acid profiles of the flour obtained from both raw and processed conophor seeds were determined using Amino Acid Analyzer (S4300, Amino Acid Analyzer, Sykam, Germany).
Procedure: The samples were hydrolysed for 24 h with 6 M HCl according to the method described by Bidlingmeyer, (1984). The cysteine and methionine contents were determined after performic acid oxidation as described by Gehrke, (1985). Typtophan content was determined after alkaline hydrolysis using the method described by Landry and Delhaye, (1992).
Fatty Acid Analysis of Conophor Oil
The fatty acid profile of the oils from raw and processed conophor seeds were determined using Gas Chromatography (Varian 450 GC, The Netherlands).
Methylation: About 0.11 g of the oil was weighed into a 5 ml vial and 1 ml of hexane was added and mixed thoroughly. A portion of the diluted oil (0.1 ml) was pipetted into an 8 ml (13 x 100 mm) screw top tube and the solvent was then evaporated under nitrogen in a warm water bath at 30-35 oC. Then 1 ml of toluene was added to the tube and vortexed for 5-10 s after which 1.2 ml of methanolic HCl was added. The tube was capped tightly with Teflon lined lids and vortexed for 5-10 s. The tube was removed from oven and allowed to cool to room temperature. Then 1 ml of distilled water was added and also 1 ml of hexane. The tube was capped and vortexed for 20 s. The aliquot was centrifuged at 2000 rpm for 3-4 min. The upper layer was transferred to a clean 8 ml tube and 2 ml of water was added to the hexane layer. The tube was capped and vortexed for 20 s. The aliquot was centrifuged for 3-4 min. Part of the hexane layer was transferred to a GC vial (approximately ½ full); the vial was capped.
GLC analysis: A Varian GC 450 gas chromatograph equipped with a flame ionization detector and an injector with a split/split less device for capillary column was used. The chromatographic column was of a chemically bonded fused silica DB225MS capillary column (30 m x 0.25 mm I.D) (Agilent J & W) and hydrogen was used as the carrier gas. The GLC operating conditions were as follows: injector and detector temperatures were 280 oC and 290 oC, respectively. After injection, oven temperature was kept at 70 oC for 2 min and then programmed at a rate of 30 oC/min to a temperature of 180 oC and held for 1 min followed by 10 oC/min to 200 oC and held for 2 min, followed by 200 oC/min to 220 oC and held for 10 min and finally 220 oC/min to 240 oC and held for 5 min.
Sterol Composition of Conophor Oils
The sterol composition of the oils from the raw and processed conophor seeds was determined using Gas Chromatography (Varian 450 GC, The Netherlands).
Sterol extraction from conophor oils: Oil (100 µl) was taken in 1 ml of 0.5 N sodium hydroxide and heated at 80 oC for 1 h. After cooling, the neutral sterols and stanols were extracted with n-hexane (3x3 ml). Hexane was evaporated to dryness and the residual product dissolved in 2 ml chloroform. Subsequently 0.5 ml aliquot was transferred to a 2 ml GC vial and evaporated to dryness.
Procedure: trimethysilyl (tms) ether derivatization of sterols and stanols: Aliquots of the biological extracts that contained the sterols (and stanols) were treated with 100 µl of Sil-Prep for 30 min at 55 oC in a screw cap tube. Solvent was evaporated at 55 oC under nitrogen, the reaction product is dissolved in 100 µl hexane and an aliquot (1-5 µl) is used for gas liquid chromatography.
GLC analysis: A Varian GC 450 gas chromatograph equipped with a flame ionization detector and an injector with a split/ split less device for capillary columns was used. The chromatographic column consisted of a chemically bonded fused silica DB17 capillary column (30 m x 0.25 mm ID) (Agilent J and W) and hydrogen was used as the carrier gas. The GC operating conditions were as follows: injector and detector temperatures are 280 oC and 300 oC, respectively. After injection, oven temperature were kept at 130 oC for 2 min and then programmed at a rate of 30 oC/min to a final temperature of 290 oC.
All analyses were carried out in triplicate and data were subjected to Analysis of Variance (ANOVA) using SPSS version 19.0 (for windows). Statistical differences between mean values were determined by Duncan’s Multiple Range Tests and accepted at P less than 0.05.
Amino Acid Content of Raw and Processed T. conophorum Seed Flours (g/100 g protein)
The results of the amino acid profile of raw and processed T. conophorum seed flours and the summary of amino acid nutritional indices are presented in Tables 1 and 2. Glutamic acid was the most abundant amino acid. In general, the amino acid content was significantly higher (p less than 0.05.
Glutamic acid was the most abundant ranging from 2.80 for raw to 3.31 for processed. However, the highest value was recorded in the cooked sample. This followed similar trend in the amino acid profile of a plant source Ogunlade et al., 2011.
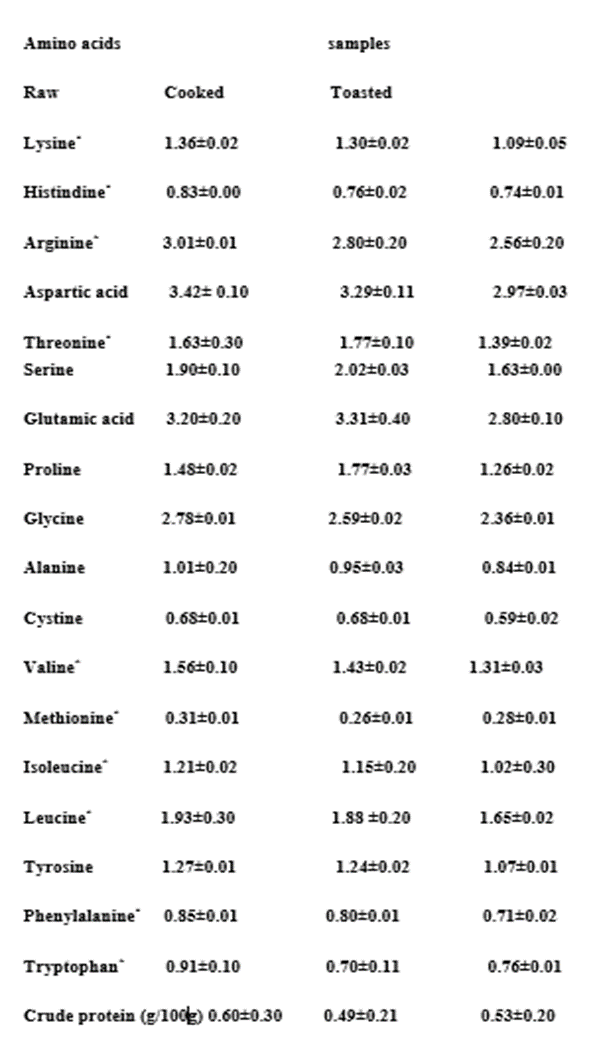
Table 1: Amino acid Profile of Raw and Processed T. conophorum Seed Flours
Values in the table are means of three determinations ±SD. Means in the same row with different superscripts are significantly different (pless than 0.05).* Essential amino acids
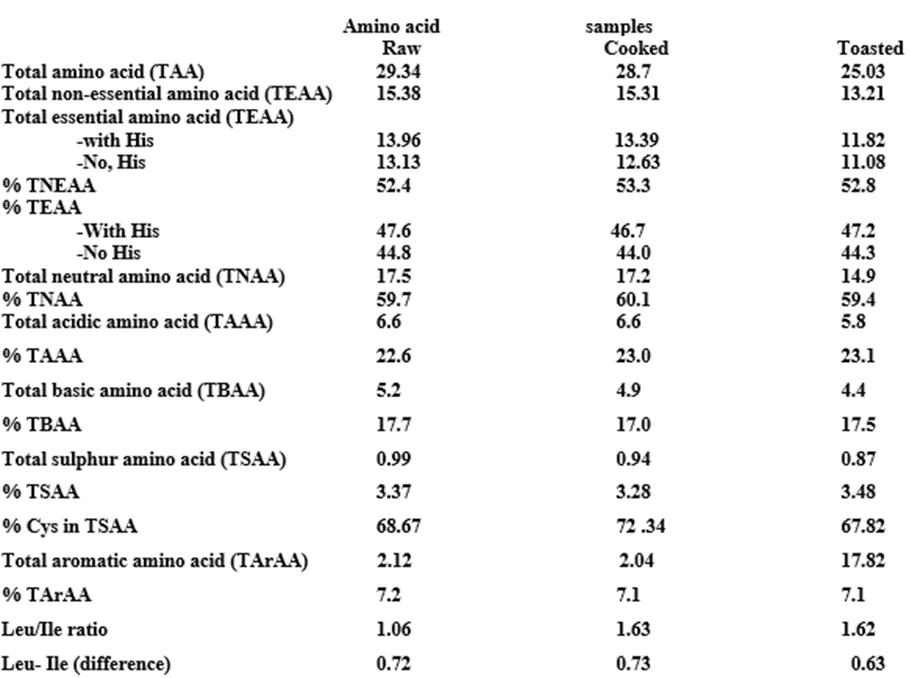
Table 2: Concentrations of essential, non-essential, acidic, neutral, sulphur, aromatic (mg/g crude protein) of Raw and Processed T. conophorum seed flours.
Glutamic + aspartic acids in the samples made up to 6.60-6.77 g/100 g crude protein on an average basis with a percentage of 22.76-26.49. Similar observation has been reported by Olaofe and Akintayo 2000 and Adeyeye and Afolabi 2004. Leucine was the most concentrated essential amino acid with values of 1.93 g/100g in the raw and 1.88 and 1.65 g/100g in the cooked and toasted sample flours respectively which is greater than that of soya beans, 0.9 g/100g (Temple and Aliyu 1994) but lower than the FAO standard for preschool children (2 to 5 years) FAO 1985. Isoleucine, (essential amino acid) values in the flours ranged from 1.02-1.21 g/100g crude protein; these values are high when compared to the FAO standards of 2.8 g/100g crude protein FAO 1985. The lysine (Lys) content of between 1.09-1.36 g/100g crude protein was low compared to the reference egg protein of 6.3 g/100g crude protein Adeyeye and Afolabi 2004. The phenylalanine and tyrosine (Phe + Try) levels ranged from 1.78-2.14 g/100g crude protein in the processed samples, showing that the cooking and toasting gave values that were comparable with FAO/WHO/UNU standards (2.3 g/100g crude protein) suggesting that processed conophor nut seeds can be exploited to enhance the protein quality of weaning/complimentary feeding, especially in dry form.
The total amino acid (TAA) ranged between 25.56 to 29.94 g/100g crude protein. The values are lower than the value of 56.16 g/100g crude protein of the reference egg protein Paul et al., 1970 and compare favorably with 21.48 to 30.70 g/100g crude protein for guinea corn Adeyeye and Afolabi 2004. In general, the amino acid content was significantly higher (P less than 0.05) in the raw seeds.
Fatty acid composition of oils from raw and processed conophor oil
The results of the fatty acid profile of oils from raw and processed T. conophorum seeds are presented in Table 3. There was a significant difference (Pless than0.05) in the fatty acid composition among the samples. The unsaturated fatty acids were the most predominant fatty acids representing up to 94.68% (Table 3). Cooking improved the content of the unsaturated fatty acids. There was a significant difference (Pless than0.05) in the fatty acid composition among the samples; this may be due to the differences in the processing conditions. The predominant fatty acid group in the conophor seed oils (Table 3) was the unsaturated fatty acids.

Table 3: Fatty Acid Profile of Oils from Raw and Processed T. conophorum seeds
Values in the table are means of three determinations ±SD. Means in the same row with different superscripts are significantly different (pless than0.05).
ND --- not determined
The percentage unsaturated fatty acids ranged from 80.28 to 94.63 % consisting mainly-Linolenic acid (61.96-62.64 %); Linolenic (14.37-16.12 %) and oleic (13.69-16.00). The saturated fatty acid in the seed oils ranged from 4.32 to 4.73 %, consisting mainly of stearic acid (2.59-2.82 %) and palmitic acid (1.65-1.66 %). Other saturated fatty acids occured in insignificant amounts. From the results, conophor oil is a good source of polyunsaturated fatty acids (PUFAs) as presented in Table 4. High levels of blood cholesterol are associated with high intake of saturated fatty acids El-mallah et al., 2011. It has been concluded that relative to carbohydrate, the saturated fatty acids elevate serum cholesterol; while the polyunsaturated fatty acids lower serum cholesterol Hegested et al., 1993. High intake of conophor seed oil with high levels of unsaturated fatty acid may not lead to the risk of increased blood cholesterol in the body. Oils from the processed seeds were significantly higher in the unsaturated fatty acids than oil from the raw seeds. Alfawax 2004 also reported linolenic acid as the predominant fatty acid in pumpkin seed oil. Linolenic acid has been reported as the most
important essential fatty acid required for growth, physiological functions and body maintenance Salunkhe et al., 1985. Conophor seed oils will participate in these functions. The very low content of trans fatty acid (trans palmitoleic acid), 0.05-0.06 % supports further the nutritional integrity of conphor seed oil, since studies have shown that the consumption of trans fatty acids elevates LDL-cholesterol (bad cholesterol) and decreases the HDL-cholesterol. The linolenic acid contents were higher than (0.53-0.60 %) those reported by Unal and Yalcin 2008 for varieties of sesame seed oils and 0.14-0.21 % reported for saftflower oils Vosoughkia et al., 2011.
Sterol composition of T. conophorum seed oils
Sterols are components of unsaponifiable compounds in fats and oils and are important to indentify blends of fats and oils Mariani et al., 1994. Sterols are also of interest because of their ant oxidative activities Dutta et al., 1994. The conophor seed oils differ significantly (Pless than 0.05).
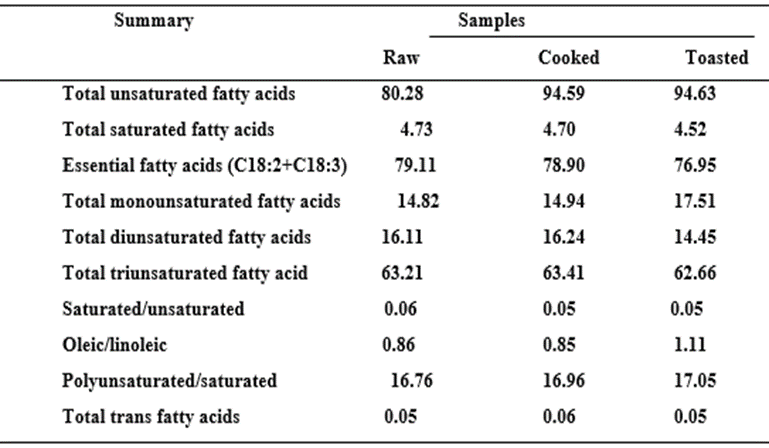
Table 4: Summary of the Fatty acid Composition (%) of Oils from Raw and Processed T. conophorum seeds.

Table 5: Sterol Composition (%) of Oils from Raw and Processed T. conophorum seeds.
Values in the table are means of three determinations ±SD. Means in the same row with different superscripts are significantly different (pless than0.05).
ND --- not determined
The campesterol in the oil from the processed seeds are higher than in the oil from the raw seeds. The levels are higher than 9.45-14.17 reported for Carthamus tinctorious seed oil Vosoughkia et al., 2011. Campesterol has been reported to be involved in controlling cholesterol and lowering the risk of heart disease. Stigmasterol content of the oil ranged from 28.12 to 23.78 %. Processing improved the stigmasterol content of the oils. Plant sterols are essential components of the membranes of all eukaryotic organisms. The commonly consumed plant sterols are sitosterol, stigmasterol and campesterol which are predominiantly supplied by vegetable oils. The nutritional benefits derived from sterols include their ability to lower plasma cholesterol and LDL cholesterol Piironen et al., 1999. A hypothesis to explain the effectiveness of sterols as antioxidants has been presented.
It can be concluded that lipid free radicals react rapidly with sterols are unhindered allylic carbon atoms forming relatively stable allylic tertiary free radical which are slow to react further and this interrupts the antioxidation chain. Cholesterol is injurious to health as it blocks the arteries leading to vascular arteriosclerosis. The levels of the cholesterol make the oils heart friendly.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti