AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2693-7247/093
State Higher Education Institution "Uzhhorod National University", Narodnaya Sq. 3, Uzhgorod
*Corresponding Author: Shuaibov O.K, 1State Higher Education Institution "Uzhhorod National University", Narodnaya Sq. 3, Uzhgorod.
Citation: Shuaibov O.K1, Minya E.Y, Hrytsak R.V, Malinina A.A, Malinin A.N et al(2022) Electrophysical Characteristics Of Gas-Discharge Synthesis Of Thin Films On The Basis Of A Superion Conductor (Ag2s) In Air; J. Pharmaceutics and Pharmacology Research. 5(7); DOI: 10.31579/2693-7247/093
Copyright: © 2022, Shuaibov O.K, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 16 May 2022 | Accepted: 27 May 2022 | Published: 21 June 2022
Keywords: electrophysical; microexplosions; inhomogeneities
The electrophysical characteristics of an overvoltage nanosecond discharge in air at atmospheric pressure between electrodes made of a superionic conductor, Ag2S, are presented. In the process of microexplosions of inhomogeneities on the working surfaces of electrodes in a strong electric field, vapors of the Ag2S compound and its dissociation products in plasma are introduced into the interelectrode space. This creates the prerequisites for the synthesis of thin structured films that have the properties of superionic conductors and can be deposited on a dielectric substrate placed near the discharge gap.
The spatial and electrical characteristics of an overvoltage nanosecond discharge, the Raman scattering spectra of the synthesized films and their surfaces are studied.
The papers [1, 2] reported on the synthesis of thin nanostructured films of oxides of transition metals (Zn, Cu, Fe) and aluminum from the products of an overvoltage nanosecond discharge with an ecton mechanism [3] of electrode sputtering in air at atmospheric pressure under automatic ultraviolet illumination from plasma a substrate with film. Under similar conditions, the electrophysical characteristics of an overvoltage nanosecond discharge in argon and air between electrodes of triple chalcopyrite (CuInSe2) [4] and an electrode of aluminum and chalcopyrite [5] were also studied, which made it possible to establish the conditions for the synthesis of thin films on CuIn2 Cu2.
The conditions for the synthesis of thin films based on zinc oxide in a magnetron discharge upon irradiation of a substrate with a film during its growth using a separate mercury lamp are given in [6]. It was shown here that under UV illumination of a rigid dielectric substrate during the growth of transparent ZnO layers, the electrical characteristics of the films are improved due to the creation of additional donor centers and a decrease in the scattering of electric charge carriers at grain boundaries. For the development of modern high-capacity capacitors, it is promising to study the synthesis of thin films based on superionic conductors, such as silver sulfide (Ag2S). Currently, there are no data on the electrophysical and emission characteristics of an overvoltage nanosecond atmospheric pressure discharge between electrodes made of polycrystalline superionic conductors.
Based on this state of research on the electrical characteristics of the plasma of nanosecond atmospheric pressure discharges between metal electrodes, which are promising for the synthesis of various nanostructures, it is currently relevant to search for new methods for the synthesis of thin films based on superionic conductors, since it is possible to improve the conditions and reduce the cost of the synthesis process. Due to the use of an overvoltage nanosecond discharge with an ecton electrode sputtering mechanism and the use of electrode material that emits in the plasma state in the UV region of the spectrum, synthesis can occur practically without significant heating of the discharge chamber and there is no need to use a separate source of UV radiation for UV irradiation during its formation on the substrate.
The article presents the results of a study of the spatial and electrical characteristics of an overvoltage nanosecond discharge between electrodes made of a polycrystalline superionic conductor (Ag2S) in air at atmospheric pressure, as well as the results of a study of the spectra of Raman light scattering by a thin film synthesized from degradation products on the film surface.
An overvoltage discharge of nanosecond duration between electrodes made of a superionic polycrystalline conductor (Ag2S) was ignited in a discharge chamber made of Plexiglas (Fig. 1.) at atmospheric air pressure. The interelectrode distance was 2 mm. The diameter of the cylindrical electrodes was 5 mm. The radius of curvature of the working end surface of the electrodes was the same and amounted to R ≈ 10-12 mm. Massive samples of polycrystals based on the Ag2S superionic conductor were synthesized in the technological laboratory of the chemical faculty of the Uzhgorod National University.
An overvoltage nanosecond discharge was ignited using a high-voltage bipolar modulator of voltage pulses with a total duration of 50-150 ns and a total amplitude of positive and negative components of ± 20-60 kV. The generator of bipolar high-voltage nanosecond pulses is built according to the scheme with resonant recharging of a storage low-inductance capacitor with a capacity of 1540 pF. A TGII-1000-25 hydrogen pulsed thyratron served as a commutator in the modulator. The frequency of repetition of voltage pulses could vary within 40-1000 Hz.
Oscillograms of voltage pulses across the discharge gap and oscillograms of current pulses were recorded using a broadband low-inductance capacitive voltage divider, a Rogowski coil, and a 6LOR-04 broadband oscilloscope. The time resolution of this system for measuring the characteristics of electrical impulses was 2–3 ns.
To record the radiation spectra of the plasma of an overvoltage nanosecond discharge, a two-channel digital spectrometer with astigmatism compensation "SL-40-2-1024USB" with an operating spectral range of 200-1200 nm was used.
The pulsed electrical power of the overvoltage nanosecond discharge was determined by graphical multiplication of the oscillograms of the voltage and current pulses. Time integration of the pulsed power made it possible to obtain energy in a single electrical pulse introduced into the plasma.
A more detailed description of the methodology and technique of the experiment is given in [7].
When a glass substrate was installed at a distance of 2–4 cm from the center of the discharge gap (Fig. 1), a thin film deposition from the products of sputtering of the electrode material was recorded on the substrate.
The discharge photographs were obtained using a digital camera (exposure time ≈ 1 s). The distance between the electrodes was used as a scale for establishing the plasma volume. At interelectrode distances of 1–2 mm, the discharge gap for air at atmospheric pressure was overstressed. The volume of the discharge plasma depended on the repetition rate of voltage pulses.
When carrying out experimental studies, a digital two-channel spectrometer with astigmatism compensation "SL-40-2-1024USB" and a Raman spectrometer "XploRA PLUS" of the center for the collective use of scientific equipment "Laboratory of Experimental and Applied Physics" at UzhNU were used.
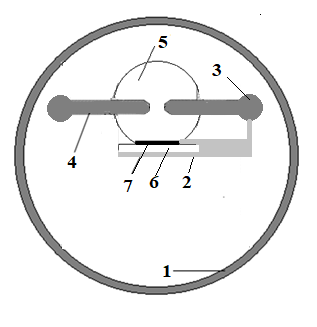
At a distance between the electrodes d = 2 mm, the nanosecond discharge
ignited at a significant overvoltage of the discharge interval, when it formed a beam of electrons- "runaways". Therefore, for this type of discharge, the role of the predionization system is performed by a beam of electrons - "runaways" and the accompanying X-rays [8].

Under the action of this beam and X-ray radiation, the discharge in air, even with a rather inhomogeneous distribution of the electric field strength between electrodes with rounding radii of hemispherical working surfaces (~10–12 mm), was quite uniform at pulse repetition rates of 40–1000 Hz (Fig. 2).
In a strong electric field between electrodes based on the Ag2S superionic conductor, microexplosions of nanowisters and the formation of ectons on the electrode surface occur [3], which contributed to the introduction of vapors of the Ag2S superionic conductor of their decay products (Ag, S) into the discharge plasma and their deposition on the dielectric substrate of a thin film. This type of nanosecond discharge is a prerequisite for obtaining uniform flows of the Ag2S compound sputtered from the electrode surface, its degradation products in plasma, as well as the flow of plasma UV radiation and the deposition of electrode material products on the dielectric substrate in the form of a thin film.
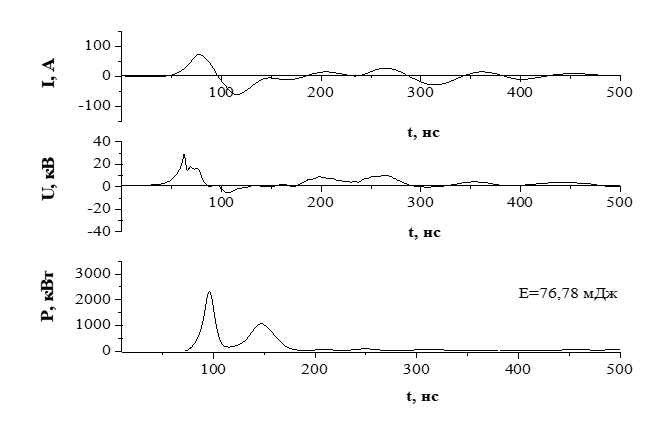
Fig.3. oscillograms of current, voltage, and pulsed power pulses of an overvoltage nanosecond discharge between electrodes made of the Ag2S compound are presented. In this experiment, the total duration of voltage pulses reached 400–450 ns, and the voltage pulse itself consisted of oscillations damped in time with a duration of about 20–30 ns. The maximum voltage drop across the discharge gap was 40–45 kV, taking into account the positive and negative components of the voltage pulse amplitude. The maximum amplitude of the current pulse reached 100 A. The highest value of the discharge pulse power was reached in the first 100 ns from the moment of its ignition and amounted to about 3 MW. The second peak of the pulsed power was 1.5 MW and was observed at the time from the start of the discharge ignition t=150 ns. The energy of a single electrical pulse was 0.77 J.
The investigated plasma radiated intensely in the spectral range 200–350 nm. The main sources of radiation in the short-wavelength region of the spectrum 200–350 nm were singly charged silver ions and atoms [9]. The presence of intense UV radiation from the discharge plasma in vapors of Ag2S compounds is promising for the synthesis of thin superionic films with automatic support of UV radiation, which can have a number of advantages in terms of characteristics (lower resistance, etc.) [6]. In powerful atmospheric pressure pulsed discharges with an electron concentration at the level of 1015-1017 cm-3, the formation of excited metal ions occurs mainly in the processes of excitation of a singly charged metal ion, which is in the ground energy state, by electrons [10], including "runaway electrons"" [8].
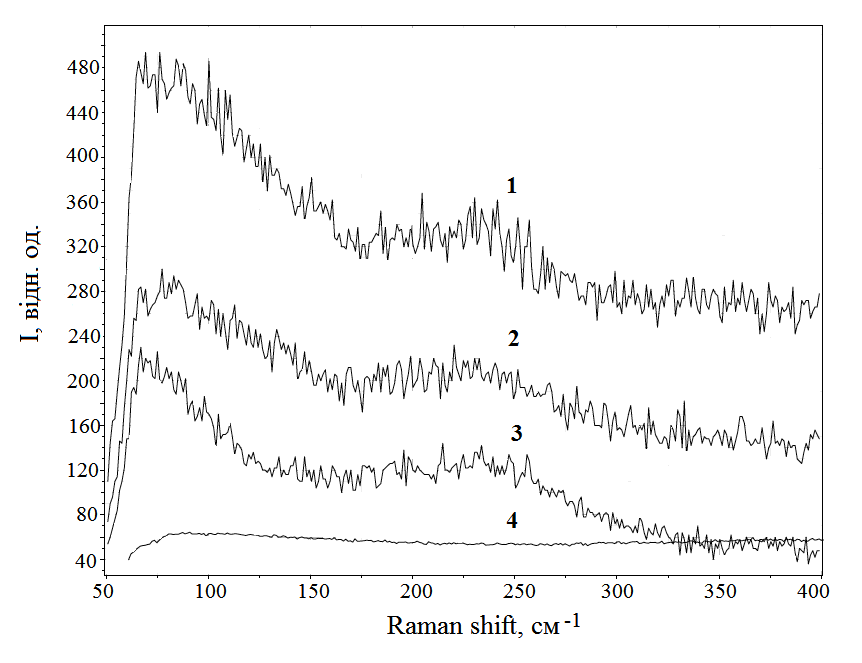
The Raman spectrum of light scattering by a film synthesized from the products of destruction of electrodes of an overvoltage nanosecond discharge between electrodes from a superionic conductor at atmospheric air pressure is shown in Fig. 4. From this Fig.4 it follows that the spectra of Raman scattering of light on the film coincide with the spectrum of the initial material from which the electrodes are made. Thus, the synthesized thin film should have the properties of a superionic conductor.

Photographs of the surface of thin films based on the Ag2S compound using an optical microscope showed that their surface is fairly uniform. Only individual inhomogeneities with sizes of the order of several micrometers were observed on it, which may be due to the destruction of polycrystalline electrodes in an overvoltage nanosecond discharge. To improve the uniformity of the synthesized films and reduce the time of their synthesis, it is necessary to reduce the energy in the electric pulse, which can be achieved by reducing the duration of the energy contribution to the plasma, increasing the pulse repetition rate, and also switching to the use of buffer gases other than air at atmospheric pressure.
Thus, the study of the electrophysical characteristics of an overvoltage nanosecond discharge between electrodes made of a superionic conductor (Ag2S) in atmospheric pressure air from the degradation products of electrodes of an overvoltage nanosecond discharge in atmospheric pressure air showed at a distance between the electrodes of 2 mm, a spatially homogeneous discharge was ignited, the shape of which was determined by the energy contribution to the plasma and the pulse repetition frequency, which is presumably due to the formation of “runaway electrons” and accompanying X-ray radiation in the discharge, which served as an automatic system for the discharge preionization; the magnitude of the pulsed electric power of the discharge reached 3 MW with an energy in a single pulse of 77 mJ;the study of the spectra of Raman light scattering by thin films synthesized in the experiment showed that they are identical to the corresponding spectra for macroscopic polycrystalline samples from which the electrodes are made; The study of the surface of the synthesized films using an optical microscope revealed their rather high homogeneity.