AUCTORES
Globalize your Research
Case Report | DOI: https://doi.org/10.31579/2641-0419/401
President of all Nations Morning star hospital, Enayam Thoppu, Kanyakumari District, India.
*Corresponding Author: Ramachandran Muthiah, President of all Nations Morning star hospital, Enayam Thoppu, Kanyakumari District, India.
Citation: Ramachandran Muthiah, (2024), Ebstein’s Anomaly and Echocardiographic Features, J Clinical Cardiology and Cardiovascular Interventions, 7(10); DOI: 10.31579/2641-0419/401
Copyright: © 2024, Ramachandran Muthiah. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 05 August 2024 | Accepted: 22 August 2024 | Published: 09 September 2024
Keywords: sail-like; deformity; ‘atretic’ ebstein’s anomaly; inverted ebstein’s anomaly; ebstein’s mitral valve; kay annuloplasty
Aim: To present the various echocardiographic spectrum of Ebstein’s malformation in adolescence and adults. Introduction: Ebstein’s anomaly has different anatomic and hemodynamic variables with clinical manifestations of cyanosis, right-sided heart failure and arrhythmias. The leaflet tethering and dysplasia, together with dilatation of the tricuspid valve ring, constitute the anatomic cause of tricuspid regurgitation observed in this condition.
Case reports: The spectrum of leaflet tethering from mild to extreme with varying degrees of tricuspid regurgitation were documented by echocardiography in a 16-year old cyanotic male with Ebstein’s anomaly associated with an atrial septal defect and mild low tricuspid regurgitation (TR), 22-year old acyanotic male with right-sided heart failure due to severe tricuspid regurgitation and an intact atrial septum, 55-year old aymptomatic female with moderate high tricuspid regurgitation. The extreme form was described as an ‘atretic’ membrane in a 28-year old cyanotic male and as a ‘blanket’ of leaflet tissue in a 30-year old cyanotic male.
Conclusion: Ebstein’s anomaly has to be suspected clinically in presence of cyanosis with a WPW (Wolf-Parkinson-White) or atrioventricular (AV) block pattern of ECG and its management is complex and must be individualized. RV (right ventricular) exclusion procedures are preferred in advanced cases.
Ebstein’s anomaly is a disease of the entire right ventricle. It is a spectrum of abnormalities, characterized by apical displacement of the valve, anomalous distal attachment of the leaflets, size of the functional right ventricle and degree of tricuspid regurgitation, with alteration in the left ventricle as well. It occurs with a prevalence of about 0.3 to 0.7% among patients with congenital cardiac disease [1] and most cases occur sporadically with an equal distribution between males and females. The anatomical hallmark of this entity is the apical displacement of the attachments of septal and posterior leaflets of the tricuspid valve. The displaced tricuspid valve divides the right ventricle into two parts. The inlet portion is usually integrated functionally with the right atrium (“functional” atrialization) and the apico-trabecular and outlet portions constitute the functional right ventricle. The proximal atrialized ventricle has a thinner wall due to partial absence of myocardium and described as “anatomical” atrialization.
In 1988, Carpentier, et al proposed a classification of Ebstein’s anomaly based on the morphology of right ventricle and tricuspid valve as shown in Table 1 [2].
| Type Right ventricle Tricuspid valve |
A Small contractile atrialized right ventricle. Moderate displacement of septal and Adequate-size right ventricle posterior leaflets. Normal anterior leaflet. B Large noncontractile atrialized right Marked displacement of septal and ventricle. Small right ventricle posterior leaflets. Hypoplastic adherent septal leaflet. Normal anterior leaflet. C Large noncontractile atrialized right Marked displacement of septal and ventricle. Very small right ventricle posterior leaflets. Hypolastic adherent septal and posterior leaflets. Restricted anterior leaflet motion. D Almost completely noncontractile Marked displacement of septal, atrialized right ventricle (except posterior and anterior leaflets. for infundibulum) Hypoplastic adherent septal and posterior leaflets. Anterior leaflet is adherent to ventricular wall. |
Table 1: Carpentier’s classification of Ebstein’s anomaly
The essence of the Ebstein’s malformation is the fact that the tricuspid valve leaflets do not attach normally at the tricuspid annulus [3]. The degree of displacement is variable and in one-third of all cases, the leaflets are adherent to the ventricular wall (“plastered down”) rather than truly displaced and so these cases had been reported.
Case 1 (16-year old cyanotic male with Ebstein’s anomaly)
A 16-year old male presented with cyanosis and he had features of an atrial septal defect such as wide, fixed splitting of second heart sound at left second intercostal space and a grade 2/6 systolic murmur at the lower left sternal border. 2D echocardiography revealed the features of Ebstein’s anomaly such as tethering of septal tricuspid leaflet (STL) to the ventricular wall associated with an ostium secundum type atrial septal defect (ASD) and low mild tricuspid regurgitation jet as shown in Figures 1 to 3

Figure 1. Tilted apical view showing the well defined septal tricuspid leaflet (STL) (lower arrow) inserting directly into the ventricular septum (IVS) and an ostium secundum type atrial septal defect (ASD) in a 16-year old cyanotic male. ATL (anterior tricuspid leaflet) is ‘sail-like’ (upper arrow) and anomalously attached to IVS. RA- right atrium.

Figure 2. Tilted apical view showing the tethering of septal tricuspid leaflet (STL) along with ventricular septum (IVS)-Becker’s grade III (>50%) and an ostium secundum type atrial septal defect (ASD) in a 16-year old cyanotic male. ATL (anterior tricuspid leaflet) is ‘sail-like’ (arrow), anomalously attached to IVS and mimicking as a thickened moderator band. RA- right atrium.

Figure 3. Tilted Apical view showing the mild low tricuspid regurgitation jet in a 16-year old cyanotic male. . ATL (anterior tricuspid leaflet) is ‘sail-like’ (arrow) and anomalously attached to IVS (interventricular septum). STL – septal tricuspid leaflet. RA- right atrium.
The patient was advised definite repair with closure of the atrial septal defect.
Case 2 (22-year old acyanotic male with Ebstein’s anomaly)
A 22-year old acyanotic male was presented with features of right heart failure and a grade 3/6 systolic murmur at lower left sternal border. 2D echocardiography revealed a normally attached septal tricuspid leaflet (STL), but tethered to the ventricular wall suggesting an Ebstein’s anomaly with severe tricuspid regurgitation swirling around the lateral wall of right atrium and interatrial septum, and a dilated atrium and atrialized RV (right ventricle) as shown in Figures 4 to 8.

Figure 4. Apical four-chamber view showing the septal tricuspid leaflet (STL) tethering (lower arrow) (Becker’s grade 2- 25 to 50%) and a dilated RA (right atrium) in a 22-year old acyanotic male. Interatrial septum (IAS) is intact. ATL (anterior tricuspid leaflet) is ‘sail-like’ and anomalously attached to IVS (interventricular septum) (upper arrows). RA- right atrium.

Figure 5. Apical four-chamber view showing the severe high tricuspid regurgitation jet (arrow) swirling around the walls of the right atrium in a 22-year old acyanotic male. IAS - interatrial septum. RA- right atrium.

Figure 6. Continuous Wave (CW) Doppler (green line) showing the low pressure TR (tricuspid regurgitation) jet in a 22 year old acyanotic male.

Figure 7. M-mode LV (left ventricle) (green line) showing the sail-like tricuspid valve (TV) (arrows) and a normal LV function (EF 61%) in a 22-year old acyanotic male.

Figure 8. Subcostal view showing the dilated atrialized RV (right ventricle) in a 22-year old acyanotic male. Septal tricuspid leaflet (STL) is displaced 57 mm from the annulus (lower arrow)
The patient was treated with antifailure measures such as digoxin and diuretics and advised definite repair (Tricuspid valve replacement with right atrial reduction atrioplasty along with plication of atrialized RV (right ventricle)
Case 3 (55-year old female with Ebstein’s anomaly)
A 55-year old asymptomatic, acyanotic female, given birth to three children, presented with grade 2/6 systolic murmur at the lower left sternal border. 2D echocardiography revealed a septal tricuspid leaflet tethering and a high moderate tricuspid regurgitation as shown in Figures 9 and 10 suggesting an Ebstein’s anomaly.

Figure 9: Apical view showing the tethering of septal tricuspid leaflet (STL) to the ventricular septum (IVS)- Becker’s grade 2 (25 to 50%) in a 55-year old acyanotic female. RA- right atrium.

Figure 10: Apical view showing the high moderate tricuspid regurgitation (TR) jet (arrow) in a 55-year old acyanotic female. RA- right atrium.
The patient was advised periodic follow up since she was remaining asymptomatic.
Case 4 (28-year old male with Ebstein’s anomaly)
A 28-year old male presented with cyanosis and auscultation revealed a ‘sail sound’ ( loud tricuspid component of first heart sound due to increased tension developed by the large anterior leaflet as it reaches the limits of its systolic excursion- an important sign of anterior leaflet mobility), a ‘cadence’ quality of quadruple rhythm due to wide splitting of first and second sounds ( due to complete right bundle branch block), atrial and ventricular filling sounds (summation of these sounds due to prolonged PR interval). ECG revealed the features of Ebstein’s anomaly as shown in Figures 11 and 12. X-ray chest revealed the Ebstein’s configuration as shown in Figure 13. 2D echocardiography revealed a ‘sail-like’anterior tricuspid leaflet forming a ‘muscular curtain’ in between the inflow and trabecular parts of the right ventricle as an ‘imperforate membrane’ with a ‘pinhole’communication, associated with a muscular VSD (ventricular septal defect) in the proximal, atrialized compartment of right ventricle suggesting an ‘atretic” (‘imperforate’) Ebstein’s anomaly as shown in Figures 14 to 27.

Figure 11- ECG revealed complete right bundle branch block in V1, QR complexes in V1-V3, precordial Q wave in V1 and a same QRS pattern in V1 and lead aVR ( since it records right ventricular intracavitary potentials unusually far to leftward to the large right atrium [4]) and first degree AV block (prolonged PR interval), suggesting Ebstein’s anomaly in a 28-year old cyanotic male.

Figure 12: ECG showing recurrent Mobitz I block in a 28-year old cyanotic male with Ebstein’s anomaly.
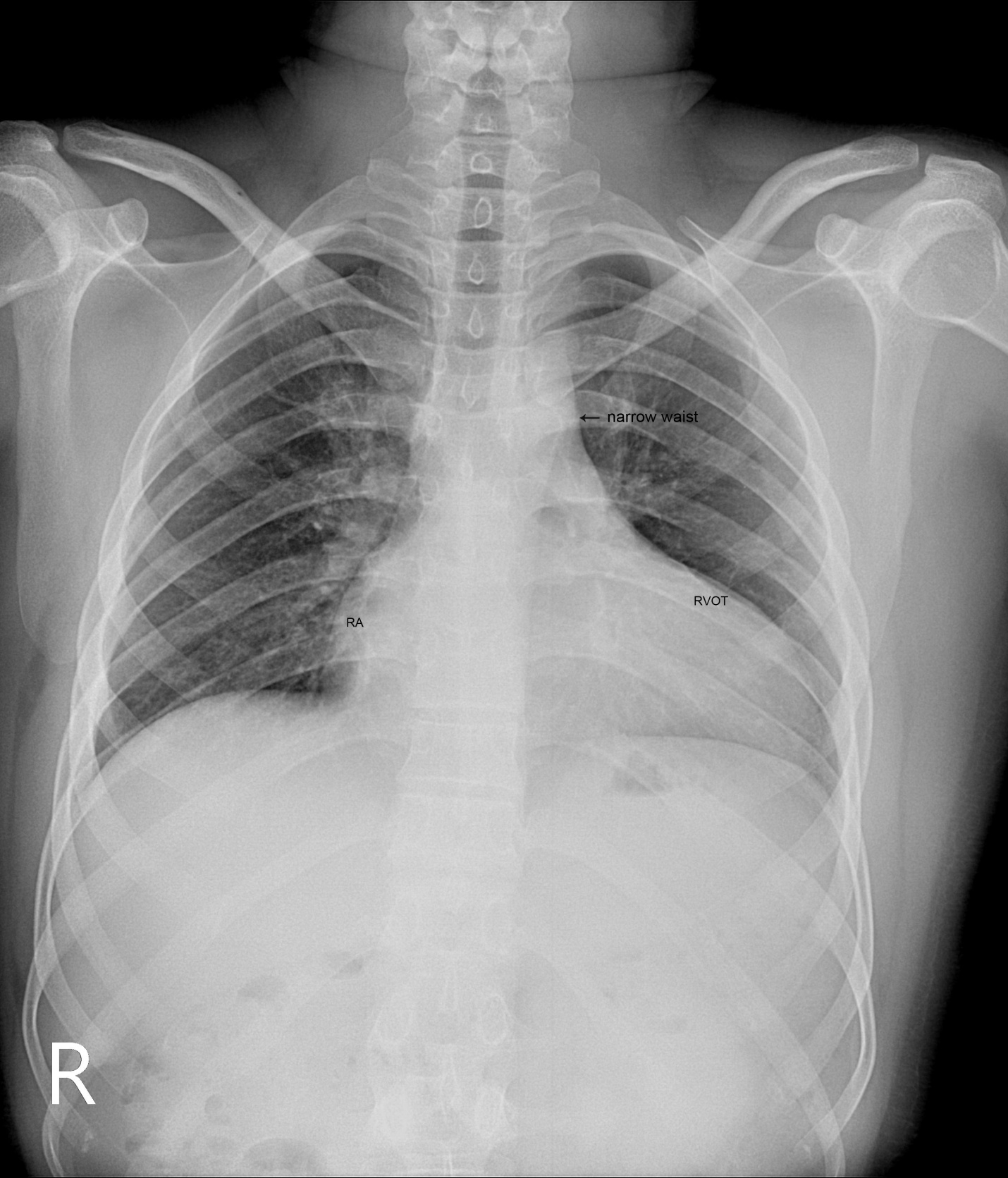
Figure 13: X-ray chest PA (postero-anterior) view showing Ebstein’s configuration, characterized by the narrow vascular pedicle (narrow waist (arrow)) (since the pulmonary trunk is not border forming and ascending aorta is inconspicuous) and a ‘box-like’ cardiac silhouette (rightward convexity of right atrium and a leftward convexity of displaced RVOT (infundibulum) with an increased cardiothoracic ratio of > 0.65 and diminished pulmonary vascular markings in a 28-year old, cyanotic male with atretic Ebsteins’s anomaly.

Figure 14: Tilted Parasternal long axis view showing the ‘sail-like’ muscular skirt of tricuspid valve (arrow) in a 28-year old cyanotic male. AO- aorta.

Figure 15: Parasternal long axis view showing the fused tricuspid valve (arrow) in a 28-year old cyanotic male. AO- aorta.

Figure 16: Apical view showing the redundant, ‘sail-like’ anterior tricuspid leaflet (ATL) (right arrow) fused with the displaced (76 mm from annulus) septal tricuspid leaflet (STL) (left arrow) as an imperforate membrane in the RV cavity in a 28-year old cyanotic male. RA- right atrium.

Figure 17: Apical view showing the ‘pin-hole’ communication across the imperforate membrane in a 28-year old cyanotic male. ‘Sail-like’ anterior tricuspid leaflet (ATL) (right arrow) and the displaced (76 mm from annulus) septal tricuspid leaflet (STL) (left arrow). RA- right atrium.

Figure 18: Apical view showing the displacement ratio with mild TR (tricuspid regurgitation) in a 28-year old cyanotic male. STL - septal tricuspid leaflet. RA- right atrium.

Figure 19: Parasternal long axis view showing the muscular VSD (ventricular septal defect) (right arrow) in the proximal compartment of RV (right ventricle) and the ‘sail-like’ motion of fused tricuspid valve (TV) (left arrow) in a 28-year old cyanotic male. AO- aorta.

Figure 20: Apical view showing the left ventricular to right atrial communication (dilated atrialized right ventricle) (lower arrow) and the displaced septal tricuspid leaflet (STL) (upper arrow) in 28-year old cyanotic male. RA- right atrium.

Figure 21: Parasternal long axis view showing the fused tricuspid leaflet during its ‘sail-like; motion’ (arrow) in a 28-year old cyanotic male. AO- aorta.

Figure 22: Tilted Parasternal long axis view showing the position of anterior leaflet during the ‘sail-like’ motion (arrow) in a 28-year old male [5]. AO- aorta

Figure 23: Right ventricular apical long axis view showing the position of tricuspid valve during the ‘sail-like’ motion (arrow) in a 28-year old cyanotic male. STL- septal tricuspid leaflet. aRV- atrialized right ventricle. RA- right atrium.

Figure 24: Right ventricular apical long axis view showing the displaced septal tricuspid leaflet (STL) (arrow) and a dilated atrialized RV (right ventricle) in a 28-year old cyanotic male. IAS- interatrial septum. RA- right atrium

Figure 25: Short axis view showing the sail like “ATL curtain” (arrow) (ATL-anterior tricuspid leaflet) in a 28-year old cyanotic male. AO- aorta.

Figure 26: Short axis view showing the ‘whipping motion’ (arrow) of tricuspid valve across the RVOT (right ventricular outflow tract) in a 28-year old cyanotic male [6]. AO—aorta

Figure 27: M-mode LV (green line) showing the ‘sail-like’ tricuspid valve (arrow) and a normal LV function- EF (50%) in a 28-year old cyanotic male.
The patient was advised Starnes’ procedure along with Kay annuloplasty and a pacemaker implantation onto left ventricular endocardium.
Case 5. (30-year old cyanotic male with Ebstein’s anomaly)
A 30-year old male was presented with marked cyanosis and no murmur and abnormal heart sounds on auscultation. 2D echocardiography revealed the features of Ebstein’s anomaly such as insertion of anterior leaflet into the trabeculated RV, forming a ‘blanket’ of leaflet tissue across the inflow and trabecular parts and bulging of ventricular septum towards leftward due to marked dilatation of RV, and LV became ‘banana’ shaped as shown in Figures 28 to 30.

Figure 28: Tilted apical view showing the insertion of tricuspid leaflets (arrows) into the trabeculated RV (right ventricle) in a 30-year old cyanotic male with Ebstein’s anomaly. RA- right atrium.

Figure 29. Tilted apical view showing the plastering of the tricuspid valve leaflets into the ventricular wall as a ‘blanket’ of leaflet tissue (arrows) in a 30 –year old cyanotic male. Right ventricle (RV) is circular and LV (left ventricle) is banana shaped. RA- right atrium.

Figure 30. Color flow imaging showing no regurgitant or stenotic lesions in a 30-year old cyanotic male. Right ventricle (RV) is circular and LV (left ventricle) is banana shaped. Tricuspid valve (TV) is plastered with fibrous stumps (arrows). RA- right atrium.
The patient was palliated with bidirectional Glenn shunt and advised periodic follow up for an RV exclusion procedure.
Case 6
(12-year old male with inverted Ebstein’s anomaly)
A 12- year old, asymptomatic boy was presented with features of an inverted Ebstein’s anomaly on routine echocardiographic screening as shown in Figure 31 and the boy was advised periodic follow up.

Figure 31. Apical view showing the ventricular inversion and Ebstein’s malformation of the inverted tricuspid valve on the left-side in a 12-year old asymptomatic male. ‘Arrow’ showing the moderator band as a feature of morphologic right ventricle (mRV). STL- septal tricuspid leaflet.
Case 7:
(Ebstein’s mitral valve in a 10-year old boy).
A 10-year old boy was presented with a grade 1/6 systolic murmur at the apex and blood chemistry revealed a positive ASO (antistreptolysin O) titer, suggesting a rheumatic involvement. 2D echocardiography revealed a displaced anterior mitral leaflet and it is thick, calcified and mildly regurgitant as shown in Figure 32.

Figure 32. Ebstein’s mitral valve, its anterior leaflet displaced downward from the annulus and it is mildly regurgitant due to rheumatic etiology in a 10 –year old boy.
The boy was advised penicillin prophylaxis (oral penicillin V 250 mg twice daily) and periodic follow up.
Review of literature
Ebstein’s anomaly was initially described by Wilhelm Ebstein at All-Saints Hospital in Breslau (now Wroclaw), Poland in the autopsy findings of a 19-year old cyanosed laborer, Joseph Prescher who died with a severe malformation of tricuspid valve in July 1864 [7],[8]. The eponym “Ebstein’s disease” was coined by Arnstein in 1927 [9]. Yater and Shapiro described the radiologic and echocardiographic features of Ebstein’s anomaly in 1937 [10]. Tourniaire, et al diagnosed this anomaly in a living in 1949 and Engle asserted the clinical recognition of Ebstein’s anomaly in 1950 [11]. Edwards emphasized the continuity of muscles between the atrium and the “atrialized” right ventricle across the atrioventricular ring in 1979 [12].
Etiopathogenesis
The exact embryology of Ebstein’s anomaly is unknown since the normal tricuspid valve is believed to be formed by a process of either ‘delamination’ of the inner layer of the inlet zone of right ventricle [13],[14] or ‘undermining’[15]. During cardiac embryogenesis, the process of valve formation is completed by the 12th week of gestation and the development of septal tricuspid leaflet is not complete until the 16th week and the valves are better delineated in the second and third trimester.
In Ebstein’s anomaly, “delamination” fails to occur, resulting in downward displacement of the origin of septal and inferior leaflets and the antero-superior leaflet is mostly ‘sail-like’, but still attached to its usual atrioventricular junction line and the mechanism for this is not entirely understood [16].
The leaflets of the tricuspid valve develop equally from the endocardial cushion tissue and myocardium. Endocardial cushion tissue lines the right atrioventricular junction and acts as an adhesive to maintain the integrity of that portion of the subjacent myocardium which forms the tricuspid valve apparatus. Expansion in the size of right ventricular inflow tract occurs by a process called “undermining”. The subendocardial portion of the ventricular free wall becomes fenestrated and spongy since the resorption of the myocardium progressively dissolves the wall and thereby enlarges the chamber size. Undermining and resorption produce a flap-like muscular skirt that is attached at the annulus and is anchored to the underlying ventricular wall by numerous branching pillars of myocardium. Progressive thinning and fibroblastic ingrowth result in the formation of tricuspid valve leaflets as anterior leaflet forms much earlier and it is not adherent to the right ventricular wall. The late forming septal and posterior leaflets remain adherent to the underlying myocardial wall and thus, Ebstein’s malformation is thought to occur as a result of incomplete undermining of the right ventricular myocardium [17].
Most cases of Ebstein’s anomaly are sporadic and familial incidence is rare [18]. There is some dysregulation of molecular and morphological events involved in the process of connection between the future right atrium to the developing right ventricle and monogenic as well as oligogenic factors play a role in its driving pathogenesis in animal models [19]. Rare cases of cardiac transcription factor NKX 2-5 mutation [20], 10p13-p14 deletion [21] and Ip34.3-36.11 deletion [22] have been described in the anomaly. Maternal exposure to benzodiazepins [23] and lithium carbonate therapy [24],[25],[26] can rarely lead to Ebstein’s anomaly in the offspring.
Morphological features
The malformation documented by Ebstein at autopsy consists of an abnormal insertion of the tricuspid valve, the septal and posterior leaflets were adherent to the ventricular wall and the mobile free parts were displaced towards the apex of the right ventricle. The two anatomic features, i.,e., valve displacement and leaflet morphology vary independently and its pathology is not uniform.
Apical displacement was determined as the distance between the anticipated normal basal attachments of the leaflets and the right ventricular apex. The degree of displacement was classified by Becker in 1971 as shown in Table 2 [27
| Grade Distance between atrioventricular junction and the apex |
Grade 1 Apical displacement of < 10> the tricuspid valve dipped below the Expected annular attachments)
Grade 2 displaced between 10% and 50% ( or 25 to 50%)
Grade 3 displaced > 50% |
Table 2: Becker’s classification of degree of valve displacement ..
The intrinsic abnormality of the tricuspid valve leaflets is generally categorized as “dysplasia” and it usually affects all leaflets with varying degrees as shown in Table 3 [28], mostly the anterior leaflet, often with displaced leaflets and sometimes, occasional [29].
Grade a focal or diffuse thickening of the leaflets
Grade b deficient development of chordae and papillary muscle
Grade c improper separation of the valve components from the ventricular wall
Grade d focal agenesis of valve tissue |
Table 3- Lev and Becker’s grading of dysplasia ‘
Apical displacement always affects the septal leaflet and also involves the posterior leaflet. The leaflets are not displaced beyond the junction between the inlet and trabecular parts of the right ventricle and the point of maximum displacement is usually at the commissure between the two leaflets. The spectrum of leaflet tethering varies from mild to extreme. The degree of dysplasia is also categorized as I to III as shown in Table 4.

(Table 4 showing the categories of dysplasia)
When chordae are absent, the free leaflets insert directly into the ventricular wall. In some cases, the greater part of the affected leaflets is firmly adherent to the right ventricular wall, drape the apical trabeculations and completely blended with ventricular wall. The communication between the atrialized and functional right ventricle is confined to slits or perforations in the anterior leaflet as Ebstein originally described. When the anteromedial commissure is fused and the anterior leaflet is intact, the tricuspid orifice is “imperforate” [30] and it occurs in 10% of hearts with Ebstein’s anomaly. In ‘imperforate Ebstein’s anomaly”, the septal, inferior and antero-superior leaflets may fuse either completely or in part, so that the inner surface of the inflow part of the right ventricle is formed by a “blanket” of dysplastic valve tissue towards the apex. The leaflets are said to be “plastered” out of the right ventricular myocardium, so that the fibrous transformation of the leaflets from the muscular precursors remain incomplete. . In severe cases, the leaflets are thickened, focally muscularized and attached to the underlying myocardium by numerous muscular stumps. In extreme cases, the fusion of leaflet tissue is so complete as a membrane-like continuum and the only identified remnants of leaflet tissue are nodular fibrous ridges at the level of the displaced functional annulus. In this setting, the entire inflow tract is atrialized and the functional right ventricle consists only of trabecular and outflow (infundibulum) components. The designation of “atretic“Ebstein’s malformation was documented by Kumar [31] to the imperforate fused tricuspid valve and its incidence becomes even higher to 29% with an inclusion of “pinhole” communication {32}.
Echocardiographic features
Echocardiogr aphy is the diagnostic test of choice for Ebstein’s anomaly and the first echocardiographic diagnosis was reported by Lundstrom in 1969. The first diagnostic criteria for Ebstein’s anomaly using a multi-crystal two-dimensional system was defined by Hagan in 1974 [33] and they were able to recognize the apical displacement of the septal tricuspid leaflet and an elongated anterior tricuspid leaflet with increased excursion. The septal leaflet of the tricuspid valve attaches chiefly to the ventricular septum , but part of its basal attachment is to the posterior wall of the right ventricle [34] and it normally exhibits a slight but distinct apical displacement of its basal attachment to the central fibrous body compared to the mitral valve. The distal displacement of septal origin of tricuspid valve seems to be the best echocardiographic criterion as the characteristic sign for Ebstein’s anomaly and the degree of maximal displacement in normal hearts varies considerably with a mean difference of approximately 6 mm with mitral valve. To define the anatomic severity of Ebstein’s anomaly, four-chamber view is the best to demonstrate the apical displacement of septal tricuspid leaflet [35]. The ratio between the mitral-to-apex distance and the tricuspid-to-apex distance varies from 1 to 1.2 in normal subjects and 1.8 to 3.2 in patients and it is 3.6 as in Figure 18 with Ebstein’s anomaly. The true distance in the level of insertion of atrioventricular valves is obtained by substracting the tricuspid-to-apex distance from the mitral-to-apex distance with a mean value of 27.25 ± 12 mm in patients with proven Ebstein’s anomaly and it is 60 mm as shown in Figure 18 compared to reference group (5.7 ± 2 mm). Kambe and coworkers calculated the distance between both atrioventricular valves directly as a mean value of 21 mm with a range of 14 to 32 mm [36]. A maximum difference in the level of valve insertion of >15 mm in children and >20 mm in adults is discriminated between normal and Ebstein’s anomaly [37],[38]. Despite this fact, a patient with an ‘unequivocal’ Ebstein’s malformation can be encountered in whom the diagnosis cannot be made with certainity solely on the basis of apical displacement of the septal tricuspid valve leaflet. Occasionally, the leaflet attaches to the trabecular part rather than the inlet part of the septum, the conventional four-chamber view will not reveal any septal insertion as shown in Figures 28 and 29.
The anterior tricuspid leaflet is not involved in the process of downward displacement, it may be abnormally inserted occasionally and Shiina, et al documented the apical displacement of anterior tricuspid leaflet in 14% of cases echocardiographically [39]. The anterior leaflet forms a large, sail-like intracavitary curtain as in Figures 14, 25 and contains muscular strands instead of consisting entirely of a fibrous membrane as in the normal tricuspid valve [40]. It is potentially mobile with a brisk sail-like movement as shown in Figure 21 to 24 [41], free bloating with a ‘whipping motion’ across the right ventricular outflow tract (RVOT) as shown in Figure 26 and in some cases, the movement is restricted due to its adherence to the ventricular wall as in Figure 1 and 2, 4 and 9. It is often fenestrated, may in part be musculaized , inserting into the trabeculations of the right ventricle (RV) as in Figure 28 and rarely, the anterior leaflet forms an ‘atretic’ membrane that spans the midportion of the right ventricular cavity as in Figure 16.
In severe cases, the inferior wall of the right ventricle may consists soley of thin fibrous tissue, devoid of myocytes and thereby represent an area of aneurysmal dilatation as in Cases 2 (Figure 8) and 3 (Figures 20 and 24). It is apparently due either to slippage of right ventricular inflow tract away from the right atrioventricular junction or to focal excessive ‘undermining’ to myocardium, transmurally to the level of epicardium. A large atrialized area causes a severe reduction in the volume of the right ventricular pumping chamber and usually produces an abnormal configuration of muscular interventricular septum, which bulges leftward and thereby compresses the left ventricular chamber, resulting in reversal of ventricular shapes with a ‘circular’ right ventricle and a ‘banana’ [42] or ‘crescentic’ [43] left ventricle as shown in Figures 29 and 30 Thus, the proximal component of the right ventricle, the ‘inlet portion’ is the part directly involved with the malformation and the distal apico-trabecular and outlet portions that constitute the ‘functional right ventricle’, which is not involved and may be of normal size, but usually markedly diminished in dimensions and in some cases, it is dilated and thin walled.
The tricuspid orifice is typically incompetent as in Figures 3, 5 and 10, occasionally stenotic, and rarely imperforate as in Figure 16 [44],[45]. The true anatomic tricuspid annulus occupies its normal position at the right atrioventricular junction and it is less well defined than in a normal heart. The annulus tends to be appreciably dilated and contribute to the development of valvular incompetence. In extreme downward displacement of posterior and septal leaflets, the closure of the tricuspid annulus depends on the size and potential excursion of anterior leaflet. When the chordal attachments are short and the leaflets contain multiple or large fenestrations, adequate valve closure is impossible to achieve and varying degrees of regurgitation results. Color flow imaging and Doppler interrogation can establish the relatively low velocity regurgitant flow as in Figure 6, which begins at the level of the displaced septal and posterior leaflets as in Figures 5 and 10 and courses through the atrialized right ventricle into the right atrium proper as shown in Figure 5. Tricuspid regurgitation increases by annular dilatation [46]. During contraction of the atrium, the atrialized portion of the right ventricle balloons out and acts as a passive reservoir. Functional improvement of right ventricle depends on the severity of tricuspid regurgitation and on the ratio of the combined areas of right atrium and atrialized right ventricle relative to the areas of functional right ventricle and left ventricle [47]. Celermajer, et al described an echocardiographic grading score for neonates with Ebstein’s anomaly as shown in the Table 5 [48].
| GOSE score | Index (RA+RV): (RV+LA+LV) | Risk of mortality (%) | |
Grade 1
Grade 2
Grade 3
Grade 4 | Ratio < 0>
Ratio of 0.5 to 0.99
Ratio of 1 to 1.49
Ratio ≥ 1.5 | 0
10
44-100
100 | |
Table 5 “Celermajer” echocardiographic grading score - GOSE (Great Ormond Street Echocardiography Score- Grade 3 and 4 have a very poor prognosis)
The functional impairement of right ventricle and regurgitation of tricuspid valve retard the forward flow and the overall effect is right atrial dilatation as shown in Figure 4. In many cases, the right atrial enlargement is extensive, but the mural thrombus is not a feature even in right ventricular dilatation. The enlarging right atrium becomes sufficiently compliant to accomodate a large volume of regurgitant flow with little or no increase in pressure as shown in Figure 6., In patients with marked Ebstein’s malformation and severe tricuspid regurgitation, liver and portal circulation are extensively affected, congestive hepatosplenomegaly and microscopic hepatic fibrosis eventually occurs in chronic cases with hypersplenism [49].
An interatrial communication is present in 80 to 94% of patients with Ebstein’s anomaly [50]. Ebstein believed that “regurgitation of blood into the right atrium caused its dilatation and prevented complete closure of the valve of foramen ovale”. The majority of hearts with Ebstein’s anomaly have a patent foramen ovale (67%) and in more than one third of cases, the interatrial communication is an ostium secundum defect as shown in Figures 1 and 2. An intact atrial septum is rare and usually seen in adults as in Figure 4. The ventricular septal defect may be localized in either the proximal as shown in Figure 19 or the distal right ventricular compartment and it can be muscular with an incidence of 4% in clinical series and 12% in autopsy studies. The hearts in which the opening is proximal to the displaced tricuspid valve, a left ventricular to right atrial shunt may occur as shown in Figure 20.
Left-sided Ebstein’s anomaly
Ebstein’s anomaly of inverted tricuspid valve has been described in 15 -50% of cases of congenitally corrected transposition of great arteries [51] as in Figure 31. The anterior leaflet of inverted Ebstein’s anomaly is usually small, malformed and the atrialized inverted right ventricle is poorly developed, not thinned and rarely dilated [52]. The posterior wall of the inflow tract of the right ventricle is normal in inverted Ebstein (discordant atrioventricular connection)[53] and it is always abnormal in Ebstein’s anomaly of concordant connection in which the myocardium is replaced by fibrous tissue to variable degree. Rarely, a morphologic mitral valve (mMV)- right sided inverted mitral valve has anatomic features of Ebstein’s anomaly [54]. The leaflets tend to be thickened and even dysplastic in appearance [55]. Ebstein’s – like anomaly can also affect a normally positioned mitral valve as shown in Figure 32, although this is exceedingly rare, first reported by Rusahhaupt, et al in 1976 [56] and its embryologic origin is obscure [57]. The left atrioventricular valve incompetence is not necessarily caused by an Ebstein-like malformation, may be congenital and it is due to rheumatic involvement in this case. The anterior mitral leaflet (the aortic leaflet of mitral valve) is not particularly redundant, and its annulus inserts along the septum normally without downward displacement (offsetting). The normal ‘offsetting’ of the mitral and tricuspid valve is maintained in the four-chamber view [58]. Only the posterior leaflet, attached to the left ventricular free wall, is involved in downward displacement. Cases have also been reported in which both the mitral and tricuspid valves were involved by Ebstein’s malformation [59],[60].
Management
The treatment plan for Ebstein’s anomaly is individualized since the hemodynamic abnormality vary considerably with the extent of tricuspid valve displacement into the right ventricle.
Medical therapy
In neonates, the disease process is much different and have a rapidly deteriorating course with severe heart failure, cyanosis and acidosis. If congestive cardiac failure is prominent, infants may require initial support with inotropic agents and long-term anticongestive measures with digoxin and diuretics. The efficacy of angiotensin-converting enzyme inhibitors in patients with Ebstein’s anomaly having right-sided heart failure is unproved and they are not recommended, including angiotensin-receptor blockers (ARBs) and β-blockers, because these drugs may increase pulmonary artery pressure, triggering further heart failure and pulmonary edema [61]. The standard heart failure therapy may be reserved for those patients who are not candidates for surgery.
In newborn infants whose primary problem is that of cyanosis, treatment may be limited to careful observation awaiting its resolution when pulmonary vascular resistance decreases. In early neonatal period, maintanance of the patency of ductus arteriosus is necessary with prostaglandin E1 to ensure adequate pulmonary blood flow.
Occasionally, arrhythmias with or without associated Wolf-Parkinson-White syndrome will be dominant in infants. Treatment is directed primarily towards delaying conduction through the AV node with class I antiarrhythmic agents (quinidine, procainamide, flecainide).
Interventional therapy
Downward displacement of the septal tricuspid leaflet is associated with discontinuity of the central fibrous body and the septal atrioventricular ring, creating a potential substrate for accessory pathways and preexcitation. The atrialized right ventricle contains right ventricular muscle fibers, which can provoke polymorphic ventricular tachycardia [62],[63] that rapidly degenerate into ventricular fibrillation since the isolated islands of myocytes cannot anchor reentrant spiral/scroll waves which break up immediately.
“Electrical instability” due to ventricular preexcitation (5 to 25% [64] or 20 to 30%) of cases with supraventricular reentrant tachyarrhythmias (the most frequent rhythm disorder –AVNRT (1to 2%)) or to atrial flutter or fibrillation due to progressive right atrial dilatation is the main clinical problem. The risk of sudden death is high due to adverse association of atrial fibrillation and preexcitation (uniformly via a right bypass tract - Type B Wolf-Parkinson White) in asymptomatic individuals.
Catheter ablation is difficult due to right atrial dilatation, atrioventricular ring distortion and large continuity of muscle in between the atrium and ventricle. Poor outcomes are mostly attributed to the presence of broad/multiple accessory pathways caused by faulty formation of the insulating tissues at the atrioventricular junction located around the orifice of the malformed tricuspid valves {65] and it can be ablated at the time of operative repair [66],[67] or valve replacement [68],[69]. More recently, catheterization techniques have been developed to ablate accessory pathways with the use of radiofrequency current [70], but the success in patients with Ebstein’s anomaly is not yet as high as in patients with structurally normal heart. Complete heart block is rare in Ebstein’s anomaly, but first-degree atrioventricular block as shown in Figure 11 occurs in 42% of patients due to right atrial enlargement and structural abnormalities of the atrioventricular conduction system. Permanent pacing is required for 3.7% of patients with Ebstein’s anomaly [71].
Selective right-sided cryo-ablation-Maze procedure may be done for patients with documented paroxysmal flutter or inducible atrial arrhythmias.
Surgical therapy
Surgical options in Ebstein’s anomaly depend on specific circumstances and the goal should be to palliate for optimum survival, accomplished with valvuloplasty, Valve repair or replacement and right ventricular (RV) exclusion procedures.
Valvuloplasty
It is favoured if the anterior leaflet is suitable for use as a functional monocuspid valve. The leaflet must exhibit adequate excursion and be free of large fenestrations. If the leaflet is ‘sail-like’ and free and when the tricuspid annulus is markedly dilated, an aggressive “Kay annuloplasty” is preferred as for case 4.
Tricuspid Valve repair
A large mobile anterior leaflet with a free leading edge is favourable for valve repair and valve reconstruction was possible in 34.4% of patients with Ebstein’s anomaly. The repair of tricuspid valve was reported in two patients in 1959 and both died [72]. Depending on the variants encountered in the anatomy of Ebstein’s anomaly, various modifications in the tricuspid valve repair have been incorporated [73]. Carpentier, et al, proposed a repair that used the mobilization of the anterior leaflet of the tricuspid valve for type B and C categories as for cases 1 to 3. Temporary detachment of the anterior leaflet and adjacent part of the posterior leaflet was followed by longitudinal plication of the atrialized ventricle and adjacent right atrium, reposition of anterior and posterior leaflets to cover the orifice area at the normal level, and remodeling and reinforcement of the tricuspid annulus with a prosthetic ring. By this procedure, early mortality was 9% and late survival rate at 20 years was 82% ± 5%. The devitalized tricuspid valve related to reattachment may develop problems, which is unclear. Current repair usually involves bringing the anterior papillary muscle towards the ventricular septum and facilitating coaptation of the leaflet edge of the anterior leaflet. An anteroposterior tricuspid purse-string annuloplasty with a plication of atrialized right ventricle or resection is performed selectively, which results in tricuspid valve repair at the level of functional annulus, in contrast to the original repair, which brought the functional annulus up to true annulus by reduction atrioplasty as for case 2 [74]. The reduction atrioplasty is performed at the time of tricuspid valve surgery when the right atrial enlargement is extensive. The right coronary artery demarcate the level of true annulus and may become kinked during plication annuloplasty procedures [75], results in acute infarction of inferoseptal wall of left ventricle and inferior wall of right ventricle.
Biventricular repair (Knott-Craig approach)
If there is moderate to severe TR and an adequate sized functional RV, then a complete biventricular repair (Knott-Craig approach) may be considered as for case 2. In this method, the tricuspid valve is repaired and the atrial septal defect is partially closed. A RV to PA valve conduit is used along with tricuspid valve repair and currently, valve repair is preferred over valve replacement whenever feasible in Ebstein’s anomaly
Definite repair
For older patients with persistent cyanosis or congestive cardiac failure, definite repair can be considered. Tricuspid valve replacement along with plication of some part of the atrialized right ventricle and closure of the atrial septal defect is recommended mostly as for case 1 Valve replacement may be done either a bioprosthetic (preferred) as for case 2 or mechanical valve. A regurgitant tricuspid valve was successfully replaced in Ebstein’s anomaly as a first time in 1962 [76].
Ventricularization
It is the reintegration of the atrialized chamber into the right ventricular cavity, which can be obtained by orthopic transposition of the detached septal and posterior leaflet of the tricuspid valve. The reimplanted septal leaflet serves as an opposing structure for the coaptation of the reconstructed atrioventricular valve [77].
Cone reconstruction
When there is greater than 50
Ebstein’s original case was an example of obstruction at the tricuspid orifice by a membrane dividing the right ventricle into two halves as shown in Figure 16 of a 28-year old cyanotic male with ECG and X-ray characteristics as in Figure 11 to 13. suggesting an advanced spectrum of Ebstein’s malformation, necessitating RV exclusion techniques such as Starnes’ procedure. The florid case of Ebstein’s anomaly with the insertion of leaflet tissue along with ventricular walls as a ‘blanket’ as in Figures 28 and 29 in a 30-year old cyanotic male may go for an initial palliation with bidirectional Glenn shunt (cavopulmonary anastomosis). The other variants of moderate degree of leaflet tethering with varying degrees of regurgitation, but an intact basal leaflet attachments with atrioventricular junction as in Cases 1 and 2 may need a definite repair. In Ebstein’s mitral valve as in Figure 32 in a 10-year old boy, the downward displacement of functional annulus > 0.8 cm/m2 is not particularly striking and tends to affect the septal leaflet (anterior mitral leaflet) alone. The valve is thickened and mildly regurgitant due to rheumatic involvement rather than an anatomic cause.
On follow-up, cases 1 and 2 showed no further deterioration and cases 3, 6, 7 remain symptomatic. Cases 4 and 5 are referred to surgical interventions.
Ebstein’s anomaly is a rare congenital heart defect occurring in 1 in 20,000 live births in general population. [88] and in neonatal Ebstein’s anomaly, the cardiac silhouette may fill the entire chest (“wall to wall” heart) in chest X-rays [89]. The spectrum of malformation in Ebstein’s anomaly may range from only minimal displacement of the septal and posterior leaflets to an imperforate membrane or a muscular shelf between the inlet and trabecular zones of the right ventricle were documented by echocardiography and various treatment options were described in this spectrum.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Giselle Pentón-Rol.
