AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2641-0419/358
1The Russian Academy of Natural Sciences (RANS), Moscow, Russia.
2Dept for Clinical Allergology & Immunology of the Russian University of Medicine, Moscow, Russia.
3EPMA, Brussels, EU.
4PMC, Washington, DC, USA.
5ISPM, Tokyo, Japan.
6AHA, Houston, TX, USA.
7Department of Neurophysiology of Kazan State Medical Academy, Kazan, Russia.
8New York Academy of Sciences, USA
9Faculty of Medicine and Life Sciences, University of Hasselt, Belgium.
10Stem Cell Biology Lab, Department of Medicine, Cardiology Division, UCSF, S-F, CA, USA.
11 Vishnevsky National Medical Research Center of Surgery, Moscow, Russia
*Corresponding Author: Sergey Suchkov, Department of cardiology, Hospital University Center Ibn Rochd, Casablanca, Morocco.
Citation: Sergey Suchkov, Lidya Kadyrova, Matt Springer, Vladimir Zemskov, Aleksandr Tyukavin, et al, (2024), Cardiac regeneration and stem cell therapy through the view of design-driven clinical applications cardiology-related practice: current knowledge and future prospects, J. Clinical Cardiology and Cardiovascular Interventions, 7(6); DOI:10.31579/2641-0419/358
Copyright: © 2024, Sergey Suchkov. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 14 March 2024 | Accepted: 09 May 2024 | Published: 03 June 2024
Keywords: cardiac regeneration; cardiac stem cells; pluripotent stem cells; cardiac SCs; personalized and precision medicine (PPM)
Cardiovascular disease is the leading cause of death, with 80% of cases occurring in developing countries. Innovative therapies are required to reduce mortality and limit or abolish the necessity for cardiac transplantation. Over the last decade, stem cells have been a promise for the cure of several diseases not only due to their plasticity but also to their capacity to act in a paracrine manner and influence the affected tissue. Human SC-based therapy derivatives are extremely attractive for therapeutic development because they have direct pharmacologic utility in clinical applications, unlike any other adult cells. Moreover, stem cell-derived paracrine factors have been shown to suppress inflammation and apoptosis, stimulate angiogenesis, and amplify the proliferation and differentiation of resident cardiac stem cells (CSCs). And SC therapies are thus viable alternatives to conventional treatments with substantial therapeutic potential; market opportunities are huge, as multiple product candidates are expected to be approved over the coming decade
The ultimate goal for regenerative medicine is to channel multipotent human cells with high proliferative capacity into pre-defined scenarios and specified differentiation programs within the human body. Current therapies do not address the underlying pathophysiology of this disease, namely, the progressive loss of functional cardiomyocytes. The notion of repairing or regenerating lost myocardium via cell-based therapies remains highly appealing.
The recent identification of adult stem cells, including both cardiac stem/progenitor cells, bone marrow stem cells and others (Figure. 1)
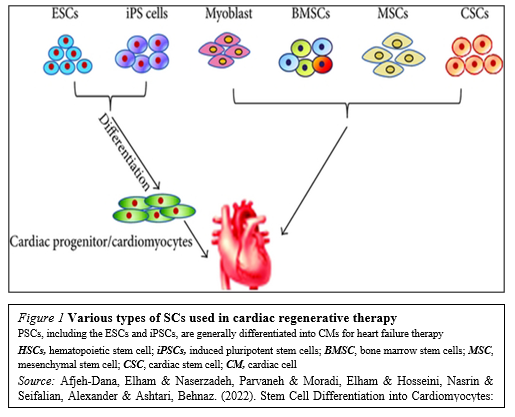
Has triggered an explosive interest in using these cells for physiologically relevant cardiomyogenesis.Unfortunately, the efficacy of cell-based therapies is somewhat limited by their poor long-term viability, homing, and engraftment to the myocardium. In response, a range of novel SC-based technologies are in development to provide additional cellular modalities, bringing CTs a step closer to the clinic. Enthusiasm for cardiac regeneration via cell therapy has further been fueled by the many encouraging reports in both animals and human studies. Further intensive research in basic science, biodesign-driven translational applications and clinical arenas are needed to make this next great frontier in cardiovascular regenerative medicine a reality.
Meanwhile, stem cell (SC)-based therapy has been considered as a promising option in the treatment of ischemic heart disease (Figure. 2).
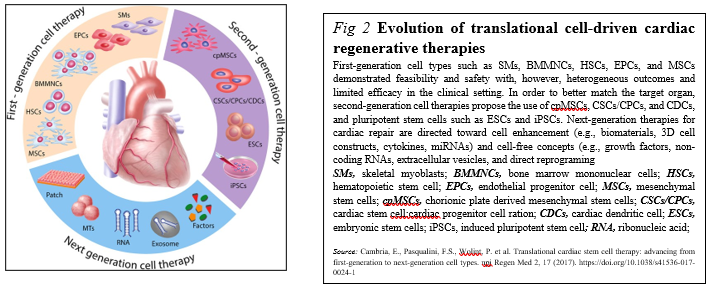
Originally, SC transplantation has emerged as a promising therapeutic strategy for acute and chronic ischemic cardiomyopathy. Regarding the current status of SC therapies for patients with myocardial infarction is critically being discussed now, describing:
(i) the current status of clinical trials of human pluripotent SCs (hPSCs) compared with clinical trials of human adult or fetal stem cells;
(ii) the gap between fundamental research and translational application of human SCs;
(iii) the use of biomaterials in clinical and pre-clinical studies of SCs; and
(iv) trends in design-driven bioengineering to promote SC therapies for patients with myocardial infarction [1,2].
Skeletal myoblasts (SMs): the pioneering steps Due to the outcome results and findings summarized, initial studies were encouraging, with SMs participating at heart muscle formation, and then were rapidly translated into the clinics with Phase I trials. However, because of the increased risk of ventricular arrhythmias potentially due to missing junctional proteins, the trials were stopped. The risk of ventricular arrhythmias is relevant now that pluripotent cell-derived cardiomyocytes aim at re-attempting heart remuscularization [3].
Bone marrow (BM)-derived cells in trials Differentiation of BM mononuclear cells (BMMNCs) into cardiomyocytes was observed but was criticized later [4].
Regarding HSCs, the researchers found no evidence of myocardial differentiation of CD34+ HSCs in preclinical models [5]. The clinical trials did not find significant beneficial effects of cell therapy [6,7].
Mesenchymal stem cells (MSCs) in trials MSCs fulfill many of the criteria for an ideal adult SC for regenerative therapy for a variety of conditions. Derived from the stromal fraction of bone marrow, MSCs can be readily isolated and identified on the basis of growth and expansion on plastic surfaces. MSCs are easily grown and expanded having multi-lineage potential, and appearing to exhibit immunological tolerance.
MSCs have repeatedly been suggested as an immunoprivileged and therefore valuable clinical cell source for cardiac repair. MSCs are multipotent stromal cells that are mainly characterized by their adherence properties, a distinct surface marker profile composed of specific markers, paracrine signaling, and the ability to differentiate into the adipogenic, chondrogenic and osteogenic lineage.
A subset of MSCs can also differentiate into cardiomyocytes under specific conditions in vitro, and thus due to the preclinical trials, adipose tissue-derived and BM-derived MSCs represent an auspicious cell source with therapeutic potential for cardiac repair, and were promising in numerous preclinical trials [8,9]. Instead of the latter, approaches using MSCs in clinical trials, are studied with promising results, but their efficacy needs to be further validated [10-12].
Second-generation therapies were aiming at orienting non-resident SCs, such as MSCs and PSCs (pluripotent stem cells), toward cardiac differentiation. Only few studies compared first-generation and second-generation cell types, which found that cardiac-committed cells displayed an improved therapeutic effect as assessed by improved engraftment, cardiac function, angiogenesis, and scar size [13-15]
Due to their enormous expansion rate and immunomodulatory properties, MSCs are considered as a potential candidate for both autologous and allogeneic SC-driven therapy with preventive, prophylactic and rehabilitative effects.
Cardiopoietic MSCs (cpMSCs) in trials The use of cpMSCs in preclinical trials with chronic ischemic disorders has shown therapeutic benefit, but the latter needs to be validated in long-term studies [16,17].
To date, clinical success of cardiac cell-therapies (including cpMSCs-driven one) remains limited. To enhance the cardio-reparative properties of SCs, the concept of lineage-specification through cardiopoietic-guidance has been recently suggested. However, so far, only results from preclinical studies and from a clinical pilot-trial in chronic heart-failure are available, while systematic evidence of its therapeutic-efficacy is still lacking [18].
Cardiac stem/progenitor cells (CSCs/CPCs) for the trials, being multipotent, clonogenic, and expressing SC markers, are usually derived directly from biopsies of the target organ, and therefore ensure a perfect match and can inhibit cardiomyocytes apoptosis [19]. Due to the preclinical studies, c-kit+ CSCs/CPCs could differentiate into cardiomyocytes, suggesting that this phenomenon occurs at a purported functionally insignificant rate [20-22].
Pluripotent SCs (PSCs) in trials PSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), constitute another source for guided cardiac differentiation (Figure. 3)
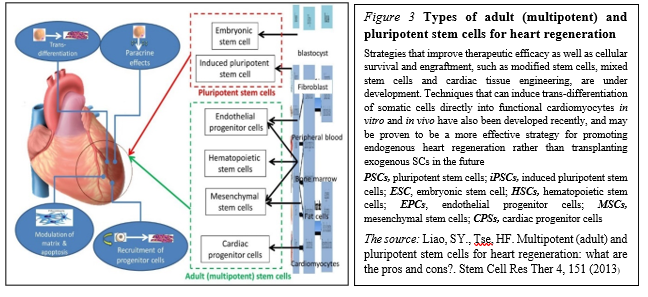
The most primitive of all SC populations are the ESCs that develop as the inner cell mass at day 5 after fertilization in the human blastocyst. When isolated and transferred to appropriate culture media, ESCs can undergo an undetermined number of cell doublings while retaining the capacity to differentiate into specific cell types, including cardiomyocytes.
Common teaching suggests that SCs emerging during late embryonic and fetal development are restricted to the production of tissue-specific cell types. And although SCs continue to self-renew in adult life, their ability to differentiate is limited to the tissue in which they reside, retaining a high degree of developmental plasticity. The latter would open a way to enter a revolutionary period in SC biology and regenerative medicine.
Meanwhile, human PSCs (hPSCs) are abundant sources of cardiomyocytes for cell replacement therapy and other applications such as disease modeling, drug discovery and cardiotoxicity screening [23]. And hPSC-derived cardiomyocytes have thus attracted attention as an unlimited source of cells for cardiac therapies to attract the strategy for cardiomyocyte production via the biphasic, involving hPSC expansion to generate adequate cell numbers followed by differentiation to cardiomyocytes for specific applications [24].
Preclinical studies with PSCs yielded mixed results depending on the animal model. But in most cases, human ESC-derived CPCs have been transplanted into animal models of MI, showing improved cardiac function, thus leading to clinical trials.
PSCs represent an appealing source from which to develop cell replacement therapies (Figure. 4).
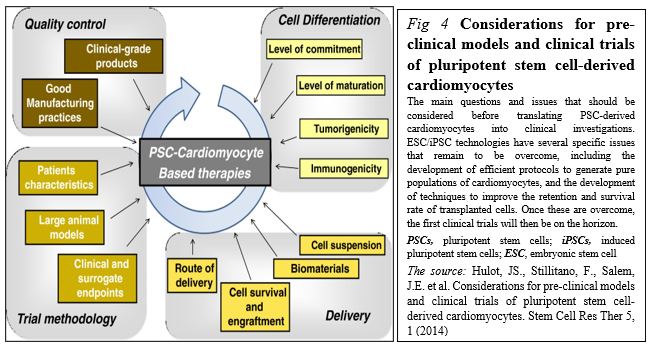
Different initiatives have been launched to promote their development toward clinical applications including stages before translating PSC-derived cardiomyocytes into clinical investigations, including the development of good manufacturing practice-level PSC lines, the development of efficient protocols to generate pure populations of cardiac myocytes, and the development of techniques to improve the retention and survival rate of transplanted cells [25].
SC-based therapies represent a possible paradigm shift for cardiac repair. However, most of the first-generation approaches displayed heterogeneous clinical outcomes regarding efficacy. Stemming from the desire to closely match the target organ, second-generation cell types were introduced and rapidly moved from bench to bedside. Unfortunately, debates remain around the benefit of SC therapy, optimal trial design parameters, and the ideal cell type [17].
Having the above-mentioned summarized and assessed, we might mention that cell (SC-driven) therapy holds potential to tackle myocardial infarction and heart failure. But the key issues such as the cell type, cell number, delivery route, timing, follow-up periods, and endpoints remain unsolved. Meanwhile, the field has rapidly evolved to address in particular the ideal cell type and does require to get the problems be surmounted to secure the full potential of cell therapy be realized in the near future to come.
1.Toward Cardiac Regeneration: Combination of PSC-Based Therapies and Design-driven Bioengineering Strategies
The potential to repair cardiac tissue by cell grafting has attracted the attention of the field, due to the scarce capacity of CMs to proliferate and replace the damaged tissue. The dispute over the existence of cardiac progenitors in the adult heart is still unsolved; on the same trail, the ability of immature cardiac progenitor cells to engraft with pre-existing CMs in vivo has not been irrefutably determined. Due to their unique capacity to produce functional CMs, a wide variety of cells have been evaluated for therapeutic
delivery, including BMCs, MSCs, and endogenous CSCs as the most promising sources for cardiac regenerative medicine application.
But despite the encouraging results from these and other in vivo studies on the potential beneficial effects of iPSC-based cell therapy for heart failure, preclinical experiments, using CMs derived from human iPSCs, have given controversial results [26]. Meanwhile, despite the fact that many different types of SCs and derivatives have been proposed as valuable candidates for regeneration of the myocardium, a general consensus on which cell type should be considered the gold standard for cell replacement therapy of the heart, remains elusive.
The problem is that the majority of preclinical studies have been conducted in 2-D culture systems, a condition that does not take into account the multiple interactions occurring in a 3-D structure, like the heart: this is among the major issues still limiting the application of cell therapy approaches into the clinics. Thus, in the last years, many productive studies have directed their efforts for the development of a 3D structure able to functionally resemble the cardiac tissue and suitable for heart transplantation.
1.1.3D culture systems as applicable to manage cardiac regeneration
In particular, the cardiac tissue is composed by contractile and non-contractile cells, that are organized as a complex 3-D structure (spheroids and organoids both referring to 3D culture systems with a specialized architecture and cell organization that typically form through self-assembling processes), which acting as tightly regulated interaction among these diverse cell types, plays a central role in the heart’s function. Thus, 3D cell cultures are emerging as a new tool for both targeted drug discovery and regenerative medicine applications, prompting and boosting the development of 3D cellular systems, as applicable to the functional features of cardiac tissue. However, those systems were found to be not ideal for developing cardiac cells for cell therapy.
The genuine true is that engineered cardiac tissue models have recently emerged as promising approaches to repair damaged cardiac tissue as well as suitable platforms for drug/toxicity testing and disease modeling (MacNeil, 2007). Meanwhile, the improvement of protocols for differentiation of PSCs toward the cardiac lineage made the production of large amounts of human CMs feasible, bursting the development of human heart surrogates for disease modeling, drug testing and autologous cell therapy applications. On this regard, embedding CMs derived from PSCs into engineered structures has been shown to positively impact cell maturation to different extents, depending on the specific characteristics of the generated 3D-constructs. In fact, use of both human iPSCs and ESCs deals with an important issue of cell maturation following cardiac induction in vitro [27]. Thus, altogether these studies strongly indicate that the above-mentioned constructs are excellent supports for maturation of CMs derived from human PSCs, since they provide a physiological-like environment and stimuli to cells. And, moreover, altogether those studies showed that cardiac engineered constructs enhance intercellular organization and crosstalk of iPSC-CMs, improving their maturation in vitro, and ameliorate cardiac performance after the transplantation in vivo, thus providing encouraging evidences for their future use to achieve cardiac regeneration in humans.
A significant number of different cell therapy strategies that employ iPSC-CMs have been proposed to restore cardiac function, ranging from injection of cells alone, to their combination with ECM-like biomaterials, and to more complicated engineered-based strategies, which aim to faithfully recreate the myocardium.
Although different types of stem cells have been proposed as potential candidates for cardiac regenerative medicine, there is no consensus about which represents the best choice. Given the complexity of cardiac tissue, which is composed by diverse cell types, human PSCs – mostly iPSCs – are certainly among the most relevant sources. In particular, CMs differentiated from iPSC may represent a realistic option for the regeneration of the injured heart (Figure. 5)
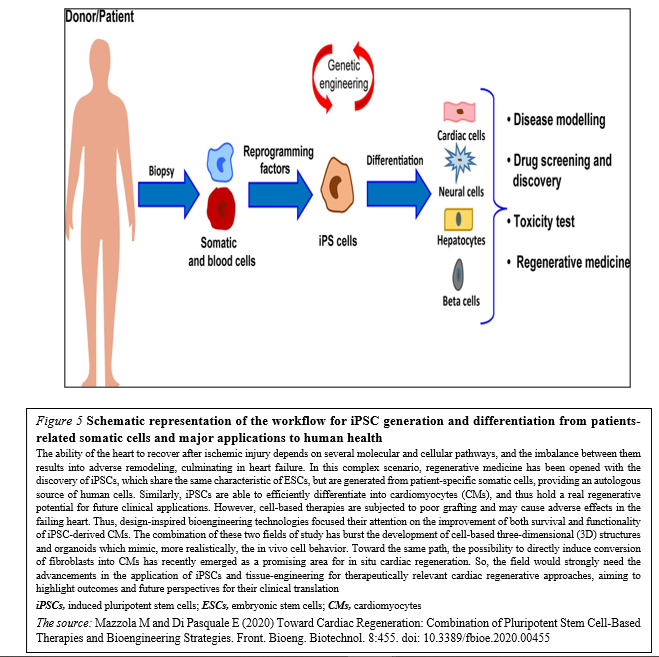
The majority of these approaches have shown – at least to some extent – success in mimicking heart cells and improving cardiac function, providing new hope for the replacement of CMs after the irreversible loss of heart tissue occurring during myocardial infarction (Figure 6).
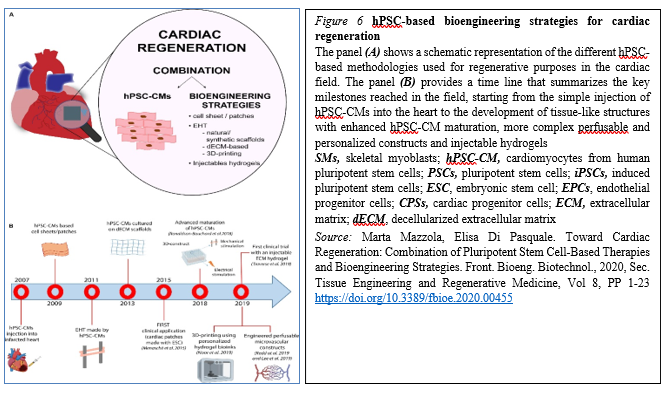
The progress in the field made by tissue engineering technologies has been encouraging and thus opened ways to create bio-supports able to incorporate the cells needed for the regeneration of cardiac tissue. However, significant improvements are still mandatory before we could consider their routine use in the clinical field for treatment of patients suffering from myocardial diseases. These improvements mainly regard scaffold production, the interactions between cells and the ECM and vascular integration with the host, hopefully opening new doors for cardiac regeneration therapy via tissue engineering strategies. However, despite the promising results obtained by preclinical research, the approach is still rather immature for its clinical translation and further investigations are needed to develop effective and more efficient strategies to achieve cardiac regeneration in vivo.
1.1.Engineered cardiac tissue constructs to be translated into clinical applications
No comments, but design-driven tissue engineering strategies are probably a more suitable option. And given the limited regenerative capacity of the heart, the design-driven bio-engineered scaffolds and tissue engineering approaches are thus ideal for cardiovascular regenerative medicine applications. The main goal is to create a cardiac graft which can be implanted and restore the functionality of the myocardium without major side effects. To this aim, tissue engineering intends to recreate the microenvironment of the cardiac tissue, in terms of cell composition, stiffness, geometry, physical and electrical stimuli and, extracellular matrix, and in order to generate constructs with an actual translational value, keeping in mind the native characteristics of the cardiac tissue [28] .And although SC therapy is a promising treatment for myocardial infarction, the minimal functional improvements observed clinically limit its widespread application. Where a need exists to maximize the therapeutic potential of those SCs by first understanding what factors within the infarct microenvironment affect their ability to regenerate the necrotic tissue, and to define a novel mechanism by which the extracellular environment of the infarction regulates the therapeutic potential of MSCs.
Moreover, within the last years, many biomaterials have emerged as good candidates for cardiac tissue engineering to fill the existing gap for their application to cardiac regeneration and treatment of myocardial infarction, and to increase cell retention, improving cell survival and coupling with the host. In general, cell homing and adhesion to cardiac tissue can occur via specific receptor-mediated interactions with the extracellular matrix (ECM) proteins, or can be initiated via physical/chemical adhesion to cardiac cells via hydrophobic and electrostatic interactions. It is widely accepted that SCs target inflammation and injury sites, including infarcted myocardium. Accordingly, many SC membrane reengineering approaches aim to promote these endogenous processes, whilst describing the development of new targeting strategies to improve homing efficiency (Figure. 7).
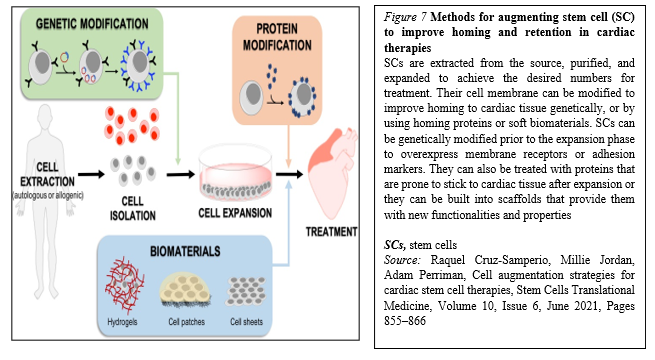
The fast-growing development of new technologies to reengineer the membrane of SCs or provide a supporting biocompatible matrix may alleviate these limitations. It is clear that genetic approaches are extremely exciting, as they can be implemented through reliable protocols that are easy to track in the preclinical phase, and have a low risk of triggering unwanted immune responses.
With respect to the developments within the biomaterial scaffold space, although they offer an effective solution to myocardium cell retention and cell number, the transplant process is generally more invasive, and challenges with effective electromechanical integration still remain. Meanwhile, the scaffolds could be modified or implemented in combination with molecules that activate the recruited cells (e.g., statins) and amplify their therapeutic potential. In conclusion, it is likely that no single approach to SC membrane reengineering will provide the “magic bullet” for cardiac cell therapies, and that the next generation of therapies will likely utilize combinations of these technologies to fully harness the therapeutic potential of transplanted SCs.
However, regardless of the employed strategy, generation of tissue-like engineered structures suitable for regenerative cardiology applications needs further considerations, specifically in relation to some aspects that may be critical for their efficacy and potential undesired effects in vivo.
2.Cardiac SCs (CSCs) as promising cell types for cardiac regeneration
Myocardial infarction results in an irreversible loss of cardiomyocytes due to aging or pathophysiological conditions, which is generally considered irreversible, and can lead to lethal conditions with subsequent adverse remodeling and heart failure. But human PSCs, including ESCs and iPSCs, can self-renew while maintaining their pluripotency to differentiate into all cell types, including cardiomyocytes. So, identifying new sources for cardiomyocytes and promoting their formation represents a goal of cardiac biology and regenerative medicine.
Meanwhile, because of the lack of strong evidence supporting the existence of resident CSCs, efforts are focused on how to mobilize and promote those few cardiomyocytes that have potential to proliferate in mammalian hearts. Whereas endogenous cardiomyocytes can proliferate at a low frequency, methods that promote cardiomyocyte proliferation may be a vital research field in the future. The aforementioned pathways could be manipulated, optimized, and synergized to achieve better functional improvement after cardiac injury. In addition to inducing cardiomyocyte proliferation, other alternative approaches could be considered (Figure. 8).
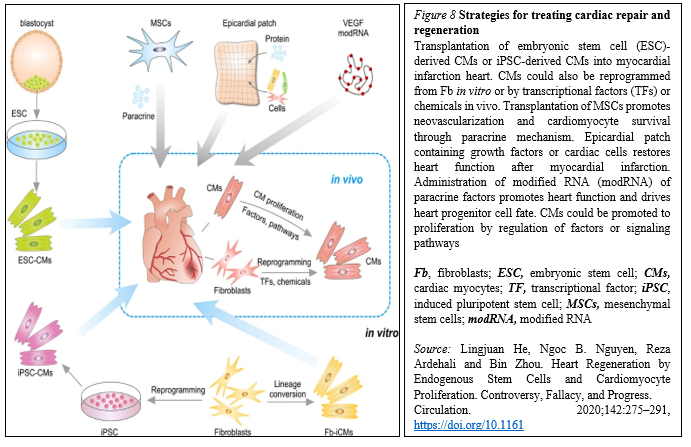
In situ reprogramming of fibroblasts to cardiomyocytes by overexpression of specific transcriptional factors represents a promising direction for future study [29].
Recent studies have also suggested that small molecules can induce reprogramming of fibroblasts to cardiomyocytes in vivo, which offers a dual pharmaceutical approach to regenerate cardiomyocytes and reduce scar formation [30].
Human ESCs or iPSCs-derived cardiac progenitors and cardiomyocytes have been successfully transplanted and confirmed the survival of transplanted cells within the host myocardium and an improvement in cardiac function after injury. Furthermore, dual SC therapy, such as human iPSC-derived cardiomyocytes and MSCs, or human ESC-derived epicardium and cardiomyocytes, synergistically improves cardiac function and augments vascularization in the injured myocardium. Moreover, recent advances in engineered epicardial patch containing multiple cardiac cell types are improved heart function and neovascularization after myocardial infarction [31-33].
In this context, CSCs are considered to be very promising cell types for cardiac regeneration. And within the past decade, among cardiac myocytes many types of putative CSCs have been reported to regenerate the injured myocardium by differentiating into new cardiomyocytes. Some of these CSCs have been translated from bench to bed with reported therapeutic effectiveness. However, recent basic research studies on stem cell tracing have begun to question their fundamental biology and mechanisms of action, raising serious concerns over the myogenic potential of CSCs [34]
In partocular, c-Kit+ CSCs represent a promising candidate for cardiac-specific SC lineages, which is likely heterogenous in nature [35]. The 1-year follow-up results from the SCIPIO trial support that intracoronary infusion of one million autologous c-Kit+ CSCs in patients with heart failure of ischemic etiology undergoing coronary artery bypass grafting is safe and feasible and may promote significant improvements in global heart function, decrease scar size, and induce regeneration of viable myocardium.
As you might see, resident CSCs are self-renewing and can give rise to cardiomyocytes. Their multipotentiality allows them to differentiate along the three main cardiac lineages: myocytes, endothelial cells, and smooth muscle cells. After their injection in the ischemic heart, the formation of the above-mentioned cell types contributes to the regeneration of myocardium and the improvement of its contractility.
But the approaches securing cardiac regeneration in post-infarction period are not available to be practiced. And the key problem is the identity of cells be born to generate functionally active cardiac myocytes replenishing those being lost during ischemia.
Previous research made by several groups has identified an extensive population of CSCs in the adult heart. Through the view of the latter, there are four ongoing clinical studies to test the benefits of autologous CSCs:
Meanwhile, with identification of resident CSCs (rCSCs), it has been supposed that the latter may be a crucial source to initiate and prompt myocardial self-renewal and regeneration. But along with the latter, endogenous cardiomyogenic SCs (CMSCs) might be better choice for cardiac repair. Ideally, those cells should be directly stimulated in situ, avoiding extraction, purification, culture and reinjection. It is therefore of uttermost importance to understand the identity and function of the cells that constitute the natural environment of cardiac progenitors and support their quiescence, self-renewal and activation. Additionally, further studies are needed to develop a deeper understanding about the properties of pericytes, as these cells have the potential to migrate to different tissues away from their perivascular location and play an active role in the activation of cardiac repair after ischaemia. This would involve a modern interpretation of the pericyte’s role as a cell type involved in reducing the threshold for the activation of an angiogenic program in cardiac repair [38].
CSC therapy holds great potential to prompt myocardial regeneration in patients with ischemic heart disease. The selection of the most suitable cell type is pivotal for its successful application. Various cell types, including crude bone marrow mononuclear cells, skeletal myoblast, and hematopoietic and endothelial progenitors, have already advanced into the clinical arena based on promising results from different experimental and preclinical studies. However, most of these so-called first-generation cell types have failed to fully emulate the promising preclinical data in clinical trials, resulting in heterogeneous outcomes and a critical lack of translation. Therefore, different next-generation cell types are currently under investigation for the treatment of the diseased myocardium [39].
Drug (including SC-driven) development continues to move in the direction of Personalized and Precision Medicine (PPM), - where ideally the most effective therapy or treatment is determined by the genetic makeup of the patient and thus by a spectrum of biomarkers to be targeted by the therapeutics (including those being based on SCs). Regarding PPM-based cardiology practice and the innovations made in the field of SC-driven technologies, the focus in the last years has been moved towards a concept of the new wave of cardiac myocyte (CM) formation via a scenario of dedifferentiation and proliferation of mature CMs. The observation that CSCs can be developed inside a pool of immature cardiac cells by formation of “cell-in-cell structures” (CICSs) has enabled us to conclude that CICSs being encapsulated are implicated into mammalian cardiac myogenesis over the entire lifespan (Figure. 9A,B).
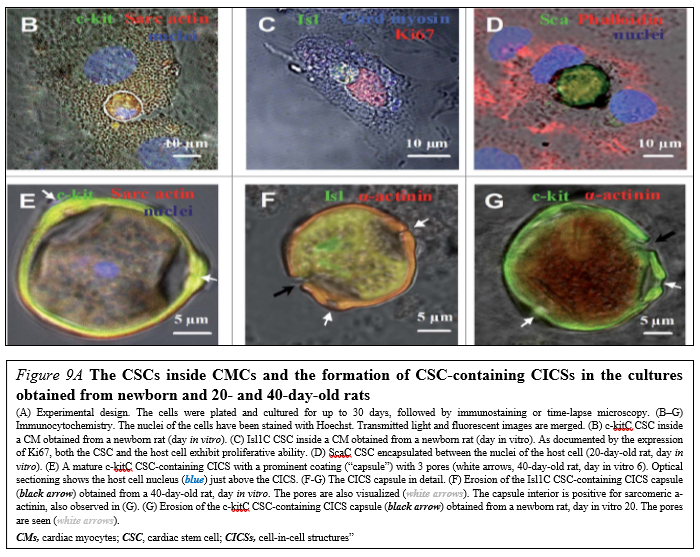
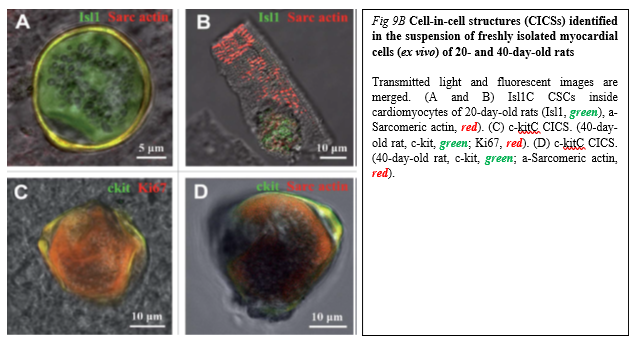
It had been demonstrated before that new CMs are generated through formation of CSC-derived transitory amplifying cells (TACs) either in the CM colonies or in a process of intracellular development of CICSs being encapsulated.
The analysis of adult rat cardiac cell suspension 1-5, 10, and 14 days after permanent coronary occlusion and ischemia/reperfusion has gifted a researcher a unique phenomenon of TAC release from mature CMs with clear sarcomeric structure. In this case a development of the intracellular CSC occurs within the vacuole pre-formed by CSC-driven sarcolemma-induced invagination. The comparison of TACs exiting the CICS with capsule with TACs just released from non-encapsulated CICS showed that non-encapsulated CICS-derived TACs are characterized by increased expression of cardiac markers and decreased expression of stemness-related markers.
Earlier on syngeneic animals using the label - GFP was shown that a local laser apoptotic effect on tissues causes an intensive transition of mesenchymal BMSCs from the bloodstream to the zone of programmed cell death (Figure. 10).
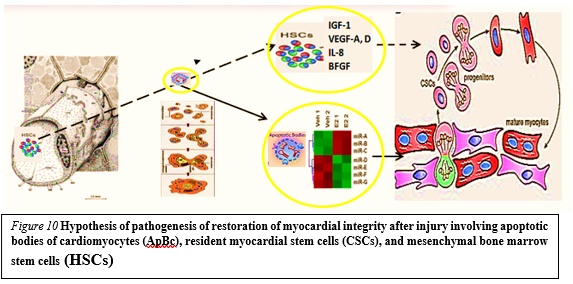
Apoptotic bodies (ApB) of cardiomyocytes combine the functions of CSC and HSCs in the areas of myocardial regeneration. Signaling molecules are located on the surface of ApB, which mediate the homing and chemotaxis of HSCs to the area of the damaged myocardium. HSCs provides targeted delivery of growth factors and cytokines required to maintain CSC proliferation.
ApB contains a complex of molecules, carriers of "epigenomic memory" about the tissue belonging of a dead cell. It is likely that simultaneously with the triggering of the effector link of apoptosis, which ends with the formation of ApB, RNA is expressed, the new profile of which is the “code” of the tissue belonging to the dead cell. When ApB enters the CSC via endocytosis, a specific set of long and short non-coding RNAs express genes that determine the direction of differentiation of resident myocardial stem cells. It can be assumed that this hypothesis is true not only for the heart, but also for other organs and tissues [40].
Those data for the first time suggest that the development of CSCs inside the encapsulated CICSs is important for cardiac self-renewal and maintenance of CSC-based pool. At the same time, the development of CSCs inside a population of mature CMs is resulting in the formation of pre-cardiac myocytes, which are able to substitute for irreversibly injured CMs, representing the major mechanism of myocardial regeneration. And that, in turn, would open up a green light to secure the targeted management of regenerative cardiac myogenesis. So, the formation of new cardiomyocytes within the injured myocardium has not been conclusively demonstrated.
Meanwhile, SC therapies are viable alternatives to conventional treatments with substantial therapeutic potential and market opportunities. And the lessons learned from our studies would yield fundamental biological insights in the repair of ischemic and other myocardial diseases whilst securing the therapeutic resources with the unique future.
To stress the above-mentioned, Biodesign-inspired and biotech-driven translational research & applications are keeping success in the field elusive in terms of return on investment and in terms of attractiveness to investors within and outside of biopharma and SC-related market as well. The SC market itself is predicted to grow to around $12.1 billion by 2024, whilst the development of the SC therapy into further applications has not yet become common practice, and the true potential of regenerative medicine has yet to be demonstrated fully. For instance, the allogeneic or ‘off-the-shelf’ business model for SC-based therapies is far more akin to current biopharmaceuticals, where the product maintains long-term stability. Meanwhile the global SC therapy market for cardiovascular disorders is likely to grow to $3.3 billion by 2030, driven by the rising incidence of those disorders.
However, the translation of SC therapy for heart diseases from bench to bed is still hampered by several limitations, covering some barriers dictated by the clinical application of SC-based therapies, the investigation of mechanisms behind SC based cardiac regeneration and also, what bioengineers can do and have been doing on the translational stage of SC therapies for heart repair. In this context, the biotech company CardioCell announced effective results in the application of SCs for the treatment of chronic heart failure indications at the European Society of Cardiology Congress, presenting the outcome of preclinical and clinical trials to study the effects of intravenous ischemic tolerance to MSCs in the treatment of chronic heart failure [41].
During the same year, at the annual meeting of the Society for Cardiovascular Angiography and Interventions (SCAI), a number of experts announced promising results for the RENEW trial and the ATHENA (Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction) trial. Although the results of these trials did not sufficiently show significant efficiency due to the early termination and limited sample size, they could still be promising development demonstrating the potential for viable SC-based heart therapies.
Meanwhile, design-driven engineering methods (Figure. 11)
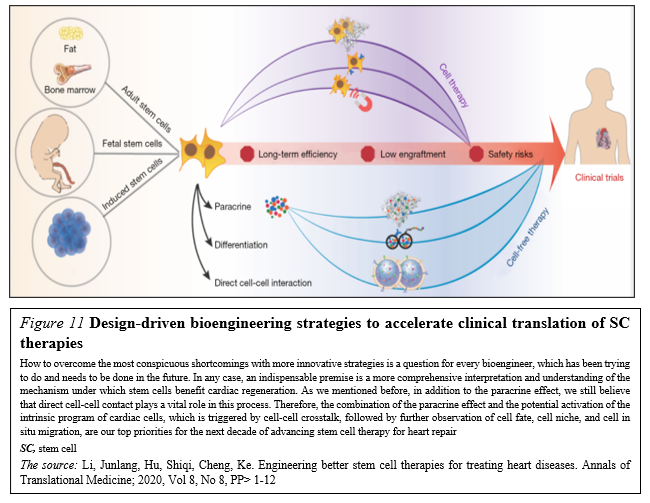
that aim to realize the multifunctionalization of SC therapies have been thriving in the past five years. With the collaboration of physicians, chemists, biodesigners and bioengineers are able to develop SC-based therapies that combine SCs, or their byproducts, with biomaterials in order to enhance therapeutic efficiency and to secure the effective delivery and biosafety [42].
So, SC research and translational applications have made significant progress in the field of cardiovascular regenerative medicine over the past few decades. However, there is still a long way to go before SC therapy can be safely and effectively applied in clinical practice. But moving forward, we must better characterize SC-based therapy and clinical trials, facilitating decision making across the sector. More basic science investigation is required to elucidate the specific mechanism(s) by which SC promote cardiac regeneration and/or repair, and how cells can be optimally delivered and engineered. As our understanding of SC therapy increases, it becomes more likely that clinical trials can produce truly meaningful results with implications for clinical practice.
Based on the new mechanisms and unique phenomenon, we are developing improvement strategies to boost the potency of SC repair and to generate the “next generation” of SC-based and regulatory biomolecules-based (bimodal) therapeutics. Moreover, our strategies should aim at more personalized SC therapies in which individual disease parameters influence the selection of optimal cell type, dosage and delivery approach. And encouraging pre-clinical and clinical studies as one and solid entity reporting significant SC-mediated cardiac regeneration would rapidly pave the way for clinical translation. So, a desire to discover innovative SC-based technologies of the next-step generation would encourage governments and companies to focus directly on regenerative medicine as a future potential economy and social insurance booster.
Thus, prospective research should focus on the development of specific responder scores and the identification of prognostic SC- and cardiac damage-related biomarkers to identify patient cohorts who benefit most from distinct SC treatments. Thereby, a higher standardization of study designs and the establishment of a global open-access database for the registration and publication of pre-clinical and clinical trials would greatly improve the comparability and access of obtained data.
In reality, the global (worldwide) SC therapy market is still in an early stage. And the developing translational pipelines for rising applications will build the competition among merchants amid the conjecture time frame.
Consequently, the focus of research in the field has since shifted to SC-derived paracrine factors, including cytokines, growth factors, mRNA, and miRNA. Notably, both mRNA and miRNA can enter into the extracellular space either in soluble form or packed into membrane vesicles. SC-derived paracrine factors have been shown to suppress inflammation and apoptosis, stimulate angiogenesis, and amplify the proliferation and differentiation of resident cardiac SCs (CSCs). Such features have led to exosomes being considered as potential drug candidates affording myocardial regeneration.
The search for chemical signals capable of stimulating cardiomyogenesis is ongoing despite continuous debates regarding the ability of mature cardiac myocytes to divide or dedifferentiate, trans-differentiation of other cells into cardiac myocytes, and the ability of CSCs to differentiate into cardiac myocytes. Future research is aimed at identifying novel cell candidates capable of differentiating into cardiac myocytes. The observation that CSCs can undergo intracellular development with the formation of “cell-in-cell structure” and subsequent release of transitory amplifying cells with the capacity to differentiate into cardiac myocytes may provide clues for stimulating regenerative cardiomyogenesis.
Indeed, human SC-based therapy derivatives are extremely attractive for therapeutic development because they have direct pharmacologic utility in clinical applications, unlike any other adult cells. The human SC as a special entity is emerging as a new type of potential therapeutic agent of cellular entity in cell-based regenerative medicine, because human SC-based therapy derivatives have the potential for human tissue and function restoration that the conventional drug of molecular entity lacks.
In this context, realizing the translational and therapeutic potential of CICSs has been hindered by some obstacles which would be surmounted and brought SC-based therapy of the future towards clinical applications, including establishing defined culture systems for de novo derivation and maintenance of clinical-grade progenitor cells and lineage-specific differentiation of the latter by siRNA-driven modulation. Such milestone advances and medical innovations in CICSs research allow generation of a large supply of clinical-grade cardiac progenitor-based therapy derivatives targeting for cardiac problems, bringing cell-based regenerative medicine to a turning point. We expect that advanced modalities that integrate cellular, bioengineering, and information (IT) technologies via clinical studies and translational applications as new consolidated entities will enhance the efficacy of cardiac cell therapy and further contribute to cardiac regenerative medicine.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
