AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2690-8808/236
1Medical microbiology department /College of Health Sciences/ Hawler Medical University, Erbil/Iraq
2Microbiology, Education College, Salahaddin University, Erbil, Kurdistan, Iraq.
*Corresponding Author: Fattma A. Ali, Medical microbiology department L College of Health Sciences/ Hawler Medical University, Erbil/Iraq.
Citation: Fattma A. Ali, Ahmed Akil Al-Daoody, Cheman H. Hamid, Sawsan M. Sorche, (2024), Bacteriology Identification of Quorum Sensing in Klesiella Spp, J, Clinical Case Reports and Studies, 5(11); DOI:10.31579/2690-8808/236
Copyright: ©, 2024, Fattma A. Ali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 11 December 2024 | Accepted: 18 December 2024 | Published: 30 December 2024
Keywords: quorum sensing; Klesiella spp
Many bacteria use quorum sensing (QS) as a communication process to control gene expression in a population density-dependent way. It is essential for coordinating a number of bacterial behaviours, like the formation of biofilms, the synthesis of virulence factors, and the resistance to antibiotics. Numerous virulence components that aid in the establishment of an infection are what mediate a bacterium's infectiousness. Quorum sensing (QS), a mechanism of cell-cell communication, regulates the development of virulence factors such lectin, exotoxin, enterotoxin, fibronectin protein, etc., which helps the bacteria penetrate the host's defense barrier and increase their pathogenicity. The technique of limiting a bacteria's ability to spread in order to adapt to a host and surroundings while still having drawbacks is known as quiescence. The QS system is generally understood to be bacterial cells synthesizing and then sensing a signaling chemical that, depending on the cell population, causes certain response events that are known to be self-inducing. Quorum sensing drives the major bacterial behavior known as biofilm development. Microbial biofilms have been posing a serious threat to the contemporary healthcare system for the past few years, and as the human population becomes more populous, so does the likelihood of them becoming resistant to antibiotics. Infectious illness research is greatly impacted by biofilms, especially when it comes to infections linked to healthcare-associated indwelling devices including prosthetic joints, implants, artificial heart valves, and catheters. Microbial aggregation membranes, or biofilms, are created when microorganisms stick to the surfaces of both live and nonliving things. Significantly, the characteristics of biofilms shield microorganisms from external stresses and strengthen their resistance to antimicrobial treatments, adding to the toxicity and persistence of microorganisms. Thus, one aspect of the bacterial survival mechanism is the creation of biofilms. On the other hand, foodborne bacteria that form biofilms have the potential to significantly increase the risk of foodborne illness infections, which can have detrimental effects on the economy and public health. In healthy individuals, Klebsiella pneumoniae colonises mucosal surfaces and accounts for one-third of hospitalised patients' Gram-negative infections. It is possible for Klebsiella. pneumoniae to acquire antibiotic resistance elements, such as transposons and plasmids that encode different β-lactamases and efflux pumps. Antibiotic resistance is also caused by mutations in several proteins, including but not limited to β-lactamases, efflux proteins, outer membrane proteins, gene replication enzymes, protein synthesis complexes, and transcription enzymes.
1. Biofilm: High-order microbial communities known as biofilms have heightened resistance to both host defenses and antimicrobial agents, such as phagocytosis, antimicrobial peptides, and the complement system. The extracellular matrix that surrounds the bacteria is composed of proteins, carbohydrates, and genetic material that is derived from the bacterium and the host. In addition to attaching to abiotic surfaces and acting as a reservoir for microorganisms during host colonisation, they also play a role in the pathogenesis of many bacterial species. (Nirwati et al., 2019). Within a biofilm, bacterial cells transition into persisted cells, a quiescent state characterized by decreased metabolic activity, sluggish rates of cell division, and strong resistance to antibacterial substances [Aboelnaga et al., 2024]. Persisted cells regain metabolic activity and antibiotic sensitivity upon detachment and dispersion. Their structure is extremely complex and heterogeneous. According to estimates, biofilms are linked to 65–80% of all bacterial illnesses, acting as a reservoir from which virulent organisms may spread, either directly or indirectly. Most biofilms within the human host are generated by multiple bacterial species; Pseudomonas aeruginosa and Pseudomonas protegens are the most frequently identified bacteria in mixed communities with K. pneumoniae (Joshi et al., 2021).The ability of these microorganisms to function in the external environment is dependent on bacterial processes such as biofilm formation, virulence factor secretion, bioluminescence, antibiotic production, secondary metabolites, sporulation, apoptosis, and horizontal gene transfer (HGT) ability [Yadav et al., 2020 and Luo et al., 2021]. Conversely, though, if same metabolic activities take place when a single bacterial cell is in its planktonic development phase, they are useless [Vishwakarma and Vavilala 2020 and Wu et al., 2015]. However, in order to live in the harsh conditions of their environment, Bacteria have effectively developed a cooperative, communicative, and regulatory "intelligent" system. [Shineh, 2023].
1.1. Formation of Biofilms: Biofilm generation and maturation are ongoing, dynamic, and complicated processes that are influenced by a variety of factors, including the culture media, matrix, intrinsic cell properties, signaling molecules, cell metabolism, and genetic regulation [Sadekuzzaman et al., 2015]. Reversible attachment, irreversible adhesion, early biofilm structure development (creation of tiny colonies), biofilm maturation, cell separation and diffusion, and biofilm maturity are the five processes that follow in the formation of biofilms (Figure 1). Bacteria start to build biofilms after absorbing organic (like proteins, lipids, polysaccharides, fatty acids, etc.) or inorganic (like inorganic salt, water, etc.) molecules to produce an appropriate surface layer. Following that, these biofilms are embedded in single or mixed communities within a heterogeneous extracellular polymer (EPS) structure. [Coughlan et al., 2016].

Figure 1: Illustrates the five basic stages that biofilm develops and forms. Reversible attachment, irreversible adhesion, early biofilm structure development (creation of tiny colonies), biofilm maturation, cell separation and diffusion, and (4) biofilm maturation are the first five processes. [22].
Over the past 15 years, research on the formation of bacterial biofilms on inanimate surfaces has gained increased attention due to its potential to contribute to persistent infections through implants and medical devices (Martin et al., 2019). These findings also show that biofilm formation plays a major role in the development of antibiotic resistance due to changes in bacterial phenotypes and its structure. (Rabin et al., 2015). The importance of biofilm for bacterial survival in hostile conditions was demonstrated by their study, which found a clear correlation between greater antibiotic resistance in the biofilm phenotype and amino acid deprivation, but not for planktonic bacteria. The significance of biofilm in the development of antibiotic resistance in E. coli was also shown by Bernier et al. A portion of the bacterial community undergoes phenotypic changes during the dispersal process, when they separate from the structure and becoming planktonic. The expression of proteins linked to motility structures, like flagella, is positively regulated by the microbial communities in response to signals from the environment, such as mechanical stress, nutrient availability, temperature variations, the presence of extracellular ATP, and other damage associated molecular patterns (DAMPs). These communities also produce various sacrolytic enzymes that aid in surface detachment. Because biofilm bacteria are less susceptible to host defense mechanisms and antimicrobial agents than planktonic bacteria are (Guilhen et al., 2016), biofilms are thought of as a repository for germs when the host is contaminated.
2. Quorum sensing: Bacteria use an extracellular signaling system based on quorum sensing (QS) to interact with one another after adhering to a live or nonliving surface [Mukherjee and Bassler, 2019]. QS Has the ability to control the entire biofilm development process by triggering specific genes in bacteria to release extracellular matrices like proteins and EPS and progressively forming a full developed biofilm structure. The maturity of biofilms is also regulated by QS intercellular communication. According to Whiteley et al. (2017), Extracellular chemical signaling molecules are produced, released, and detected by the bacterial quorum sensing system., or"autoinductors." Once these signals reach the proper threshold concentration in the environment, they aggregate locally and engage in interactions with the receptor protein to bring about coordinated changes in the expression of specific genes (Abisado et al. 2018). Quorum sensing (QS) is a process that mediates the creation, release, and accumulation of extracellular signal molecules, which is the chemical exchange between bacterial cells. Signal molecules are necessary for the QS system to function normally. Different bacteria secrete different signal molecules. For example, Both Gram-positive and Gram-negative bacteria release autoinducer-2 (AI-2), while Gram-negative bacteria secrete acylated homoserine lactones (AHLs) and Gram-positive bacteria emit auto inducing peptides (AIPs). This suggests that the regulatory processes by which Gram-positive and Gram-negative bacteria build biofilms may differ. Auto inducers are molecules that act as chemical signals. Since bacterial cells constantly manufacture these auto inducers, the quantity of auto inducers rises as the number of cells does. By identifying the quorum, bacteria cantransition between two distinct strategies for gene expression. The first (1) encourages antisocial behaviour on an individual basis and is favourable at low cell densit (LCD). The second (2), which is preferred at high cell density (HCD), encourages group behaviour, commonly referred to as community behavior which shown in (Figure 2). [17]. The autoinductorfunction of N-acylated homoserine lactones (AHLs), which are produced by an enzyme of the LuxI type, is observed in Gram-negative bacteria. These molecules pierce the membrane of the bacterial cell, and the density of the bacterial population is determined by the quantity of proliferating cells. The LuxR receptor protein is released once it reaches the proper threshold concentration. Triggered, causing target effector gene transcription to take place. The bacterium Pseudomonas aeruginosa shows how the QS system is used in Gram-negative bacteria. It has two pairs of LuxI/LuxR homologs: LasI/LasR and RhlI/RhlR. The quorum sensing system of this bacterium regulates the development of numerous virulence components, including elastase, protease, alkaline phosphatase, and exotoxin A, as well as the creation of biofilm. Another illustration is the Vibrio fischeri bacteria, where the lux CDE genes and lux AB genes control the QS system and encode luciferase. Encoding enzymes that generate luciferase's substrates, which results in bioluminescence. Short oligopeptide signals and two-component systems made up ofcytoplasmic transcription factors and membrane-b n n nound sensor kinase receptors are used by gram-positive bacteria to modify gene [removed]Papenfort and Bassler 2016). An example of Gram-positive bacterium using the quorum sensing system is Staphylococcus aureus with an agrsystem that controls the production of virulence factors such as exotoxins or biofilm. Auto inducers can cause triggered signal transduction cascades in microbial populations, which result in multicellular responses, if they exceed a minimum threshold range. This method may play a role in controlling the formation of biofilms in other multicellular responses, particularly when extracellular polysaccharides are produced and channels or columnar structures are formed. The delivery of nutrients to cells within a biofilm community is ensured by the creation of these structures [Liu et al., 2023]. Furthermore, in order to facilitate inter-microorganism communication, bacteria typically incorporate the data contained in some QS automated induction factors into the regulation of gene expression [Papenfort and Bassler, 2016]. Signal molecules are necessary for the QS system to function normally. Different signal molecules are secreted by different microorganisms. For instance, gram-positive and gram-negative bacteria both secrete autoinducer-2 (AI-2) while gram-negative bacteria secrete acylated homoserine lactones (AHLs) and auto inducing peptides (AIPs). This suggests that the regulatory processes by which Gram-positive and Gram-negative bacteria build biofilms may differ.

Figure 2: An illustration of quorum sensing (QS). A chemical used in signalling is autoinducer. Since bacterial cells constantly manufacture these autoinducers, the quantity of autoinducers rises as the number of cells does. [Liu et al., 2023].
Microorganisms change their phenotypic through the process of biofilm development in order to adapt to immunological responses or environmental challenges. Biofilm regulating genes are active during the production of multispecies biofilms and function as necessary. Moreover, interactions among different species enhance the likelihood that biofilms will control genetic alterations. Thus, the method by which multispecies biofilms form is intimately associated with QS, EPS, biofilm regulatory genes, and other constituents. There is now proof that interactions between several species can greatly increase multispecies biofilms' resistance to biocides. [Liu et al., 2023]. Compared to single-species biofilms, multispecies biofilms exhibit higher resistance to disinfectants. Therefore, to lower the microbiological hazards associated with biofilm formation, it is essential to completely understand the mechanisms and environmental factors that influence biofilm formation. Bacteria use quorum sensing (QS) to synchronously regulate global gene expression in response to variations in species complexity and cell density [Saini et al., 2015]. Getting Used to The bacterial community must adapt to changes in the environment by integrating external signals and coordinating internal responses through global regulatory networks. The bacterial community must integrate external signals and coordinate internal responses based on global regulatory networks in order to adapt to changes in the environment. The fundamental procedures having to do with identifying and responding to variations in the quantity of bacterial cells are similar in every bacterial quorum sensing system that is currently in use [Sharma 2020 and Uruén et al., 2021]. First, intracellular synthesis produces signal molecules known as auto inducers (AIs). Secondly, these compounds are secreted or released in a passive manner from the cellular environment. The concentration of extracellular autoinducer rises with the population's cell count. Third, when the quantity of signalling molecules rises above the minimal level necessary for identification, their When homologous receptors attach to the auto inducer, a signalling cascade is set off, altering the bacterial population's gene expression [Zhou et al., 2020]. Therefore, quorum detection increases the likelihood of bacterial cell population survival in unfavourable environmental conditions by assisting the cell population's coordinated operation. It is commonly recognized that the QS system controls the formation of biofilms by bacteria [Hawas, 2022]. The development of pathogenic biofilms by bacteria and their defense mechanisms against antibiotics, antimicrobial agents, and host innate immunity are covered in a number of outstanding studies. [Azevedo et al., 2020]. A list of enterococcus strains, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and others, was compiled by the World Health Organisation (WHO) in 2017. Can become ineffective in conventional antibiotic therapy due to various molecular pathways of antimicrobial resistance (AMR) [Salam et al., 2023]. The biofilm and pathogenicity of P. aeruginosa are tightly associated. Vancomycin and fluoroquinolones were among the drugs that E. faecium and S. aureus were resistant to [Breijyeh and Karaman, 2020].
2.1. QS in Gram-Negative Bacteria:
2.1. 1. AHL Signaling: Homoserine lactones are the main signalling molecules found in Gram-negativebacteria. (AHLs), also referred to as AI-1 auto inducers and acyl-homoserine lactones [Sharma and Singh, 2020]. Biofilm-forming bacteria use AI-1 for intraspecific communication, but certain bacteria are also able to identify rival bacterial species in their surroundings [Khelissa et al., 2017]. The QS based on AHLs is essential for controlling global gene expression in Gram-negative bacteria inresponse to bacterial cell density. There are more than that form of QS signal present in 70 different types of bacteria, the majority of which are harmful [Zhou, 2020]. Among Gram-negative bacteria, the most well-known AHL-mediated QS mechanism is the LuxI/LuxR system, first discovered in V. fischeri. (Figure 3). AI-1, mostly 3-oxo-hexanoyl-l-homoserine lactone (3OC6-HSL), is synthesized by LuxI-type proteins and passively permeates cell membranes to transfer signals across cells. AI-1 is recognized and bound by the LuxR protein's N-terminal domain. However, in the lux-box region of their palindromic sequence, which is located about 40 bp upstream of the ATG codon, the promoter of a number of target genes interacts with the conserved helix-turn-helix motif of the C-terminal domain. [Mirghani et al., 2022]. Following threshold crossing, AHLs and LuxR combine to form the LuxR–AHLs complex, which stimulates luxI transcription by identifying the “lux box” of luxI [Wu and Luo, 2021].

Figure 3: shows the general process of QS in Gram-negative bacteria, specifically how luxI and luxR in Vibrio fischeri activate the lux operon. At low cell density, the autoinducers (3OC6-HSL: red dots), generated by LuxI, permeate into the growth media across the cell membrane. The autoinducers in the medium build up in a small space as the cell development proceeds. It is possible to detect very low light intensity. Autoinducers can re-enter the cell and directly bind the LuxR protein to activate the production of luxICDABEG after a sufficient number have accumulated in the media. [Wu and Luo, 2021].
3. Quorum sensing of Klebsiella pneumoniae Mixed Biofilms: The encapsulated, Gram-negative bacteria Klebsiella pneumoniae causes a wide range of illnesses, including meningitis, bacterialemia, liver abscesses, pneumonia, and urinary tract infections. (Paczosa and Mecsas, 2016). Newborns, the elderly, and those with impaired immune systems are among the risk groups for K. pneumoniae infections; yet, the bacterium is also accountable for a rising number of illnesses that are acquired in the community (Bengoechea and Sa Pessoa, 2019). The bacteria can be found on abiotic surfaces like medical equipment as well as in the environment (soil and shallow seas). It begins by colonising human mucosal surfaces, particularly those of the gastrointestinal tract and oropharynx, from which it may spread to other tissues (Paczosa and Mecsas, 2016). The incidence of multidrug-resistant K. pneumoniae has significantly increased over the past ten years, underscoring the need for a deeper comprehension of the pathogenesis of K. pneumoniae. According to Wang et al. (2020), it is common practice to classify K. pneumoniae strains as hypervirulent (hyKp), opportunistic, or multidrug-resistant (MDR). K. pneumoniae (cKp) strains that are hypervirulent are regarded as community-acquired germs that can infect people of all ages, including healthy ones. In contrast, the classic strains of Kp are opportunistic strains that are frequently associated with nosocomial infections (Chew et al., 2017; Russo and Marr, 2019). The current review examines the bacterial factors involved in K. pneumoniae biofilm formation, the regulatory channels governing this mechanism, and the various model systems used to study biofilm formation in light of the critical role that biofilm formation plays in the virulence and dissemination of this bacteria. Quorum sensing is essential for the production of biofilms in a variety of bacteria, including Klebsiella pneumonia. Bacteria in biofilms are extremely robust because they are protected from drugs and immunological reactions. Biofilm-related bacterial infections are thought to account for 65–80% of cases. (Paluch et al., 2020).The capacity of K. pneumoniae to produce biofilms bacterial communities made up of one or more species included in an extracellular matrix made of proteins, DNA, and polysaccharides is a crucial virulence feature. Increased resilience to external stresses and antimicrobial agents results from the production of biofilms (Brindhadevi et al., 2020; Wang et al., 2020). Formation of Biofilms by Klebsiella pneumoniae, one essential regulatory mechanism for regulating the production of biofilms is quorum sensing. Once attached to a surface, bacteria utilize quorum sensing signals to interact with other bacteria in the vicinity and initiate the formation of biofilms. Quorum sensing influences the production of biofilms in K. pneumoniae via influencing the synthesis of lipopolysaccharides, for example, two often coexisting organisms in the environment and possibly the gastrointestinal system, K. pneumoniae and P. aeruginosa, work together to generate biofilms that differ structurally from their individual mono-biofilms (Lee et al., 2014). These two species' mixed biofilms also show improved resistance to detergent treatment and antibiotics such tobramycin. According to Subramon et al. (2021), Las and Rhl, two P. aeruginosa quorum sensing systems, had little effect on these interactions. However, Pseudomonas type IV pilus, which is essential in both motility and K. pneumoniae's ability to create extracellular matrix, was required for the best possible biofilm development. because K. pneumoniae's non-mucoid variant did not grow well in the presence of P. aeruginosa (Booth and Rice, 2020). In a different investigation, environmental biofilms taken from sinks in hospital ward patient rooms were used to analyse carbapenemase-resistant K. pneumoniae (Santiago et al., 2020). K. pneumoniae grows in biofilms on both host tissues, such as the mucosa of the respiratory, urinary, and gastrointestinal tracts, and abiotic surfaces, such as catheters and medical equipment. In K. pneumoniae, biofilm development is influenced by a number of factors. As seen in Figure 4, these include the polysaccharide capsule, fimbriae and pili, iron metabolism, and the existence of many bacterial species. [Guerra et al., 2022].
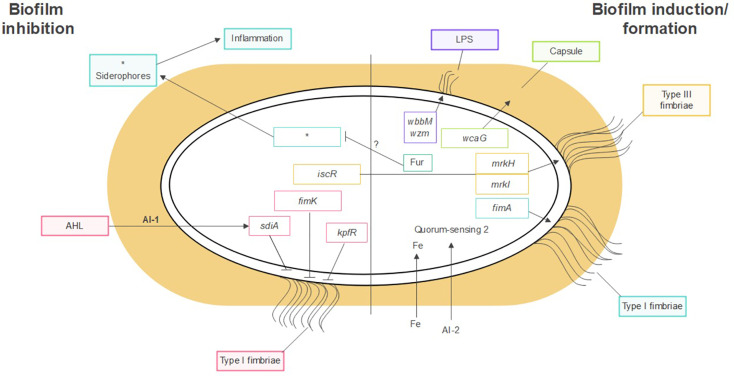
Figure 4: Factors influencing the production of K. pneumoniae biofilms. quorum sensing, iron metabolism, fimbriae, pili, polysaccharide capsule, and LPSsiderophore-related molecules. [Guerra et al., 2022].
3.1. Capsule: According to Piperaki et al. (2017), the polysaccharide capsule serves as a crucial defence mechanism for the bacteria, preventing complement deposition, opsonization, and phagocytosis. It has a very flexible structure as well. Thus far, 134 distinct capsule synthesis loci (K-loci) have been identified in K. pneumoniae isolates by means of genome sequencing and comparative genomics. (Wyres et al., 2016). It has been shown that the polysaccharide capsule influences the initial surface adhesion and maturation stages of K. pneumoniae biofilm formation. Impaired biofilm formation was seen in mutant strains with abnormalities in capsule synthesis (Zheng et al., 2018). Additional research has validated the role of capsular polysaccharides in the production of biofilms. Zheng et al. (2018) observed a favourable correlation between the expression level of the virulence gene wcaG, which is involved in capsule manufacture, and biofilm development in K. pneumoniae bacteremia strains. Moreover, these bacteria's ability to build biofilms was decreased by wcaG silencing. Interestingly, Zheng et al. (2018) found no evidence linking the hypermucoviscosity phenotype, which is frequent in bacteremic K. pneumoniae strains, to enhanced biofilm formation. Buffet et al. showed that the presence of the capsule was a disadvantage in a nutrient-rich environment but an advantage in a low nutrition environment for the encapsulated strains in a study investigating the role of the capsule in K. pneumoniae fitness under varied growth conditions. This study further claimed that whilst the encapsulated strain's creation of capsules covered the fimbriae and inhibited biofilm formation, non-encapsulated strains exhibited greater adhesion in all settings. This further highlights the association between capsule and biofilm formation. However, they contend that the quantity of capsules produced reduces the capacity to build biofilm, rather than the capsule's presence having any effect on biofilm formation (Buffet and Rendueles 2021).
3.2. Lipopolysaccharide (LPS): Lipopolysaccharide (LPS) is a crucial part of K. pneumoniae and other Gram-negative bacteria's outer membrane. The production of K. pneumoniae biofilms is also facilitated by LPS. Balestrino et al. showed that LPS is essential for the early stages of biofilm development because it encourages K. pneumoniae's initial adhesion to abiotic surfaces. The scientists demonstrated that mutant strains of K. pneumoniae that lack the wbbM gene or the wzm gene, which are linked to LPS production, exhibit a delay in the formation of biofilms. They postulate that the proper folding of Type 1 pilli requires LPS charge, which could account for their observations. In a more recent study, Vuotto et al. highlighted the role of the wbbM and wzm genes in the formation of K. pneumoniae biofilms by demonstrating that the expression of both genes was higher in Biofilms of K. pneumoniae growing than in planktonic K. pneumoniae cells during the exponential phase (Vuotto et al., 2017).
3.3. Fimbriae: Fimbriae of types I and III are the two primary kinds that K. pneumoniae expresses Fimbriae operate as adhesins, facilitating adhesion to biological surfaces (causing tissue invasion) as well as abiotic surfaces like medical equipment where bacteria develop biofilm. (Piperaki et al., 2017). The genome of K. pneumoniae contains at least eleven gene clusters that encode the chaperones, ushers, and adhesin proteins required for the construction of fimbriae, including fim, mrk, ecp, and kpa to kpg (Khater et al., 2015). The genes for fimbriae of type I and type III. (Paczosa and Mecsas, 2016), common pilus and type I-like fimbriae are among the most characterised experimentally among them. These are fim, mrk, ecp, and kpf. Type I and type III fimbriae are the two primary kinds of fimbriae that K. pneumoniae expresses. In vitro, type III fimbriae have been shown to adhere to kidney, lung, and bladder epithelial cells, among other cell types. Type I fimbriae attach themselves to mannose-containing receptors found in a variety of human host tissues.
4. Molecular Mechanisms of the Formation and Functioning of Bacterial Biofilm
4.1. The Role of QS in the Global Control of Gene Expression Profiles: Several stages of biofilm formation occur, depending on the type of bacteria and the colonized surface [Shineh et al., 2023]. Increased resilience to external influences including temperature, drugs, and nutrition changes characterizes bacterial cells that are an intrinsic element of the biofilm. These features originate from the diversity of phenotypic subpopulations of bacterial cells that comprise the biofilm structure.Complex ecological and structural heterogeneity, genetic variety, intricate connections, and the presence of extracellular materials are characteristics of biofilms [Yan and Bassler, 2020,]. QS regulates a substantial number of genes, maybe surpassing 10% of the bacterial genome. [Wu and Luo, 2021]. With the advent of high-throughput sequencing cDNA technology (RNA-seq) using next-generation sequencing (NGS) platforms, research on the molecular underpinnings of biofilm formation and the involvement of the QS in this process gained speed. Transcriptomics offers a global examination of gene expression in contrast to conventional methods of analysing individual genes, and it has been effectively applied to the research of biofilm development. [Abdelghafa, 2023 and Ghosh et al., 2022]. Serious results showed that dangerous bacteria, such as Salmonella [Zheng, 2022], S. pneumoniae [Wu et al., 2022], S. aureus [Tomlinson and Prince, 2021], V. parahaemolyticus [Rubio-Mendoza et al., 2023], and C. difficile [Jiang et al., 2021], that are developing in biofilms display differential gene [removed]DEGs) in comparison to the planktonic stage. According to their biological roles, the genes regulated by QS may generally be divided into four groups based on many transcriptional studies [Yan and Bassler, 2020]. Genes involved in cell development and life are in the first group; genes involved in cell life are in the second group. Regulating the actions of cells in their surroundings; genes linked to HGT are included in the third group; and genes whose expression is connected to the production of virulence factors are included in the fourth group [Sánchez-Jiménez and Llamas, 2023]. A number of gene groups that are expressed through QS system induction include Pqs operon (pqsE), Rhl operon (lecA, Lecb, rhlAB), Igs operon (lasA, lasB, hcnA, rhlAB), and Las operon (lasB, aprA, toxA, rhlR). Proteins linked to proteases, elastases, coagulases, exotoxins, lectins, and other virulence factors are encoded by these gene families. [Abdelghafa, 2023 and Ghosh et al., 2022]. Of the mRNA transcripts regulated by genes involved in the cellular metabolic pathway (metK, artI, hyaA, fruK, gadB) and stress response pathway (hslS, hslT, soxS) were found to express at different levels in the QS system. As of late, Jiang et al. showed that the differential expression of the genes most likely to influence biofilm formation, artM, artQ, ssrS, pflA, and hutX, was substantially linked with the in vitro colonisation and adhesion capacity of Haemophilus parasuis. These findings indicate that the formation of biofilms is a complex process that involves stress response, structural development, and regulatory mechanisms. It should be remembered, though, thatphosphorylation cascades that are not detected at the level of global expression analyses can control a number of significant signalling pathways. [Asfour, 2018].
4.2. Molecular Typing: MLST it’s a technique which is used for 400-500 base paring, sixty-two isolates STs were found by MLST analysis of the 72 isolates of K. pneumoniae sensu lato complex; the most common type was ST107 (n = 4, 5.6%) (Table 2). In addition, eighteen additional STs were found. Capsule locus typing also revealed genetic variety (Supplementary Table S1). Depending on reviews of [Klaper et al., 2021]. Out of 38 different capsule types, the capsule locus KL30 (n = 7, 9.7%) was the most common (Figure 6). Moreover, no correlation was seen between any particular ST and type of capsule and isolation sources. Only three little clusters of genetically closely linked isolates were found using cgMLST. There were only two isolates in each cluster (Figure S1), and the changes in alleles were limited to eight. Overall, no common genetic lineage was found using cgMLST analysis (Figure 6).
| Sequence Types | Novel Sequence Types |
| ST n = 72 | ST n = 72 |
| ST11 1 (1.4%) | ST5-1LV 2(22.8%) |
| ST13 1 (1.4%) | ST23-1LV 1 (1.4%) |
| ST18 1 (1.4%) | ST63-1LV 1 (1.4%) |
| ST20 2 (2.8%) | ST70-2LV 1 (1.4%) |
Table 2: shows the variety of multilocus sequence types found in the 72 isolates of K. pneumoniae sensu lato. . [Klaper et al., 2021].
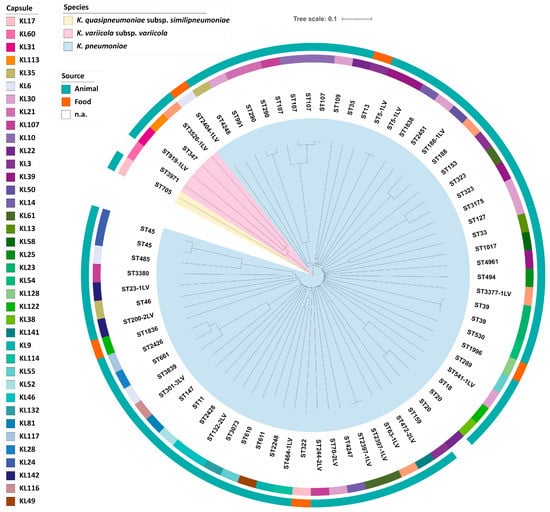
Figure 5: Phylogeny of 72 isolates of K. pneumoniae sensu lato. A neighbour joining is depicted in the inner circle. . [Klaper et al., 2021].
4.3. Presence of Virulence Factors: Only K. pneumoniae isolates showed evidence of genetic variables linked to hypervirulence. In total, one or two siderophore systems were detected in 22 K. pneumoniae genomes (Figure 6). Nine (9.6%) isolates had yersiniabactin (ybt), whereas aerobactin (iuc) was present in 18 isolates (19.1%) (Supplementary Table S1). It's interesting to note that aerobactin, which belongs to iuc lineage 3, was only found in isolates of wild boar and domestic pigs, although yersiniabactin was not linked to any particular isolation source. Sequencing in length for strain 30312, 2 also showed the presence of a 171 kb iuc3-carrying IncFIBK plasmid. All iuc3 plasmids had the IncFIBK replicon, according to further sequencing analysis. In the isolates under investigation, no additional genes for virulence that code for salmochelin, colibactin, or RmpA/A2 were found. [Klaper et al., 2021].
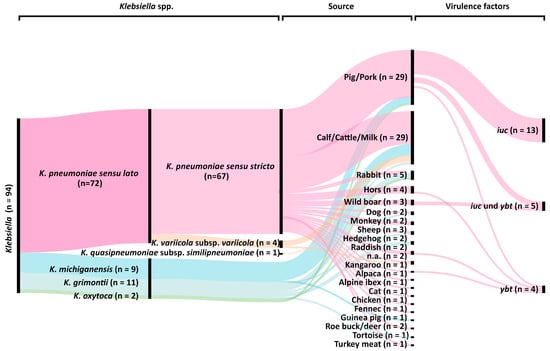
Figure 6. Classification of the 94 isolates of Klebsiella spp. from food and animals using their genomes. Species (first column), phylogroups (second column; [3, 4]), isolate sources (third column), and the existence of certain virulence genes (fourth column) are all included in the graph. [Klaper et al., 2021].
5. Identification of gyrA and parC Sequencing and Plasmid-Mediated Quinolone Resistance Genes:Following the manufacturer's instructions (Wizbio, Korea), DNA was extracted, and genomic DNA was eluted by adding 50 EB buffer (10 mM Tris-HCL, pH 9, 0.5 Mm EDTA). Electrophoresis on horizontal gels containing 1% agarose and ethidium bromide staining were used to visualise the results. The PMQR genes qepAqnrBqnrS and aac(6)Ib were found by PCR screening isolates with decreased susceptibility to ciprofloxacin. Furthermore, these isolates' gyrA and parC were amplified by PCR and then sequenced. In accordance with the manufacturer's instructions (Promega/USA), a PCR mixture was created by adding 3 µL of DNA template, 1.5 µL (0.6 pmol) of both forward and reverse primers, 12.5 µL of 2 X GO TaqGreen mastermix, and nuclease-free water to get the volume up to 25 µL. The amplification operation was carried out using the TechNet-500/USA thermocycler. The following were the conditions for the PCR reaction: 35 cycles of denaturation (30 seconds at 95C), annealing (30 seconds at 72C), and extension (1 minute at 72C). The last extension is at 72C for 10 minutes. The NCBI database was utilised for the sequencing study of the gyrA and parC PCR results, which was performed by Macrogen DNA Sequencing (Seoul, Korea). [Catalán-Nájera, 2017].
6. Hypermucoviscous and hypervirulent variants of Klebsiella pneumoniae (hmvKpn/hvKpn): Taiwan was the first place where a novel, hypervirulent strain of Klebsiella pneumoniae was originally reported in the mid-1980s. Clones of K. pneumoniae that are hypermucoviscous have been described as hypervirulent.9. Mucoid colonies are generated by K. pneumoniae in a nutritive media, much like by all other capsulated bacteria.A hypermucoviscous phenotype is not the same as this mucoid phenotype in during the'string test,'which involves using a loop to extend a colony of Klebsiella spp. on an agar plate, theproduction of a viscous filament measuring around 5 mm is used to indicate hypermucoviscosity (Fig. 7). [Catalán-Nájera, 2017].

Figure 7: Test with strings. When a K. pneumoniae colony is stretched utilising a loop on an agar dish, a viscous filament measuring at least 5 mm is generated, indicating a hypermucoviscous phenotype (positive string test). [Catalán-Nájera, 2017].
A positive string test result is not always seen in K. pneumoniae mucoid colonies. This occurrence highlights a clear distinction between the hypermucoviscous variations and mucoid capsular strains.The initial findings from the Far East suggested that these variations could result in invasive infections that lead to primary abscesses in the liver, prostate, kidney, bone, and lung; necrotizing fasciitis has also been reported in a few cases. [Catalán-Nájera, 2017]. The main differences between the hvKpn and cKpn variations are therefore explained by the invasive capability and the hypermucoviscous phenotype which shown in (Figure 8). However, there hasn't been much research done on the hypermucoviscous K. pneumoniae phenotype in the absence of a hypervirulence genotype. Other serotypes have also been linked to the hvKpn/hmvKpn phenotypes, in addition to serotype K1/K2.6, 19 S. Variants of (hvKpn/hmvKpn) have been noted in Asian individuals with immunocompromised immune systems and in diabetics with inadequate glucose regulation. Furthermore, the majority of these strains were of the K1 serotype, which is the most common in the Far Eastern region. [Namikawa et al., 2016]. The hvKpn/hmvKpn strains exhibit sensitivity to multiple antibiotic classes, with the exception of ampicillin, to which K. pneumoniae possesses inherent resistance.
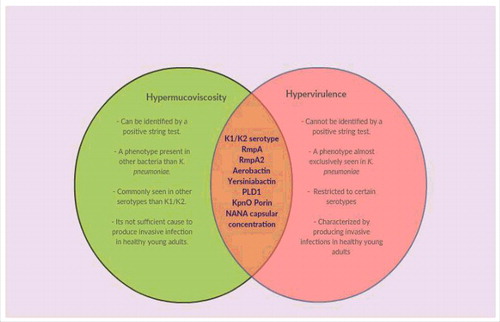
Figure 8: Venn diagram illustrating the distinctions and parallels between phenotypes that are hypervirulent and hypermucoviscous. According to recent data, hypervirulence and hypermucoviscosity are two distinct phenomena. The hypermucoviscous phenotypic's primary traits, which set it apart from the hypervirulent phenotype, are depicted in the green area. The factors that have been substantially linked to both phenotypes are highlighted by the intersection. [Catalán-Nájera, 2017].
7. Quorum sensing [QS] Inhibition Pathways:
Quorum-sensing inhibitory substances may find use in environmental engineering, agriculture, and medicine, among other sectors. This is crucial when it comes to treating and preventing infections brought on by the harmful biofilm that is resistant to conventional antibiotics. It is well recognised that a large number of bacterial pathogens that cause infectious illnesses are capable of forming biofilms. QQ is a viable antibacterial strategy since human and animal infections are becoming more resistant to antibiotics. One benefit of preventing biofilm formation by inhibiting the QS signal is that there is no direct bactericidal action, which reduces the likelihood of bacterial resistance developing. By preventing the manufacture of certain virulence factors, it boosts the efficacy of antibiotic therapy in conjunction with it [Juszczuk-Kubiak, 2024]. Thus, to inhibit specific virulence factors and matrix-targeting enzymes that cause biofilm formation, next-generation antibiofilm drugs are being identified and produced (Figure 9). Each pathway has multiple mechanisms for QS inhibition, including: (1) blocking AHL synthesis; (2) blocking AHL receptor antagonism; (3) blocking targets after receptor binding; (4) sequestering AHL; (5) AHL degradation; and (6) blocking AHL secretion and/or transport. [Jiang et al., 2021].

Figure 9. Diagrammatic illustration of a potential mechanistic strategy to prevent the creation of biofilms. Bio Render (https://biorender.com/, 4 February 2023) was used to produce the figure. [Jiang et al., 2021].
Since the discovery of quorum sensing more than 40 years ago, the mechanistic understanding of many QS systems and the awareness of the role of QS in the pathogenicity of multiple bacterial species have grown. Several investigations have verified the development of biofilms in both Gram-positive and Gram-negative bacterial strains was controlled by the QS system. Thus, a therapeutic approach that targets QS systems and is gaining traction in drug research is the use of QS-inhibiting drugs. Many synthetic or natural QS-inhibiting techniques that successfully prevent the formation of biofilms have been created recently, mostly because to the development of advanced micro- Regretfully, it's important to note the possible risk associated with utilizing each of the QQ [20]. Worldwide epidemics have been brought on in recent years by K. pneumoniae infections, particularly those that produce carbapenemases. [8, 41]. Because there aren't many therapeutically available antimicrobial medicines, infections brought on by these bacteria frequently have high fatality rates, which makes clinical anti-infective treatment extremely tough and challenging. One particular phenomena that arises when bacteria grow in clusters is called bacterial biofilm. Not only do biofilms facilitate bacterial colonization of the host, but they also induce resistance via inhibiting the binding of antimicrobial medicines to the target areas of bacteria (Li and Ni, 2023). Numerous research have suggested that quorum sensing could be used to treat infections caused by Pseudomonas aeruginosa. The development of antibiotic resistance makes the search for novel treatments that go beyond traditional antibiotics a top priority.Health research; conventional medications that had previously been categorized for their antibacterial activities and checked to see whether they had any characteristics that could hinder communication or, in theory, have inhibitory activity for quorum sensing. (Yu et al., 2018).
Gram-negative bacteria that are clinically important frequently exhibit efflux-mediated drug resistance. (Assoni et al., 2021). Klebsiella variicola has also been shown to exhibit the hypermucoviscous phenotype. A rod bacterium that is Gram-negative and closely related to the K. pneumoniae and K. quasipneumoniae species is called K. variicola. At first, it was found as a symbiont in leaf-cutting ants after being characterised as endophytic and harmful to humans. K. variicola has been generally classified as a human pathogen in several regions of the world throughout the past year. [15]. The entire genome of a K. variicola hypermucoviscous clinical isolate that was recovered from the sputum of an elderly man in Mexico was sequenced by (Garza-Ramos et al. 2015). The rmpA gene and the genes encoding salmochelin and yersiniabactin, two siderophores, were found within the mobile genetic element. These findings imply that the spontaneous horizontal gene it is alarming to consider the likelihood of Klebsiella virulence clusters linked to interspecies pathogenicity. In addition, the bacterial genome was found to have the iutA gene, which codes for the aerobactin receptor, and the clusters mrkABCDFHIJ and fimABCDEFGHI, they, in turn, encode type I and type III fimbriae. et al., Breurec (2016). Although both K. quasipneumoniae subsp quasipneumoniae strains were sensitive to different kinds of antibiotics, only b-lactamase blaOKP, a b-lactamase characteristic of K. quasipneumoniae subsp quasipneumoniae, was found in these two experiments. [2]. Numerous studies have connected the K1 and K2 capsular serotypes, transcriptional regulators like the rmpA/rmpA2 genes, and even specific siderophores like aerobactin and yersiniabactin as the causative agents of the hypervirulent characteristic, despite the fact that hypervirulence is associated with clones rather than serotypes. However, several studies have reported the existence of non-K1/K2, rmpA/rmpA2-negative strains that cause invasive infections and have unique siderophore distribution. Therefore, before the genetic. The factors connected to hypervirulence are well-defined, seldom are Klebsiella spp. found in food samples and animals. [Wyres and Holt, 2020]. However, studies exist of the isolation ofsequence types ST11, ST15, ST25, and ST23 from companion animals and livestock [Zhang et al., 2019]. These widely dispersed STs are connected to the most K. pneumoniae infections in humans that are acquired through community and nosocomial means.
Quorum Sensing QS play a major part in virulence expression and host defense communication. The investigated antibiotics can be used with other anti klebsiella spp. Treatments, to lessen pathogenicity, aid in overcoming bacterial resistance, and steer clear of the main adverse consequences of excessive dosages. It is well known that K. pneumoniae isolates with a hypermucoviscous phenotype are the ones that rarely result in enteric Gram-negative microorganisms-induced invasive infections in otherwise healthy individuals. Furthermore, given the term "hypervirulent" refers to the apparent relationship between this phenotype and the clinical differences of the illness. The phrases hypervirulent and hypermucoviscous are frequently used interchangeably in medical literature. Also, a string test that is positive has been seen as a sign of extreme pathogenicity. It is alarming that hypervirulent K. pneumoniae variations have been found that can still cause invasive infections in healthy people even when they do not exhibit the hypermucoviscous phenotype. However, recent research indicates that in at least three Klebsiella spp. bacterial species, a hypermucoviscous phenotype alone is not sufficient to induce a hypervirulent state. Therefore, it is not reasonable to rule out hypervirulence based solely on the lack of hypermucoviscosity. Molecular testing to identify virulence factors associated with this phenotype, such as aerobactin, yersiniabactin, and the rmpA/rmpA2 genes, as well as the clinical signs of the illness are required. However, hypervirulence cannot be identified by a straightforward molecular test.