AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2637-8892/234
Department of Pharmacology, Anand College of Pharmacy, Sharda University, Agra-282007, India
*Corresponding Author: Kashmira J. Gohil, Department of Pharmacology, Anand College of Pharmacy, Sharda University, Agra-282007, India.
Citation: Roshan sah., Manish Pal Singh., Kashmira J. Gohil, (2023), Cognitive Guardians: Exploring Flavonoids as Potential Therapeutic Agents in Alzheimer’s Disease Treatment, Psychology and Mental Health Care, 7(6): DOI:10.31579/2637-8892/234
Copyright: © 2023, Kashmira J. Gohil, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 September 2023 | Accepted: 26 September 2023 | Published: 04 October 2023
Keywords: alzheimer's disease; presenilin; apolipoprotein e;neurofibrillary tangles
Alzheimer's Disease (AD) is a devastating neurodegenerative disorder characterized by progressive cognitive decline. With no cure in sight, there is an urgent need to identify novel therapeutic strategies. This abstract summarizes the emerging research on the potential of flavonoids, a class of natural compounds found in various fruits, vegetables, and beverages, as promising agents for AD treatment. Flavonoids have gained attention due to their antioxidant and anti-inflammatory properties, both of which play crucial roles in AD pathogenesis. This review examines the accumulating evidence suggesting that flavonoids may exert neuroprotective effects through multiple mechanisms. Flavonoids demonstrate the ability to enhance synaptic plasticity and promote neurogenesis, crucial processes for memory and learning, which are severely impaired in AD. These compounds show promising effect in reducing the accumulation of amyloid-beta plaques and tau tangles, the pathological hallmarks of AD. Furthermore, their potent antioxidant capabilities help combat oxidative stress, a hallmark of AD, by neutralizing harmful free radicals and also, flavonoids modulate inflammatory pathways, reducing neuroinflammation and its detrimental effects on the brain. This abstract provides an overview of preclinical studies and epidemiological evidence supporting the potential of flavonoids in preventing or slowing the progression of AD. Additionally, it discusses challenges in translating these findings into clinical practice, including issues related to bioavailability and standardization of flavonoid-rich interventions. In conclusion, flavonoids represent a promising avenue in the search for therapeutic agents against Alzheimer's Disease. Their multifaceted neuroprotective properties make them attractive candidates for further investigation and potential inclusion in future AD treatment strategies. However, more rigorous research, including well-designed pre-clinical and clinical trials, is necessary to establish their efficacy and safety in AD patients.
NFTs - amyloid plaques and neurofibrillary tangles
Aβ - amyloid peptideβ
AD - Alzheimer's disease
PS – Presenilin
APP - Amyloid precursor protein
ApoE- Apolipoprotein E
NFTs - neurofibrillary tangles
MCI - mild cognitive impairment
PPARγ - Peroxisome proliferator-activator receptor-γ
GPx - Glutathione peroxidase
Erk - Extracellular regulated kinase
AMPK - AMP- activated protein kinase
GSK3β – Glycogen synthase kinase-3β
Cdk-5 – Cyclin-dependent kinase-5
PP2A – Protein phosphatase 2A
PGC-1α – Peroxisome proliferator-activated receptor-γ coactivator
GSHpx – Glutathione peroxidase
Er-α - Estrogen receptor-α
ACHE - Acetylcholinesterase
BCHE - Butyrylcholinesterase
GSH - Glutathione
NLRP3 – Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing3
Around the world, there are at least 50 million people who have dementia. [1] This is expected to triple by 2050 as a result of longer life spans, yet there are still no treatments available to stop or delay the onset of dementia. Alzheimer's disease (AD), the most prevalent cause of dementia, is a neurodegenerative condition that is often characterised by the buildup of NFTs, which are brought on by tau aggregation and Aβ respectively. More recently, gliosis and chronic neuroinflammation have been added to the list of AD symptoms along with plaques and tangles. [2] One of the main challenges in halting the progression of AD, by the time it is clinical diagnosis, brain atrophy is already established and now pathogenic cascade are well established. Its complex pathology is characterised by a memory loss that often develops beyond the age of 60. Rarely, the symptomatology may appear between 40 and 50 years of age, in which case the disease advances quite quickly. [3] In most cases, the "familial" early-onset form of the disease is linked to particular mutations in the genes that code for PS1 and PS2 as well as APP, while occasional late-onset disease is linked to mutations in the gene that codes for ApoE and includes a number of environmental risk factors. [4] The extracellular Aβ deposits, which take the form of neuritic plaques, are the histopathological characteristics of AD. Aggregates of increased phosphorylation Tau protein make up intraneuronal NFTs. [5] Language, focus, and orientation problems that develop into motor problems and personality abnormalities are the hallmarks of the symptomatology, which has a significant negative influence on the general public's health and places a heavy load on the medical community. [6]
A number of medications have been chosen to treat the condition, but regrettably none of them alter the course of the disease; instead, they only provide milder management and temporary symptoms due to the diverse nature of the pathogenic targets involved in AD progression. [7] More research is required to fully characterise the element of danger that expose one to the advancement of AD and to find medications that can stop the disease's progression or protect against it. [8] In this regard, natural remedies and diet offer significant promise in preventing the onset of neurodegenerative illnesses. [9] In reality, using natural chemicals can be an excellent replacement medicine, unlike manufactured products, which have major negative effects. This review's objectives are to summarise the present level of knowledge regarding AD, the molecular targets that cause it to progress pathologically, as well as to emphasise the beneficial correlations that flavonoids may have for the prevention and treatment of AD.
According to the amyloid cascade hypothesis (ACH), the buildup of Aβ oligomers is what starts a chain reaction that results in neuroinflammation, tau-induced toxicity, synaptic dysfunction, and neuronal death. This hypothesis chain reaction is occurred in two ways such as non-amyloidogenic pathway and amyloidogenic pathway. [10] In the non-amyloidogenic pathway involves the splitting of APP by α-secretase between Lys16 and Leu17 in the Aβ domain, resulting in the production of a soluble N-terminal fragment (APPsα) and a membrane-bound C-terminal fragment, C83, which can be further broken down by γ-secretases to produce a soluble extracellular p3 peptide and prevent the formation of intact Aβ. In contrast to the non-amyloidogenic pathway, the amyloidogenic process involves the internalisation and delivery of APP to endosomes. The enzyme β-secretase cleaves APP during the processing of amyloidogenic APP, and producing both a membrane-bound C-terminal fragment (C99) and a soluble N-terminal fragment (APPsβ). [11] A set of enzymes called γ-secretase then breaks down C99 inside the membrane, releasing a cytoplasmic polypeptide called AICD (APP intracellular domain) at the luminal side and Aβ peptides. γ-secretase breaks down APP at different locations in the transmembrane domain, resulting in Aβ peptides with lengths ranging from 38 to 43 residues. (fig.1) According to the amyloid cascade hypothesis, the aberrant buildup of amyloidβ (Aβ) plaques in multiple parts of the brain that triggers neurodegeneration in AD. Numerous Aβ-targeting treatments have been developed on the basis of this theory, with the hope that by focusing on this early Aβ-accumulation, the ensuing harmful pathways can be prevented. [12] Unfortunately, therapies that directly target APP processing have failed in Phase III, most likely as a result of side effects. [13] Examples of these therapies include BACE1 inhibitors. Aducanumab, an Aβ-targeting antibody, is said to have met its primary endpoint, according to recent reports, however approaches to diminish aggregating forms of Aβ via antibody techniques have also had poor results. It is becoming more and more obvious that lowering Aβ levels in the brain is unlikely to have a meaningful clinical impact if administered at MCI stage or later. The protracted prodromal period of AD will probably necessitate the use of Aβ-targeting therapies from the onset and throughout in order to become most successful.[14] This restriction calls for a tactical change, either focusing on targets that better correlate with later stages of disease progression, like neuroinflammation or tau, or sticking with the current targets while putting the emphasis on biomarker development to improve the classification of patients for earlier treatment. Finding and validating safe and affordable lifestyle therapies that might be widely used throughout midlife to lower AD risk throughout the public is another strategy. (Figure 1)
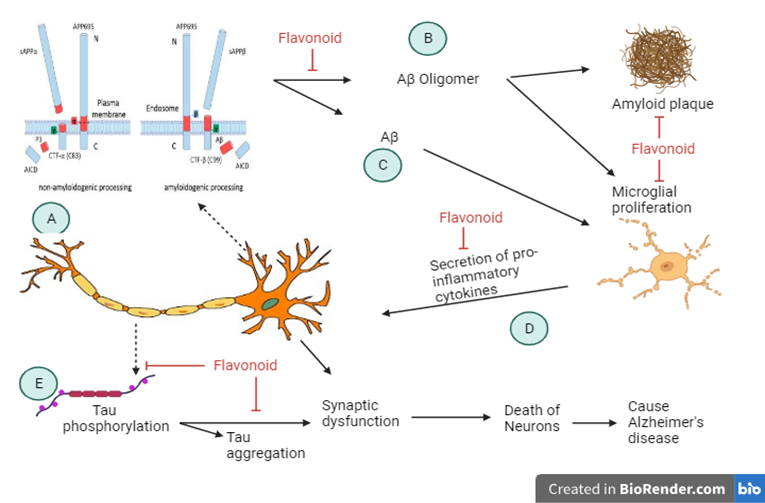
Figure 1: Mechanism of action of Alzheimer’s Disease.
Recent research has looked at the effectiveness of plant-derived natural substances in preventing AD. Fruits, nuts, and drinks are the main sources of the flavonoids and more than 6000 distinct flavonoids have been found. [15] Phenol has the capacity to pass the blood-brain barrier and has multitarget activity. Flavonoids have been shown in numerous studies to have biological effects as neuro anti-oxidants, as well as anti-amyloidogenic, metal-chelating, anti-inflammatory, and improvements in cognition and neuroprotection qualities. [17] According to epidemiological research, diets high in flavonoids are directly linked to a lower risk of dementia and the potential for improved symptoms in AD patients. Although there isn't any concrete evidence that flavonoid consumption lowers the risk of AD, only the epidemiological research indicate that flavonoid consumption may reduce the pathology of AD and improve symptoms. [18] Flavonoids can be categorised into six different groups: anthocyanidins, flavones, isoflavones, flavonols, flavanones, and flavan-3-ols (also known as flavanols). Although flavonoids are well-known for their antioxidant properties, it is also evident that they have the power to control intracellular reactions, primarily through altering protein-Kinase signalling pathways. [19] Furthermore, there is currently a lot of data in favour of flavonoids' potential to obstruct AD-related pathways. [20] But due to the high level of uncertainty surrounding absorption, metabolism, and fundamental drug kinetics, flavonoids have not yet been developed as treatment options for AD. With an emphasis on research where the potential for bioavailability of flavonoids has been seriously taken into account, growing mechanistic evidence supporting the modulation of AD pathways by flavonoid-mediated mechanisms is assessed in this review.
| S. No. | Dietary | Flavonoids | Model | Route of administration | Mechanism of action | References |
| 1. | Onion | Quercetin | Rats | Gastric intubation | Apoptosis. | 127 |
| 2. | Garlic root | Quercetin | Rats | Gastric intubation | Apoptosis. | 128 |
| 3. | Lactuca capensis | Catechin | Rats | Intraventricular | By altering the cholinergic response. | 129 |
| 4. | Hot peppers | Zeaxanthin | Rats | Intraperitoneally | By lowering the Aβ-peptide levels. | 130 |
| 5. | Orange’s peel | Naringenin | Rats | Orally | Stimulating Ache activity | 131 |
| 6. | Broccoli juice | Quercetin | Cellular model | Injectable | Triggering the Nrf2 signalling pathway | 132 |
Table 1: Dietary’s flavonoid in Alzheimer’s Disease
Several studies show that flavonoids can act as acetylcholinesterase (AChE) inhibitors but, as AChE inhibitors are only symptomatic interventions and not preventative or disease modifying. [21] But the study of flavonoids in AD mouse models has made significant strides in the last few years. The evaluation of these in vivo studies will primarily focus on those that used an oral route of phenolic treatment, in food or drink. A summary of the research that used oral means of delivery are presented in the table. 2 The processes are demonstrated by flavonoids through the pathophysiology of Aβ and tau, inflammation of the brain, oxidative stress, and neurogenesis. [2]
4.1 Flavonoid suppression of APP processing and Aβ accumulation
Both in vitro and in vivo studies have indicated that a number of flavonoids can improve Aβ pathogenesis such as- ECG, myricetin, genistein (Figure 1). This conclusion could be attributable to an increase in Aβ degradation, inhibition of aggregation, and a shift towards a non-amyloidogenic processing route.
Epigallocatechin gallate (EGCG): A widely researched catechin that is prevalent in tea, is a well-known illustration of a flavonoid-mediated blocking of APP synthesis. It has been demonstrated in vitro that EGCG increases the ratio of mature ADAM10 (mADAM10) to pro-ADAM10 in a dose-dependent manner, which is associated with a change in processing from amyloidogenic to non-amyloidogenic and reduction in Aβ formation. [22-24] The levels of both soluble and insoluble Aβ were shown to decrease via oral administration of EGCG, by upregulating the α-secretase activity and downregulate the β- and γ-secretase activity. In-vivo, it has been demonstrated in numerous AD-related mice models that EGCG causes APP processing to shift in favour of a non-amyloidogenic route. [132,133]]
Myricetin: Myricetin is a naturally occurring flavanol that is found in a variety of fruits and vegetables, and also available in red wine, blackcurrant tea, and medicinal plants. [25-28] Myricetin has an anti-amyloidogenic properties by lessens the formation of Aβ-aggregation by creating H-bonds between its hydroxyl group and the carbonyl group on the surface of the β-sheet. As a result, myricetin limits the expansion of Aβ fibrils, inhibits the inter-strand hydrogen connections, and inhibit Aβ hazardous alterations. [29-33] Myricetin has been demonstrated to boost α-secretase level and enzyme activity, while inhibiting β-secretase activity, in cortical nerve cells of the cultured rat. It has been demonstrated that an H-bond is formed between the hydroxyl group at C7 of the A ring of myricetin and the pair of Asp 32 and 228 of BACE-1, resulting in a significant decrease in the enzyme's activity. [34,35]
Genistein: Genistein is a flavonoid that is mostly derived from the plant Glycine max soybean and is found in a variety of crops, including beans, green peas, and nuts. Numerous studies suggest that genistein may be used therapeutically to prevent the onset of Alzheimer's disease by enhancing cognitive ability and synapse formation. [36-38] By lowering the production and aggregation of Aβ, genistein guards against the advancement of AD. According to a study it has been shown that genistein's inhibitory efficacy against the degree of Aβ25-35 self-aggregation decreased when given after 24 hours of incubation. [39] Genistein altered the PPARγ receptor to promote the synthesis of ApoE in rat hippocampus neurons by decreasing presenilin levels, increasing αsecretase while decreasing βsecretase and BACE1 activity. [40-43]
Baicalein: Baicalein show its mechanism via a GABAAA receptor-dependent mechanism, promotes cleavage of APP that is not amyloidogenic while blocking cleavage that is amyloidogenic.[44] GABAA activation promote the non-amyloidogenic processing of APP and baicalein may control GABAA receptor activation by inhibiting the GABAA transaminase that breakdown GABAA and also inhibit the non-amyloidogenic processing of APP. [45-47]
4.2 Flavonoid inhibit aggregation and phosphorylation of tau
Physiologically, the microtubule-associated protein tau binds to microtubules primarily in axons. [48,49] Tau is assumed to have a major role in microtubule structure and stabilising, which facilitates the movement to and from synapses.889 Tau.

Table 2: Mechanism of flavonoid in Alzheimer’s Disease
On the other hand, becomes hyperphosphorylated and separates from the microtubule in AD. [48] Tau's mis-localization to the somatodendritic area, where it has been demonstrated to impede nuclear import and synaptic function, is facilitated by hyperphosphorylation. Local translating of tau mRNA, which is triggered by Abeta application via an FYN/ERK/S6 pathway, has also been linked to tau aggregation in the somatodendritic area. [50] Additionally, hyperphosphorylated tau forms oligomers that eventually form insoluble helical filaments and tangles of neurofibrillary cells (NFT), which are one of the distinguishing features of Alzheimer's disease (AD). [51] More precisely, numerous different kinases, including Erk, Akt, p38, AMPK, GSK3β, cdk5, and PP2A, influence the phosphorylation of Tau. An activation of MAPK, a heterotrimeric protein kinase, results in Tau being hyperphosphorylated in neurons. Additionally, it monitors Aβ's metabolism and participates in cell division, apoptosis, and inflammatory reactions. [52-55]

Figure 2 holecular target of flavonoid in Alzheimer’s Disease
Inhibiting the MAPK pathway is therefore a viable strategy for slowing the onset of AD and can considerably enhance synaptic remodelling, consciousness, and mental abilities. Numerous signalling pathways in cells are connected by GSK3β. It is regarded as the primary cause of the phosphorylation of the Tau protein in AD.
Quercetin: According to the research it has been shown that the main target of quercetin is MAPK. [56] As per study, by affecting GSK3β and reducing kinase activity, quercetin indirectly inhibits the hyperphosphorylation of Tau protein, which contributes to the strengthening of its neuroprotective effects. By pre-incubating HT22 cells with 5 mol/L of the medication for 12 hours, researchers were able to determine quercetin's anti-apoptotic effects through MAPKs and PI3K/Akt/GSK3ß signalling pathways. [57,58]
Genistein: In rat models of AD, genistein inhibits the formation and deposition of Aβ aggregates along with the excessive phosphorylation of the tau protein. [59-61]
Naringenin: In PC12 cells from AD-rats, naringenin increased learning and spatial memory via controlling the PI3K/Akt/GSK-3K pathway and lowering the hyperphosphorylation of TAU. The activation of PI3K/Akt, the modification of the signalling pathway GSK3β, and blocking the caspase 3 activity are all components of the intracellular system that enables this neuroprotective function and is essential for the survival of neuronal cells. [62-64]
Proanthocyanidins: It were given orally through a gavage, and this decreased the amount of lead-induced tau phosphorylation that was connected to the activation of p38 kinase and JNK, however not any direct correlation was found. [65]
Epicatechin: Exercise and dietary polyphenols epicatechin may improve brain function and decrease the course of a moderate- or mid-stage AD. [66]
Baicalein: The flavonoid baicalein's potential as a cutting-edge and effective oral bioactive treatment for Alzheimer's that inhibits memory loss. [67]
Hesperidin: In the hippocampus of APP/PS1 mice, EGCG administration decreased TNF-/JNK signalling and enhanced Akt and glycogen synthase kinase-3 phosphorylation. [68] Hesperidin may enhance cognitive performance in the APPswe/PS1dE9 transgenic mice model of AD by reducing mitochondrial dysfunction via suppression of GSK-3 activity, along with an improvement in anti-oxidative defence. [69] Through the stimulation of Akt/Nrf2 signalling and the suppression of RAGE/NF-B signalling, hesperidin reduces inflammation and oxidative stress while also providing neuroprotection. [70]

Figure 4: Mechanism of flavonoid inhibit aggregation and phosphorylation of tau
4.3 Flavonoid enhanced antioxidant reaction in AD
Generally speaking, flavonoids are renowned for their antioxidant properties [71], which may be helpful in AD given that oxidative damage has been linked to neurodegeneration. [72] According to evidence from post-mortem investigations on AD-brain, there is a net decline in the capacity to lower H2O2 levels and activate anti-oxidative stress pathways when compared to control brain. [73] H2O2 either activate MAPK signaling pathway or can react with metallic cation such as Fe2+, due to this it has been reported of abnormal brain-iron levels in AD. [74,75]
Although flavonoids have the ability to act as reactive oxygen scavengers by donating hydrogen ions, it is unlikely, that this is how they elicit their antioxidant potential because of the low potential to cross blood-brain barrier of flavonoids in the brain in comparison to better marked ROS scavengers. [76,77] It is more likely that the manipulation of intracellular pathways like the MAPK pathway by flavonoids, such as those found in plants, is how they suppress oxidative stress. [78]
In another study it is concluded that, nitric oxide synthase (NOS) activity is regulated by p53, and one of the most potent inducers of biosynthesis of mitochondria and respiration, and PGC-1a, is suppressed. The decrease in PGC-1α expression in AD is one of the factors contributing to the disease's progression because it is associated with an increasing in the production of peptide Aβ. It is another mechanism through which flavonoid show its anti-oxidant property in AD via inhibiting p53. [79,80]
Myricetin: Remarkable antioxidant properties as well as radical detoxification activities are ademonstrated by myricetin. Myricetin has been shown to be able to prevent the production of ROS, myeloperoxidase, and ATP depletion associated with oxidative stress in mouse models. But in animal models, it raises the degree and activities of the major antioxidant enzymes, including SOD, CAT, and GSHpx. As per study, treatment with myricetin for 3 hours enhanced the cell survival of isolated, inebriated cardiomyocytes, decreased aluminum phosphide, and also inhibited mitochondrial dysfunction. [81-83]
The pyrogallol group in myricetin is responsible for its antioxidant properties. This property helps to break the chain of events started by ROS. The molecule has a tendency to combine with radicals that are free to create radical semiquinones. [84-86] Moreover, the substance possesses chelating characteristics on metal ions like Cu2+ ions and Fe2+; this, on the one hand, significantly boosts its antioxidant activity since the Fenton reaction is hindered and, as a result, the creation of ROS is decreased. On the other hand, the toxicity of Aβ complexes is decreased by myricetin, which works directly on them, by limiting the number of metal ions that can interact with them. [87-89]
Genistein: Due to its strong antioxidant capacity and excellent ROS scavenger activity, genistein has been successfully demonstrated to alleviate oxidative stress in vitro. [90] The binding of the ER-α and activation of AMPK are both known to increase the production of antioxidant enzymes including SOD, CAT, and GPx. Genistein's antioxidant properties are also linked to these two processes. [91-93]
Quercetin: Because p53 is a substantial promoter of the ROS-mediated apoptosis pathway, quercetin's effect on p53 has an impact on the reduction of oxidative stress. By significantly scavenging ROS, quercetin has been proven for regaining the cellular redox equilibrium. [94,95] GSH levels and the expression of certain antioxidant enzymes, such as SOD, catalase CAT, and GPx, were raised in the mice by feeding a 1% quercetin diet for 20 weeks. [96-99]
Apigenin: Apigenin pretreatment effectively reduced the generation of intracellular ROS, reduced cellular DNA damage, decreased lipid peroxidation, alleviated protein carbonylation, and reinstated cell apoptosis. In another study, and it is also shown that apigenin enhanced the cellular antioxidant defence mechanism. [100-101] In another study, it has been shown that the apigenin demonstrated superoxide anion scavenging properties and enhanced glutathione peroxidase and superoxidase dismutase antioxidative enzyme activity in AD. [102]

Figure 5: Mechanism of flavonoid enhanced antioxidant reaction in AD
4.4 Flavonoid inhibiting AChE and BChE in AD
Acetylcholinesterase (AChE) is a particular esterase that is a member of the carboxylesterase class of enzymes. Acetylcholine (ACh), a neurotransmitter, is what it mostly hydrolyzes. AChE is primarily present in high quantities in red blood cells, as well as at cholinergic brain synapses and neuromuscular junctions in the brain. [103] The pathophysiology of AD is influenced by the decline in cholinergic activity in the brain regions in charge of higher cognitive tasks. The hallmarks of AD include localised A production and accumulation, intracellular neurofibrillary tangles, and extracellular senile plaques.mExtracellular deposits of the human beta-amyloid (Aβ) peptide are detected in senile plaques that are found in the brains of AD patients. [104,105]
According to research, a region close to the human AChE's C-terminus and the Alzheimer's disease Aβ peptide's N-terminus are only weakly homologous. The development of amyloid fibrils in plaques of senility and the toxicity linked to such deposits are further indications that AChE may be implicated. [106] BChE appears to be involved in the brain's conversion of risky amyloid plaques into the pathogenic formations that cause dementia and AD. [107]
Genistein: Genistein supplementation has been shown to affect dopaminergic and cholinergic activity, assisting in memory restoration and neuroprotection in mice and also alleviated brain insulin resistance. [108] As per study, the two variants of genistein inhibit the enzyme AChE, and demonstrating the medicinal potential of genistein. [109] According to the study, in MPTP-induced PD mice, genistein exhibits neuroprotective effects on dopaminergic neurons; this effect may be explained by increasing Bcl-2 gene expression. [110]
Quercetin: ACh levels drop and communication between neurons slows down in the brain. Through the phenyl ring's OH groups, quercetin establishes hydrogen bonds with certain amino acids in the AChE active site. Memory loss symptoms are reduced by AChE and BChE inhibition, which improves neuronal signalling and activates cholinergic pathways in the brain. [111,112] As per study, it has been shown that via using in vitro models, quercetin treatment caused the inhibition of AChE and secretase enzymes, blocking the breakdown of acetylcholine and reducing the synthesis of A, respectively. [113]
Myricetin: According to the study, myricetin have capacity to suppress AChE significantly lessened the mice's loss of learning and memory in the scopolamine-induced mouse model of Alzheimer's disease. [114]

Figure 6: Mechanism of flavonoid inhibiting AChE and BChE in AD
4.5 Flavonoid regulating neuroinflammatory response in AD
Alzheimer's disease (AD) causes complicated and poorly understood neuroinflammatory reactions. They relate contact among the central nervous system (CNS) and the periphery and involve diverse cellular and molecular actors. In the brains of AD patients and in mouse models of the disease, amyloid peptides seen in senile plaques and improperly phosphorylated tau in tangles of neurofibrillary cells can trigger inflammatory responses. These reactions' impact on pathophysiology of AD varies on a number of variables and may be advantageous or harmful. Thus, comprehending the function of neurological inflammation in AD may aid in the creation of safer and more effective therapy approaches. [115]
Myricetin: Myricetin decreases the amounts of proinflammatory mediators, such as IL, NF-kB, TNF, iNOS, and COX2, in the microglial BV2 cell line, which prevents lipopolysaccharide (LPS) from causing neuroinflammation. [116] Myricetin has the ability to suppress NLRP3, decrease microglia activation, and directly lower inflammatory factor levels. [117] By interfering with the NF-kβ signaling pathway, myricetin's anti-inflammatory activities primarily aim to lower levels of IL, TNF-, iNOS, COX-2, as well as other inflammatory mediators in the brain and lessen the damage these inflammatory factors cause to the neurological system. [118] Myricetin’s anti-inflammatory properties may eventually aid in easing AD symptoms.
Genistein: As per study, genistein suppresses neuro-inflammation in the RAW 264.7 cell culture by controlling the transcription of cytokines such TNFα, IL-1β, IL-6, and IL-12. [119,120]
Naringenin: Naringenin effectively modulates the (NF-kB) signaling system, which is linked to inflammatory processes, and lowers interleukin (IL)-6, IL-1, and tumour necrosis factor (TNF). [121] By significantly reducing the phosphorylation process and nuclear translocation of P65, an NF-kB subunit, as well as by raising sirtuin 1 (SIRT1) levels in the hippocampus, naringenin causes the lowered expression of NF-kB. [122]
Quercetin: It has been demonstrated that quercetin exerts anti-inflammatory effects via lowering the production of cytokines and other chemicals that cause inflammation. [123]

Figure 7: Mechanism of flavonoid regulating neuroinflammatory response in AD
The discussion of challenges in translating findings into clinical practice, including concerns regarding the bioavailability of flavonoids and the standardization of flavonoid-rich interventions, is pivotal in the treatment of AD. Addressing bioavailability underscores the importance of optimizing the delivery of flavonoids to ensure they reach therapeutic levels effectively, while acknowledging the variability in flavonoid content necessitates standardized approaches to ensure consistent dosing and research reproducibility. These challenges not only highlight the need for further investigation but also raise questions about the practical implementation of flavonoid-based interventions in Alzheimer's Disease treatment, emphasizing the complexities involved in transitioning promising research into effective clinical strategies. [124]
The inclusion of a discussion on the challenges related to translating findings into clinical practice, particularly focusing on issues of bioavailability and standardization of flavonoid-rich interventions, is a crucial aspect for effective pharmacological action. Here's an evaluation of this section:
5.1 Bioavailability Concerns: Addressing the bioavailability of flavonoids is essential because it reflects how effectively these compounds are absorbed and utilized by the body. This issue is relevant because some flavonoids may have low bioavailability, meaning they might not reach therapeutic levels in the brain or other target tissues. Discussing this challenge highlights the need for research to identify delivery methods or formulations that enhance flavonoid bioavailability. [124]
5.2 Standardization of Interventions: The mention of standardization is important as it recognizes the variability in flavonoid content among different sources, such as fruits, vegetables, and beverages. Standardization ensures that the intended dose of flavonoids is consistent across studies or clinical applications. It's a valid concern as inconsistent dosing can lead to inconsistent outcomes and hinder the reproducibility of research results.
5.3 Practical Implementation: While not explicitly mentioned, addressing these challenges also raises questions about the practicality of incorporating flavonoid-rich interventions into the daily lives of individuals at risk of or suffering from Alzheimer's Disease. It's not just about efficacy but also feasibility and acceptability to patients. [125]
5.4 Future Research Directions: Enhancement of flavonoid effect in AD, emphasizes the need for further rigorous research, including clinical trials. It would be beneficial to briefly suggest potential research directions to overcome these challenges. For example, exploring novel delivery methods to enhance bioavailability or developing standardized flavonoid supplements could be avenues for further investigation. [126]
In summary, discussing the challenges related to bioavailability and standardization of flavonoid-rich interventions in the context of Alzheimer's Disease treatment adds depth and realism to the further research in the treatment of AD. It highlights the practical hurdles that researchers and clinicians must address when considering the use of flavonoids as potential therapeutic agents.
In conclusion, this abstract presents a compelling case for exploring flavonoids as potential therapeutic agents in the treatment of Alzheimer's Disease. It effectively highlights their multifaceted neuroprotective properties, including antioxidant and anti-inflammatory effects, modulation of key pathological hallmarks, and promotion of critical brain processes like synaptic plasticity and neurogenesis. However, it judiciously acknowledges the challenges ahead, particularly regarding flavonoid bioavailability and the standardization of interventions. These obstacles underscore the necessity for rigorous research, including well-designed clinical trials, to assess the safety and efficacy of flavonoids in Alzheimer's Disease treatment. Despite these challenges, flavonoids represent a promising avenue for further investigation and potential inclusion in future Alzheimer's Disease therapeutic strategies, offering hope in the quest to combat this devastating neurodegenerative disorder.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.

Dear Editorial Team, Journal-Clinical Cardiology and Cardiovascular Interventions, “Publishing my article with Clinical Cardiology and Cardiovascular Interventions has been a highly positive experience. The peer-review process was rigorous yet supportive, offering valuable feedback that strengthened my work. The editorial team demonstrated exceptional professionalism, prompt communication, and a genuine commitment to maintaining the highest scientific standards. I am very pleased with the publication quality and proud to be associated with such a reputable journal.” Warm regards, Dr. Mahmoud Kamal Moustafa Ahmed

Dear Maria Emerson, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews’, I appreciate the opportunity to publish my article with your journal. The editorial office provided clear communication during the submission and review process, and I found the overall experience professional and constructive. Best regards, Elena Salvatore.

Dear Mayra Duenas, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews Herewith I confirm an optimal peer review process and a great support of the editorial office of the present journal
Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. I am really grateful for the peers review; their feedback gave me the opportunity to reflect on the message and impact of my work and to ameliorate the article. The editors did a great job in addition by encouraging me to continue with the process of publishing.

Dear Cecilia Lilly, Editorial Coordinator, Endocrinology and Disorders, Thank you so much for your quick response regarding reviewing and all process till publishing our manuscript entitled: Prevalence of Pre-Diabetes and its Associated Risk Factors Among Nile College Students, Sudan. Best regards, Dr Mamoun Magzoub.

International Journal of Clinical Case Reports and Reviews is a high quality journal that has a clear and concise submission process. The peer review process was comprehensive and constructive. Support from the editorial office was excellent, since the administrative staff were responsive. The journal provides a fast and timely publication timeline.

Dear Maria Emerson, Editorial Coordinator of International Journal of Clinical Case Reports and Reviews, What distinguishes International Journal of Clinical Case Report and Review is not only the scientific rigor of its publications, but the intellectual climate in which research is evaluated. The submission process is refreshingly free of unnecessary formal barriers and bureaucratic rituals that often complicate academic publishing without adding real value. The peer-review system is demanding yet constructive, guided by genuine scientific dialogue rather than hierarchical or authoritarian attitudes. Reviewers act as collaborators in improving the manuscript, not as gatekeepers imposing arbitrary standards. This journal offers a rare balance: high methodological standards combined with a respectful, transparent, and supportive editorial approach. In an era where publishing can feel more burdensome than research itself, this platform restores the original purpose of peer review — to refine ideas, not to obstruct them Prof. Perlat Kapisyzi, FCCP PULMONOLOGIST AND THORACIC IMAGING.

Dear Grace Pierce, International Journal of Clinical Case Reports and Reviews I appreciate the opportunity to review for Auctore Journal, as the overall editorial process was smooth, transparent and professionally managed. This journal maintains high scientific standards and ensures timely communications with authors, which is truly commendable. I would like to express my special thanks to editor Grace Pierce for his constant guidance, promt responses, and supportive coordination throughout the review process. I am also greatful to Eleanor Bailey from the finance department for her clear communication and efficient handling of all administrative matters. Overall, my experience with Auctore Journal has been highly positive and rewarding. Best regards, Sabita sinha

Dear Mayra Duenas, Editorial Coordinator of the journal IJCCR, I write here a little on my experience as an author submitting to the International Journal of Clinical Case Reports and Reviews (IJCCR). This was my first submission to IJCCR and my manuscript was inherently an outsider’s effort. It attempted to broadly identify and then make some sense of life’s under-appreciated mysteries. I initially had responded to a request for possible submissions. I then contacted IJCCR with a tentative topic for a manuscript. They quickly got back with an approval for the submission, but with a particular requirement that it be medically relevant. I then put together a manuscript and submitted it. After the usual back-and-forth over forms and formality, the manuscript was sent off for reviews. Within 2 weeks I got back 4 reviews which were both helpful and also surprising. Surprising in that the topic was somewhat foreign to medical literature. My subsequent updates in response to the reviewer comments went smoothly and in short order I had a series of proofs to evaluate. All in all, the whole publication process seemed outstanding. It was both helpful in terms of the paper’s content and also in terms of its efficient and friendly communications. Thank you all very much. Sincerely, Ted Christopher, Rochester, NY.
