AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2690-1919/289
1 Infectious Diseases and Tropical Medicine Research Center (IDTMRC), Department of Aerospace and Subaquatic Medicine, AJA University of Medicinal Sciences, Tehran, Iran
2 Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
*Corresponding Author: Mohammad Darvishi. Infectious Diseases and Tropical Medicine Research Center (IDTMRC), Department of Aerospace and Subaquatic Medicine, AJA University of Medicinal Sciences, Tehran, Iran.
Citation: Mohammad Darvishi, Fatemeh Hajilou. (2023). Diagnostic Biomarkers of Diabetic Neuropathy, Journal of Clinical Research and Reports, 13(1) DOI:10.31579/2690-1919/289.
Copyright: © 2023 Mohammad Darvishi. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 10 December 2022 | Accepted: 22 December 2022 | Published: 09 January 2023
Keywords: biomarker; diabetic; neuropathy; diagnostic
Diabetes mellitus (DM) is a chronic disease that occurs due to inadequate production of insulin or decreased effect of available insulin. Considered one of the most critical life-threatening diseases of the 21st century, the number of patients has been increasing over the past few decades. These patients suffer many problems such as neuropathy, nephropathy and rethinopathy which the first can decrease their life expectations.
Diabetic peripheral neuropathy (DPN) is the most common complication in diabetic patients and more than one half of these patients develop nerve dysfunction through their life. The primary cause of diabetic foot disease is DPN which sleep disturbances, poor quality of life, depression and finally unemployment are the results of it.
In this article, we are studying different biomarkers of diabetic neuropathy using related papers in order to consider them in diabetic patients and prevent further complications in them.
Diabetes mellitus (DM) is a chronic disease that occurs due to inadequate production of insulin or decreased effect of available insulin. Considered one of the most critical life-threatening diseases of the 21st century, the number of patients has been increasing over the past few decades (World Health Organization 2019). One of the complications in these patients is diabetic neuropathy.
Diabetic peripheral neuropathy (DPN) is the most common complication in diabetic patients and more than one half of these patients develop nerve dysfunction through their life [1], [2]. One of the results of diabetes mellitus is diabetic foot which many factors affect recurrence of it [3]. The primary cause of diabetic foot disease is DPN [2], [4] which sleep disturbances, poor quality of life, depression and finally unemployment are its results [2] , [6], [7], [8], [9].
1-Nerve conduction studies (NCS)
NCS has been used as a diagnostic, staging, and prognostic biomarker for DPN. Historically, it has been gold standard for the diagnosis of DPN. This study includes supramaximal transcutaneous stimulation of defined upper and lower limb peripheral nerves. It has been done with surface electrodes noninvasively.
One of the prognostic features of DPN is slowing of motor nerve conduction velocity which can led to death [10],[2]. There are some limitations for NCS, too. Most of the time, neurophysiology departments have normal ranges but it is not the same for NCS and also there are not specified standards for it [11],[2].
2-Pain-related Evoked Potentials
It is involved recording of cortical activity through scalp electrodes in response to selective stimulation of nociceptive skin afferents. Distinct waveforms can be obtained for A-delta and C-fiber stimulation, but for the purposes of clinical evaluation only assessment of A-delta waveforms is currently believed to be reliable [12],[13],[2]. Nociceptor stimulation delivered by using a laser or a brief thermal stimulus.
The most reliable neurophysiological method to assess nociceptor pathways is Laser evoked potentials (LEPs) [12],[13],[2]. LEPs have a lot of advantages such as being noninvasive, almost rapid to perform and are an objective neurophysiological biomarker for SFN [14],[2] There are some downsides for LEP, too. This technique is expensive, not common and not available, and certain safety measures (eg, wearing of eye protection) are required during the investigation. Also, the waveform amplitudes are dependent on the participants’ attention [15],[2] and it cannot differentiate between central and peripheral nervous system pathology.
Mechanism of contact heat evoked potentials (CHEPs) is the same as LEPs but they are noninvasive and do not have safety issues as with LEPs. However, they require specialized equipment and are subject to significant habituation effects [15],[2].
Intra-epidermal electrical evoked potentials (IEEPs) using specialized electrodes that deliver a high current density to epidermal fibers. They do not need a specialized stimulator device such as a laser or thermode. Also, they are noninvasive and easy to perform but it is currently experimental, with few studies that address the use of IEEPs in DM and none that compare their use with other SFN biomarkers [16], [2]
3-Nerve Excitability Testing
One of noninvasive methods in which properties of human peripheral nerve axons can be assessed is using nerve excitability testing (NET) [17],[2]
NET is performed in a similar manner to NCS but uses submaximal as well as supra-maximal stimulation according to standardized excitability protocols (stimulus–response curve, strength–duration properties, threshold electrotonus and the current/threshold relationship, and the recovery cycle) to obtain a reflection of firing threshold [17],[18],[19],[2] The results provide a surrogate of ion channel and axonal membrane potential properties at the stimulation site. Although motor axons are more commonly investigated, because recordings are more stable and less affected by artifact, sensory nerves can also be assessed. NET has the advantage in that it assesses functional measures which may occur before pathologic axonal or demyelinating features and their NCS corollary become evident.
In diabetic patients, changes in excitability, including features compatible with sodium channel dysfunction, have been reported in association with subclinical neuropathy [20],[21],[2] These alterations progress with increasing neuropathy severity and are seen in patients with normal NCS, raising the possibility that NET could provide an opportunity to detect abnormalities before they become irreversible [21],[2]. The types of alteration are different between sensory and motor axons and occur at an earlier stage in sensory nerves [2].
NET is not widely available and requires specialist equipment and software. There are no clinically relevant normative ranges and, although alterations in NET can be shown despite normal NCS, it has yet to be validated as a biomarker for either DPN or pDPN. As with NCS, it does not provide information about the status of small nerve fibers. However, it is a useful, noninvasive method to explore disease pathophysiology and may prove a useful biomarker when assessing response to treatment for DPN or pDPN such as in trials of therapeutic agents that act on voltage-gated ion channels [2].
4-Microneurography
Microneurography involves inserting a fine tungsten electrode into a peripheral nerve. For evaluation of peripheral neuropathy, this typically involves recording from the peroneal or superficial peroneal nerves. The electrode is micromanipulated to enable recording of unmyelinated fibers. A typical paradigm involves stimulation of the cutaneous receptive field(s) of the unmyelinated fibers by using electrical stimuli. Using this method, several C-fibers can be recorded at one time. A raster plot is generated showing action potentials time-locked to the electrical stimulus with latencies appropriate for the slow conduction velocity of C fibers. This enables differentiation of nociceptor subtypes (eg, polymodalnociceptorsvs silent nociceptors [22],[2]. Normally, there is a stable baseline latency to low-frequency stimulation of the receptive field, but when a fiber is spontaneously active an irregular “saw-tooth” baseline is seen [22],[23],[24],[25],[26],[2] Also, assessment of “abnormal”sensitivity to cutaneous applied mechanical and thermal stimuli can be recorded [23],[28],[2]. The method can provide evidence of both spontaneous activity and sensitization. Patients with painful peripheral neuropathy have been shown to have abnormal hyperexcitability of nociceptor fibers [23], [26], [27], [28], [29],[2]. Microneurographic studies in DPN have shown variable findings. In patients with documented large fiber neuropathy, one study found an altered distribution of nociceptor subtypes with a higher proportion of mechanically insensitive to mechanically sensitive C-nociceptors, as well as evidence of loss of mechanical sensitivity in normally sensitive fibers [30],[2] Although a small proportion of nociceptor fibers were spontaneously active, this did not significantly differ between pDPN and DPN. Other studies have shown a higher proportion of hyperexcitablenociceptor fibers in patients with pDPN [26], [28], [2].
Although microneurography is an invaluable tool for investigating the presence of nociceptorhyperexcitability, its use as a biomarker is limited. The technique is invasive, albeit minimally and time consuming for both the patient and investigator. Furthermore, it requires specialist equipment and highly trained operators, and is performed only in a limited number of centers worldwide. These factors significantly affect the availability and cost effectiveness of microneurography. Also, age and potentially disease-specific definitions of normative and abnormal data are needed so that its sensitivity and predictive value can be determined, and it can be established as a clinical tool. Anotherdownside of it is that each session may yield only small numbers of nociceptor recordings that are suitable for analysis. For example, in one study, only 1 to 3 suitable nociceptor fibers were recorded per session [26],[2] This limits diagnostic applicability and capacity for monitoring interventions on an individual level. It is not known how microneurography compares with the indirect, but consider more widely available, QST techniques in identifying patients with hyperexcitablenociceptors. Furthermore, the relationship between abnormalities detected by microneurography and structural changes of small fibers in the skin is unknown. The method is potentially of use in clinical trials, especially those with a focus on underlying pain mechanisms, in which patients with and without evidence of hyperexcitability can be segregated [31],[26],[2]
5- Monocyte chemoattractant protein-1 (MCP-1)
MCP-1 protein also known as C–C motif ligand 2 (CCL2)/Monocyte Chemotactic and activation factor (MCAF), belongs to the chemokine family. MCP-1 is a monomeric polypeptide secreted by monocyte, macrophages, and dendritic cells at the site of infection, damage, and injury [32],[33]. MCP-1 secretion is induced by pro-inflammatory mediators like TNFa, interferon-gamma (INF-c), interleukin 1 beta (IL-1b), and platelet-derived growth factor (PDGF) during inflammation. According to scientific reports, MCP-1 aids mononuclear phagocytes relocation during hypoxia and inflammation. Investigators reported that serum, urinary levels of MCP-1 are significantly upregulated in early and late stages in type 2 diabetes. An increase in expression or upregulation of MCP-1 is mainly associated with monocyte binding. MCP-1 contributes to the treatment and management of diabetic neuropathic pain via C–C motif ligand receptor 2 (CCR2) receptors. MCP-1 acts by augmenting excitatory synaptic transmission. The literature showed that spinal astrocytes play a crucial role in neuropathic pain sensitisation by activation of c-Jun-N-terminal kinase (JNK). Some reports showed that TNF-a could activate JNK via TNF receptor 1. This activated TNF-a/JNK pathway transiently stimulates MCP-1. This upregulated levels of MCP-1 can be considered as a potential biomarker in the diagnosis of diabetic neuropathy pain during its early stage to avoid its diverse progression and damage of neurons. MCP-1 inhibitory activity is linked with the management of inflammation as well as the treatment of neuropathic pain [33].
6-Vascular endothelial growth factor (VEGF)
VEGF is an angiogenic factor secreted by various cells, including platelets, macrophages, renal mesangial cells, and keratinocytes. VEGF plays a critical role in cellular activities like haematopoiesis, bone formation, wound healing, and development [33],[34]. Hyperglycaemia modulates the VEGF pathway at protein and mRNA levels in Schwann cells and dorsal root ganglion. Various therapeutic approaches, like VEGF-A165b and VEGF gene transfer, are used to treat diabetic neuropathy. The VEGF-A165b initiates extravasation in dorsal root ganglia, plantar skin of hind paw, and saphenous nerve. This potentiates transient receptor potential cation channel, subfamily A, member1 (TRPA1) channel at the initial stages of diabetic neuropathy leading to altered neuronal stress and pain [33],[35]. A study by Veves et al. showed that VEGF gene transfer studies had reversed severe diabetic neuropathic conditions [33],[36]. DM also influences neuropeptide Y and VEGF during inflammation; both of them play a crucial role in the pathophysiology of diabetic neuropathy. VEGF exhibits polymorphism and halts the potential regulation of gene expression [33], [37] VEGF also reported for its association with atherosclerosis by increasing vascular permeability to low-density lipoproteins in the cardiovascular system, phosphorylation of Murine thymoma viral oncogene Akt and stimulation of neurons linked with neuroprotection in the central nervous system [33]. It is also reported that patients with diabetic peripheral neuropathy in the symptomatic stage have downregulated serum levels of VEGF [33],[38]. Studies also reported that Schwann cells, when cultured in the hyperglycaemic medium, resulted in impairment of neurite outgrowth. This impairment was due to alteration in VEGF receptors regulation in dorsal root ganglion. This causes decreased intracellular secreted protein levels without altering the mRNA level in Schwann cells. This leads to reduced expression of the VEGF both in Schwann cells and dorsal root ganglion [33].
7-Transient receptor potential vanilloid 1 or transient receptor potential cation channel subfamily V member (TRPV1)
TRPV1 is a transducer protein associated with noxious inflammatory and thermal stimuli. TRPV1 proteins are mainly found in nociceptive neurons of the peripheral nervous system and some tissues of the central nervous system. TRPV1 plays a crucial role in pain sensation. It is activated in the presence of capsaicin and acidic condition [33],[39]. It is also activated by ligands like phosphatidylinositol, tyrosine kinase, and proton influx, specifically in peripheral and nociceptive neurons [33],[40]. Diabetic peripheral neuropathy is mainly characterised by allodynia and hyperalgesia [33],[41] The severe pain in diabetic neuropathy is primarily associated with the upregulation of TRPV1 in peripheral nerves [33],[42]. The elevated levels of TRPV1 were reported due to enhanced reflex arc functioning in diabetics [33], [43] It is also said that in peripheral sensory neurons, the inhibitory activity of TRPV1 is due to the activation of the m-opioid receptor. This inhibition of TRPV1 is due to impaired function in the dorsal root of the ganglion and TRPV1 activity [33], [44] This impairment of TRPV1 was confirmed in human embryonic kidney cells. These cells heterologously expressed TRPV1 by stimulating PKA and PKC mediated phosphorylation and resulted in the modulation of TRPV1. Scientific reports also showed that the TRPV1 activation is mainly associated with increased stress in dorsal root ganglion.TRPV1 also has a potential role in pain and post-traumatic neuropathy [33],[45]
The involvement of C fibres was assessed through microneurography. The mechanism of transduction channels TRPA1 and TRPV1 in methylglyoxal-induced pain sensation was investigated by using specific ion channel blockers. The study showed that the selective pharmacological blockade of TRPV1 showed that TRPV1 is crucially involved in methylglyoxal- induced chemical pain sensation and heat hyperalgesia.
The study concluded that methylglyoxal could be a mediator of diabetes-induced neuropathic pain through TRPV1 activation and sensitisation of the voltage-gated sodium channel subtype 1.8 [33],[46]
Cellular oxidative stress plays a key role in the post-translational modification of proteins via SUMOylation. The effect of SUMOylation on the TRPV1 ion channel was carried out by Agarwal and group in diabetic mice and patients. In this study, they have identified the novel molecular targets, that is, key enzymes involved in the regulation of metabolic pathways and ion channels of SUMOylation. In the study sensory neurons of diabetic mice and diabetic patients showed significant changes in the SUMOylation status of metabolic enzymes and ion channels in western blot analysis. These changes lead to metabolic dysfunction, sensory loss, and accelerated neuropathology in diabetic gene-targeted mice selectively lacking the ability to SUMOylate proteins in peripheral sensory neurons. The study showed that diabetes induced de-SUMOylation can impair the functions of TRPV1. The diabetes-induced and metabolic imbalance is caused by de-SUMOylation of various metabolic enzymes that facilitate diabetic sensory loss. The study concluded that endogenous post-translational mechanism regulates TRPV1 function in diabetic neuropathy [33], [47]
It is documented that a-lipoic acid has the ability to regulate TRPV1 in dorsal root ganglion neurons of rats with diabetes. Alpha-lipoic acid acts via alleviating the neuropathic pain in diabetes by regulating TRPV1 expression [33],[48]. All scientific reports insist that the regulation of TRPV1 will provide the new strategy for the management of painful diabetic neuropathy.
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-jB) NF-jB is a pro-inflammatory mediator predominantly involved in adaptive immunity, stress responses, lymphoid organogenesis, and B-cell development. The pro-inflammatory mediators, namely TNF-a and IL-6, are responsible for the activation of NF-jB during cellular damage and inflammation [33], [49]. NF-jB is involved in pro-inflammatory cytokine production during immunological responses. Hyperglycaemia causes activation of NF-jB. This activated NF-jB further triggers expressions of inflammatory mediators. Literature showed that NF-jB is associated with activation, survival, and differentiation of inflammatory T cells and innate immune cells. NF-jB is mainly expressed in endothelialcells linked with its dysfunction and progressing of diabetic neuropathy. The overexpression of NF-jB triggers damage to endothelial cells [33],[50 ]
8-Oxidative biomarkers Nuclear factor (erythroid-derived 2) (NFE2L2)
Nrf2 or NFE2L2 is a dynamic controller of antioxidant responses and cellular damage. Oxidative stress is a factor involved in many metabolic disorders, including diabetes and its complications. Oxidative stress is linked to insulin resistance [33],[51]. The activation of “NFE2L2” plays an important role in maintaining blood glucose level, vascular complications, and endothelial dysfunction by reducing oxidative stress. Hyperglycaemia induced oxidative stress leads to disturbance in the normal physiology of nervous tissue. Increased oxidative stress is one of the early events in the development of insulin resistance, inflammation, and alteration of the aldose-reductase pathway [33] The endogenous disturbance body stimulates major reactive oxygen species defense machinery, NFE2L2. Itscavenges free radicales and increases insulin sensitivity and lipid metabolism. The excess oxidative stress during prolonged hyperglycaemia cannot be controlled by NFE2L2. This leads to the downregulation of NFE2L2 in the sciatic nerve of diabetic animals [33], [52]
9-Adiponectin
Adiponectin hormone is made of 147 amino acids with 30 kDamolecular weight. Adiponectin involved in homeostatic control of blood glucose circulation, lipid oxidation, insulin sensitivity, coronary heart diseases and atherosclerosis [33],[53]. Adiponectin secreted by adipocytes and white adipose tissue is associated with the progression of diabetic neuropathy. Adiponectin gene polymorphism and glypican secretions are significantly involved in diabetic peripheral neuropathy. The upregulation of serum adiponectin levels is positively correlated with aching diabetic neuropathy [33]. Increased levels of adiponectin were associated with a decrease in heart rate and an increase in heart rate velocity leading to cardiovascular autonomic neuropathy. Type 1 diabetics are more prone to cardiovascular autonomic neuropathy, but these findings were found to be limited in type 2 diabetics.Researches have been shown that the adiponectin polymorphism at G276T and T45G is associated with a significantly increased risk of diabetic neuropathy in Type 2 diabetic patients. There is a strong connection between adiponectin and decreased nerve conduction velocity in inflammation and progression of diabetic sensorimotor neuropathy [33],[54]. Recently, ketogenic diet is reported to control upregulatedadiponectin levels in diabetic neuropathy. Ketogenic diet (High-fat, adequate-protein, and low carbohydrate) strategy is usually used to control several diseases such as diabetes, neurological disorders, and cancer. Ketogenic diet replaces plasma glucose with ketone bodies.
The ketogenic diet gives the body the capacity to fight against several diseases such as metabolic disorders, epileptic seizures, neurodegeneration to skeletal muscle atrophy, autosomal dominant polycystic kidney disease, and peripheral neuropathy. A ketogenic diet control the diabetic neuropathy via reducing oxidative stress, enhancing mitochondrial efficiency via regulating the phospho-AMP-activated protein kinase, Naþ/ Kþpump, ketogenesis, gamma-aminobutyric acid-glutamate, ghrelin and leptin levels, lipolysis, lipogenesis, and gluconeogenesis. Anti-inflammatory and antioxidant activities of the ketogenic diet reduce metabolic syndromes-associated allodynia and stimulate peripheral sensory and axonal regeneration [33],[55]. Further studies should be planned to study the detailed molecular mechanism of adiponectin in diabetic neuropathy.
10-Nicotinamide adenine dinucleotide phosphate oxidase (NOX1)
A multicomponent enzyme, NOX, mediates electron transfer from NADPH to molecular oxygen.Mostly they are present in the phagocytic cells and primary cells in the brain, such as vascular endothelial cells, microglia neurons and astrocytes. NOXs mediate neuropathic painby production of reactive oxygen species like superoxide in phagocytic cells. The prolonged hyperglycaemic condition develops upregulation of NOX 4 via tyrosine kinase and MAPK pathway. Reactive oxygen species (ROS) is a triggering factor for activation of NOX 4 via angiotensin II (Ang II). NOX is involved in the generation of peroxynitrite (powerful oxidant), which acts via Ang II in endothelial nitric oxide synthase uncoupling in nerve tissue. Hyperglycaemia-induced oxidative stress plays a crucial role in the progression of diabetic complications. NOX-ROS significantly contributes to the activation of oxidative stress-associated inflammatory pathways that lead to vascular tissue damage including nerves [33],[34] These pathways are basically associated with hypertrophy, angiogenesis, and inflammation in nerve cells. These pathways are responsible for the upregulation of the NOX in diabetic neuropathy [33]. Recently, an in vivo study was carried out by Oghbaei and group to observe the effect of a low dose of sodium nitrate on the diabetic peripheral neuropathy in male Wistar rats.
Diabetes was caused using STZ (60 mg/kg i.p.). Diabetic animals were treated with 100 mg/L sodium nitrate solution (SC) administered for 60 days. Behavioural studies, mechanical allodynia like von Frey filament test, thermal algesia like tail withdrawal test, hot plate test were carried out. Blood samples were analysed for serum insulin and NOX levels at the end of the study. The NOX levels were upregulated in animals with diabetic neuropathy. The study revealed that sodium nitrate has a protective effect on diabetic neuropathy [33].
11-Ceruloplasmin
Copper is an essential factor necessary for homeostasis. Ceruloplasmin, , also known as a 2- glycoprotein, is a serum ferroxidase produced by liver cells and contains almost 95% of copper found in plasma [33], [56]. It plays a key role in copper transportation and metabolism within the plasma. Ceruloplasmin also monitors iron efflux from cells with the enrollment of iron [33], [57]. Recently, scientific data reported that ceruloplasmin gene abnormality is associated with various neuronal diseases, including neuropathy and neurodegenerative disorders [33]. It is also known as “moonlighting protein” for its versatile pro-oxidant and antioxidant activities. The pro-oxidant activity is linked with amine oxidase and antioxidant activity linked with glutathione peroxidase activity. The upregulation of ceruloplasmin is linked with the acute phase of trauma, inflammation, diabetes, and its complications [33]. The damage to ceruloplasmin is coupled with extrapyramidal diseases, progressive dementia, cerebellar ataxia, and diabetes mellitus [33]. Scientists also expected significantly increased levels of ceruloplasmin in diabetic neuropathy. More studies are required to understand the mechanism of action of ceruloplasmin in diabetic neuropathy [33].
Haem oxygenase-1 (HO-1)
Haem oxygenase-1, is associated with stress response and is widely distributed in systemic tissues. HO-1 is mainly regulated by the Nrf2 gene and induced by haem, inflammatory cytokines, prostaglandins, heat shock protein, endotoxins, and heavy metals. HO-1 helps in the regulation of haem metabolism, cellular homeostasis, and vascular inflammation [33],[58]
HO-1 is associated with the rate-limiting step involved in oxidative cleavage of haem degradation, which generates biliverdin, carbon monoxide, and free iron. HO-1 is reported for different activities, namely anti-inflammatory, antioxidant, immunomodulatory, antiapoptotic, and antiproliferative activities. The upregulation ofHO-1 is associated with the activation of multiple signaling pathways like PI3K/Akt, p38 MAPK, and JAK-STAT pathway. HO-1 is implicated in oxidative stress and cellular defense, specifically in neuro-inflammation and also in the progression of diabetic neuropathy
The function of HO-1 in diabetic neuropathy was studied by Leng and group. Diabetes was induced in male C57 mice with a high-fat diet (8 weeks) and STZ (100 mg/kg, i.p. for 2 successive days). Diabetic animals were treated with diosgenin (50 & 100 mg/kg) for 14 days (after 6 weeks of induction). The study showed that diosgenin significantly increased tail withdrawal latency and mechanical hyperalgesia. Diabetic animals showed down-regulation of HO-1 compared to normal control in western blot analysis. Diosgenin treatment upregulated the HO-1 expression as compared to diabetic animals. The study concluded that diosgenin has a neuroprotective effect in diabetic peripheral neuropathy via reducing oxidative stress in Nrf2/HO-1 pathway [33[,[59]. Similarly in vitro studies were carried out in dorsal root ganglionic cells. The cells exposed to high glucose displayed downregulation of HO-1 in diabetic peripheral neuropathy. The cells treated with hirudin protected dorsal root ganglion neuronal cells from apoptosis by scavenging ROS, upregulating Nrf-2/HO-1 expressions, inhibiting NF-j B, and Caspase-3 pathway [33],[52]
12-Dipeptidyl peptidase-4 (DPP4)
DPP4 is an immune regulated enzyme involved in signal transduction in glucose metabolism. It also helps in the degradation of glucose like peptide-1 (GLP-1), cytokines, and growth factors. DPP4 inhibitors improve levels of incretin hormones and insulin sensitivity, which regulate glucose level. The higher levels of serum DPP4 have been reported during a condition called metabolic syndrome which obesity, non-alcoholic fatty liver disease, and diabetes are its signs .
Alterations in regulations of DPP4 are linked with neuroinflammation in hyperthermia [33],[60] The mechanism of action of DPP4 is still not understood clearly.
Poly ADP ribose polymerase a (PARP a) Eukaryotic cells manage various endogenous and environmental genotoxic agents, reactive nitrogen species (nitrosative) and oxidative stress through PARP a. The PARP repair DNA by the removal of base and cell proliferation mechanism [33],[61]. PARP activation is an essential downstream effector in metabolic changes in the diabetic neuropathy. Enzyme PARP can cleave nicotinamide adenine dinucleotide and give rise to poly (ADP-ribose) polymer and nicotinamide. These end products have a considerable role in neurodegenerative diseases. It is well known that the relation between PARP activation and oxidative-nitrosative pressure is unidirectional, and it is just the outcome of scavenging free radicles and oxidation [33],[62]. The mechanism of PARP involves the reduction of NADþ and moderates ATP generation, electron transport, and glycolysis. Overall results revealed in acute endothelial dysfunction in blood vessels during diabetic neuropathy. It is discovered that PARP activation leads to NADþdepletion, which further leads to alteration in transcriptional regulation, impaired signal transduction, gene expression, and damage to neurons and neuroglial cells.
In diabetic neuropathy, PARP activation was basically found in peripheral nerves, dorsal root ganglia and spinal cord. This activated PARP causes up-regulation of inducible nitric oxide synthase (iNOS) as a pro-inflammatory response. This nitrosative stress may cause damage to nerves and nerve fibres, which leads to diabetic neuropathy [33],[63]
The effect of Lipoic acid was studied on PARP expression in type 2 diabetic Sprague Dawley rats by Chen and group. Type 2 diabetes was induced using a high-fat diet followed by STZ (35 mg/kg, i.p.). Diabetic animals were treated with100 mg/kg i.p. lipoic acid for 8 weeks. The results showed that diabetic animals showed demyelinating changes to sciatic nerve fibres.PARP expression and the apoptosis index of sciatic nerve cells were significantly higher than normal animals in immunohistochemistry. Lipoic acid treatment showed significant improvement in the symptoms of diabetic neuropathy by reducing PARP activity and inhibiting apoptosis [33],[63]
13-Sirtuin 1 (SIRT1)
SIRT, also known as metabolic sensor, belongs to the family of Nicotinamide adenine dinucleotide (NAD)-dependant histone deacetylases. It plays a critical role in the assessment of the energy status of the body by regulation of mitochondrial function, oxidative stress, and inflammation. The imbalance between the energy requirement and supply may lead to the progression of various forms of diabetic neuropathy [32],[64]. This epigenetic enzyme act by deacetylation of multiple factors. Inactivation of SIRT1 is one of the underlying causes of pathogenesis in hyperglycaemia associated with vascular complications and insulin resistance. Studies have been shown that the inhibitory activity of SIRT1 is due to the interaction between pro-inflammatory mediators like IL-6, TNF-a, and NF-jB [32]. The downregulation of SIRT1 has been majorly documented in type 2 diabetic neuropathy. Downregulation of SIRT1 may lead to hypoxia, oxidative, and metabolic stress which leads to the progression of diabetic neuropathy. SIRT1 associated antioxidant signalling in experimental diabetic neuropathy works by reducing oxidative stress and improving mitochondrial impairment [32],[65] . SIRT also regulates the synaptic plasticity in the brain and spinal dorsal horn neurons. The role of SIRT in synaptic plasticity is still unclear. But literature showed that the upregulation of SIRT is linked with the neuroprotective activity of diabetic neuropathy.
14-Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)
MALAT1 which is the first identified non-protein-coding long noncoding RNA (lncRNA), has a highly conserved gene [32]. MALAT1 is located in various tissues like kidney, liver, lung, brain, and heart. The p53 gene regulates the MALAT1 expression during haematopoiesis. MALAT1 helps in retaining the proliferation of haematopoietic cells [33],[66]. Glucose imbalance leads to activation of MALAT1 by inflammatory mediators and may increase serum amyloid antigen 3 significantly [33]. MALAT1 overexpression occured during the progression of diabetic neuropathy. Scientific data showed that the MALAT1 was also associated with an anti-inflammatory role during nerve damages. The study revealed that MALAT1 can be the potential biomarker for diabetic neuropathy [33].
15-microRNA (miRNA)
MiRNAs whichare small (19–25 nucleotides) single-stranded, noncoding RNA transcripts, are present in tissue and plasma samples. They act by inducing messenger RNA (mRNA) degradation or blocking protein translation during post-translation modifications of genes.While the mechanism of miRNA in the progression of diabetic neuropathy is still unclear [33],[67] it has been shown that the degree of miRNA aberration is directly associated with painful diabetic neuropathy. For instance, miRNAs -146, -199a-3p and -499a can be used as circulating biomarkers for the detection of diabetic neuropathy. Some miRNAs -25, -190a-50, -23a, -9 and -29c have a potential role as therapeutic targets in diabetic neuropathy.
Recently, the literature showed that miRNA plays a key role in the progression of diabetes-associated with neuropathic pain. But the mechanism ofmiRNA accumulation and diabetic neuropathy is unclear. The role of miR-155 in diabetic peripheral neuropathy in vivo and in vitro was studied by Chen and group. The study was done in Schwann cells. Hyperglycaemia was caused in Schwann cells by exposing the cells to 5.5mM glucose. Functional studies were carried out to determine the effect of miR-155 on cellular function, reactive oxygen species, Nrf2 and inflammation.In an in vivo study in type 1 diabetic rats,diabetes was induced by using STZ (60 mg/kg, i.p.). Diabetic rats were treated with miR-155 antagomir or agomirin order to study the role of miR-155 on nerve conduction velocities, angiogenesis, and inflammatory response. In vitro silencing of miR-155 was carried out in Schwann cells. The results of the study showed the inhibition of apoptosis and alleviated inflammation. In vitro and in vivo treatment of miR-155 antagomir-induced inhibition enhanced nerve conduction velocities, strengthened angiogenesis, and alleviated inflammation. The study showed that miR-155 was involved in the regulation of Nrf2 in diabetic peripheral neuropathy. The study concluded that the silencing of miR-155 alleviate sciatic nerve injury in diabetic peripheral neuropathy. Hence miR-155 would be considered the potential therapeutic target for the management of diabetic peripheral neuropathy [33]. Further studies should be done to confirm the mechanism of miRNA in the progression of diabetic neuropathy.

Image1-Summery of some of the biomarkers
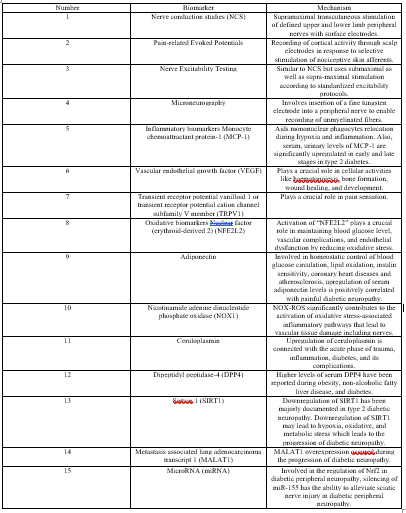
Table 1-Summery of biomarkers of DPN
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
