AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2690-1919/256
1 Department of Basic Medical Sciences, Inaya Medical College, Riyadh, Saudi Arabia.
2 Department of Physiology, Faculty of Medicine, Sabratha University, Libya.
3 Department of Biochemistry, Faculty of Medicine, Sabratha University, Libya.
*Corresponding Author: Azab Elsayed Azab, Department of Physiology, Faculty of Medicine, Sabratha, University, Libya. Email: azabelsaied@yahoo.com
Citation: Ata S.I. Elsayed, Azab E. Azab and Khawla A. Etwebi. (2022). An Overview of Oxytocin: Chemical Structure, Receptors, Physiological Functions, Measurement Techniques of oxytocin, and Metabolism. J. Clinical Research and Reports, 11(4); DOI:10.31579/2690-1919/256
Copyright: © 2022, Azab Elsayed Azab. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 06 June 2022 | Accepted: 20 June 2022 | Published: 30 June 2022
Keywords: oxytocin; structure; receptors; physiological functions; measurement techniques; measuring receptors; metabolism
Background: Oxytocin is a peptide hormone comprising 9 amino acids. It is produced in the hypothalamus and stored and secreted by the posterior lobe of the pituitary gland and synthesized in other organs such as the uterus, ovaries, placenta, heart, blood vessels, skin, kidneys, and testis. Receptors of oxytocin are present on myoepithelial cells, heart, blood vessels, macrophages, thymus, pancreas, kidneys, and adipocytes.
Objectives: The current review aimed to give highlight the oxytocin structure, receptors, physiological functions, measurement techniques, and metabolism. Oxytocin is a small peptide that consists of nine amino acids in a six–amino acid ring formed by cysteine bonds and a three–amino acid tail with a terminal amine. It is synthesized in brain regions that are critical to behavioral and physiologic homeostasis. Oxytocin is involved in uterine contraction during labor and ejection of milk during breastfeeding and plays a role in social behavior, emotions love and affection, the period after childbirth, and metabolic functions. The action of oxytocin in facilitating human bonding and social relation is well known. The effects of Oxytocin on metabolism and food intake suggesting its potential effects in treating obesity. The half-life time of oxytocin in the brain is triple as long as its half-life time in the periphery. The sensitivity and density of oxytocin receptors increase during labor. After birth, the neonatal baby sucks on his mother's breast, causing the release of milk by stimulating hypothalamic neurons to produce oxytocin. Oxytocin neurons have been heavily implicated in mediating sexual behavior in both humans and animals. The social memory was enhanced by central oxytocin administration in male rats. The action of oxytocin affects social memory in multiple brain regions, including the ventral hippocampus, amygdala, olfactory bulb, and lateral septum. Oxytocin neurons may mediate MC4R-driven sexual behavior in male mice. MC4R signaling in oxytocin neurons permits ejaculation. A decreased latency to ejaculate in rabbits and rats after administration of oxytocin. The effect of oxytocin receptor ligands on the ejaculatory response may be due to the modulation of dopamine serotonin neurotransmission. Oxytocin lowers the threshold for the initiation of maternal behavior but is not involved in its maintenance. The oxytocin and the melanin-concentrating hormone (MCH) systems may interact to modulate maternal behavior. Oxytocin regulates maternal- or mating-regulated mood. Initial measurements of oxytocin by using radioimmunoassay and bioassays suggested that oxytocin concentration in blood is very low, 5 pg/ml, with small increases as pulses,15 pg/ml, during lactation and uterine contractions. Variations in oxytocin concentration, especially in rapid response to specific experiences, such as anticipation of breastfeeding, sexual stimulation, exercise, affiliative social contact, and psychologic stress. Oxytocin is rapidly removed from the plasma by the liver and kidney. Oxytocinase activity increases throughout pregnancy and peaks in the plasma, placenta, and uterus near term. It is also expressed in mammary glands, the heart, the kidney, and the small intestine. Lower levels of activity can be found in the brain, spleen, liver, skeletal muscle, testes, and colon. The plasma half-life of oxytocin ranges from 1 to 6 minutes. The half-life is decreased in late pregnancy and during lactation.
Conclusion: It can be concluded that oxytocin is a peptide hormone that is synthesized in brain regions and other organs and posse's receptors in many organs. It plays a role in social behavior, emotions love, and affection, the period after childbirth, and metabolic functions. Its potential effects in treating obesity. The half-life time of oxytocin in the brain is triple as long as its half-life time in the periphery. Oxytocin neurons may mediate MC4R-driven sexual behavior. Variations in oxytocin concentration, especially in rapid response to specific experiences. Oxytocin is rapidly removed from the plasma by the liver and kidney. The plasma half-life of oxytocin ranges from 1-6 minutes. The half-life is decreased in late pregnancy and during lactation.
All animal cells are exposed to a variety of extracellular signals that need interpretation and translation into the appropriate response to the surrounding environment. These signals can be soluble chemical substances factors generated locally or from distant glands, such as hormones. Achieving this ligand cells maintained a diversity of cell surface receptors responding specifically to this ligand. These receptors are classified into many families according to the way in which they generate the intracellular signals pathway to give the particular responses and functions. The activity of any receptor can be modulated by other signaling pathways in different ways, generating the flexibility needed for such a complex system. the major classes of receptors included G protein-coupled receptors, ligand-gated ion channel receptors, tyrosine kinases receptors, integrins, and cytokine receptors [1].
Oxytocin hormone is produced in the hypothalamus and stored and secreted by the posterior lobe of the pituitary gland. oxytocin is involved in uterine contraction during labor and ejection of milk during breastfeeding, it also has many effects on social behavior, emotions love, affection, and metabolic functions. Many studies were conducted on its effects in weight loss by decreasing food consumption and increasing energy consumption. The effects of Oxytocin on metabolism and food intake suggesting its potential effects in treating obesity [2].
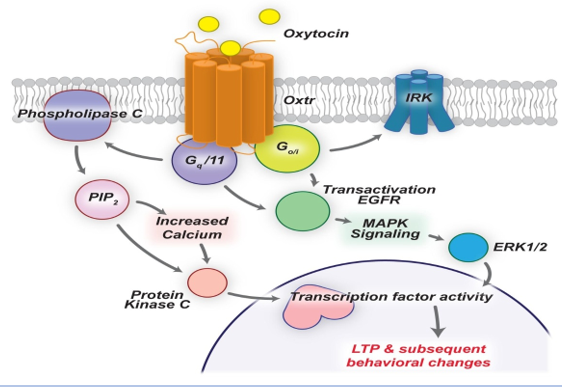
Oxytocin is a peptide hormone comprising 9 amino acids produced mainly in the brain and many peripheral organs. It binds to its receptor, which is G protein-coupled receptor. Synthesis of oxytocin occurs in paraventricular and supraoptic nuclei in the hypothalamus. The magnocellular neurons extend axons to the posterior lobe of the pituitary gland. The hormone is secreted into the circulation and cannot back to the brain due to the presence of the blood-brain barrier. Other axonal connections exist between the paraventricular oxytocin-producing neurons and regions of the brain, brainstem, and spinal cord. Parvocellular neurons in the paraventricular nucleus connect to the nuclear bed of the ventral tegmental area, stria terminalis, and the nucleus accumbens which are involved in eating behavior and reward behavior regulation. G protein-coupled oxytocin receptors are expressed throughout the brain in structures such as the hypothalamus, amygdala, olfactory nucleus, and anterior cingulate cortex in the limbic system. The interaction between oxytocin and other neurotransmitters, such as serotonin, increases oxytocin concentrations. The reaction of dopamine and oxytocin regulates the activity of the reward circuitry in the brain (Figure 1).
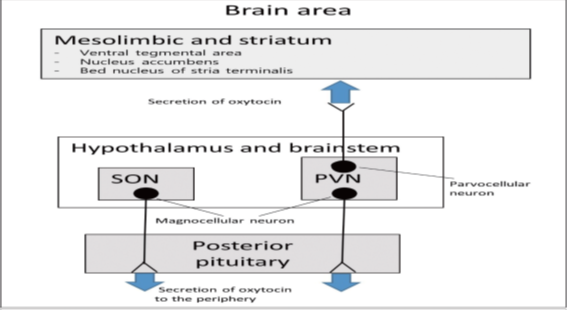
SON: supraoptic nucleus; PVN: paraventricular nucleus [3].
Also, oxytocin is synthesized in other organs such as the uterus, ovaries, placenta, heart, blood vessels, skin, kidneys, and testis. Receptors of oxytocin are present on myoepithelial cells, heart, blood vessels, macrophages, thymus, pancreas, kidneys, and adipocytes.The receptors of oxytocin are involved in uterine contractions, nitric oxide production, and lipolysis through G protein coupling and C-beta pathway activation. Secretions of oxytocin in GIT are associated with paracrine and autocrine effects.Positive feedback mechanisms were noticed from the peripheral or central administration of oxytocin, stimulating autoreceptors in the magnocellular neurons in the supraoptic nucleus.Oxytocin receptors are found throughout the central nervous system and peripheral regions, providing a clue for the regulation of metabolism and food consumption. In addition to the anterior pituitary gland, oxytocin receptors have been found in pancreas, gastrointestinal tract, adipocytes, and studies have reported their association with eating behaviors (Figure 2) [3].
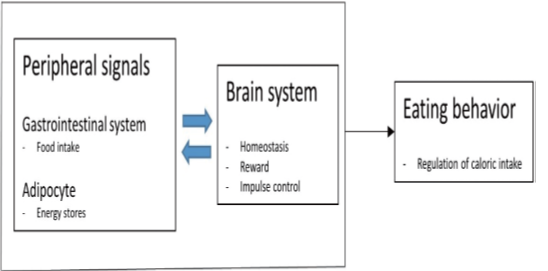
The current review aimed to give highlight the oxytocin structure, receptors, physiological functions, measurement techniques, and metabolism.
Chemical structure of oxytocin
Oxytocin is a small peptide that consists of nine amino acids in a six–amino acid ring formed by cysteine bonds and a three–amino acid tail with a terminal amine. Oxytocin is synthesized in conjunction with carrier proteins (neurophysin 1). The gene for oxytocin is located on human chromosome 20, adjacent to the gene for vasopressin. These genes lie on opposite strands and have opposite transcriptional orientations [4].
It is synthesized in brain regions that are critical to behavioral and physiologic homeostasis. Different cells in specific brain regions produce this hormone, including the supraoptic nucleus and paraventricular nucleus in the hypothalamus. The neurons of the paraventricular nucleus termed magnocellular neurons, synthesize oxytocin and extend their processes to the posterior lobe of the pituitary gland to release it into circulation. The hypothalamus paraventricular nucleus is a major site of integration and convergence for neural communication relating to stress with effects on the hypothalamus-pituitary-Adrenal axis and autonomic nervous system functions. Oxytocin is co-localized in at least some of its cells of origin with corticotropin-releasing hormone, which in turn regulates the hypothalamus-pituitary-Adrenal axis. Corticotrophin-releasing hormone actions also have been implicated in some of the detrimental effects of chronic stress. Oxytocin may be co-released with Corticotrophin releasing hormone or vasopressin as an adaptive response to a variety of challenges and facilitating coping (Figure 3) [5].
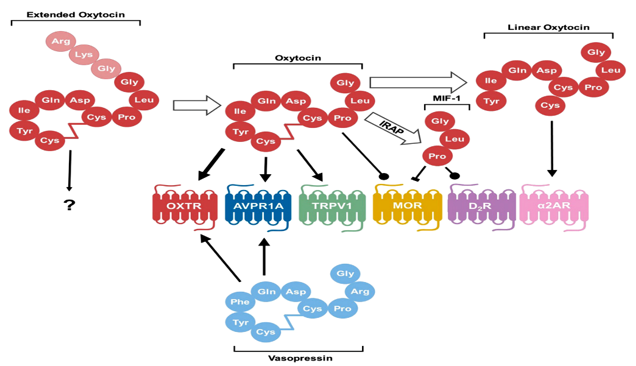
Conventional arrow = agonist; circle-tipped arrow = positive allosteric modulator; block-tipped arrow = antagonist [6].
After conversion from the prohormone form, oxytocin exists in an extended form with three extra amino acids. The conversion from extended oxytocin to oxytocin consisting of nine amino acids occurs with the maturation of the hypothalamus in neurotypical individuals. It is not known how this extended form interacts with receptors. In their canonical nine–amino acid forms, both oxytocin and vasopressin bind and act as agonists to both oxytocin receptors and arginine vasopressin receptor1A, although oxytocin has a higher affinity for oxytocin receptors than the arginine vasopressin receptor1A, as denoted by the thicker arrow. Oxytocin also acts as an agonist to the pain-sensing transient receptor potential vanilloid-1 (TRPV1) receptor and as a positive allosteric modulator at the MOR. After degradation by IRAP, the C-terminal tail is cleaved from oxytocin to form MIF-1, which can both inhibit MOR and act as an allosteric modulator on the D2 subtype of dopamine receptors (D2R). Oxytocin can also be degraded by other as of yet unspecified peptidase activity into a linear form that stimulates activity of the a-2 type adrenoreceptors (a2ARs) [6].
Oxytocin receptors
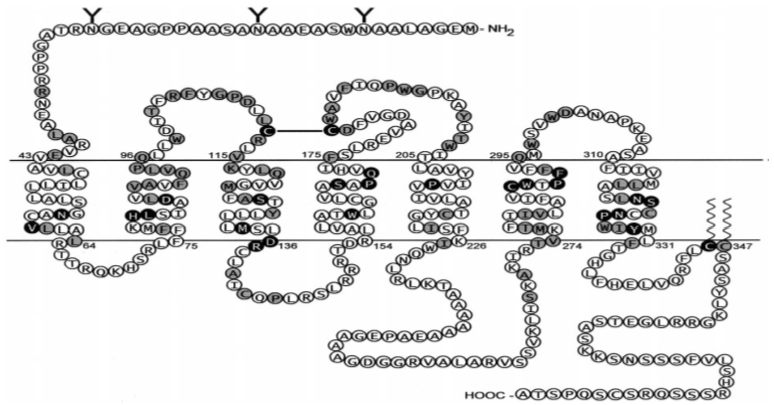
The receptor of oxytocin is a member of the G protein-coupled receptors family, rhodopsin-type (class I). The seven transmembrane α-helices are most highly conserved among the G protein-coupled receptors family members. Conserved residues among the G protein-coupled receptors may be involved in a common mechanism for activation and signal transduction to the G protein. The switching from the inactive to the active conformation is associated with a change in the relative orientation of transmembrane domains 3 and 6, which then unmasks G protein binding sites. In the class, I G protein-coupled receptors, and Asp in transmembrane domain 2 (Asp-85 in human oxytocin receptor and a tripeptide v (E/D RY) at the interface of transmembrane 2 and the first intracellular loop are believed to be important for receptor activation. With respect to Asp-85, this was confirmed for the human oxytocin receptor. When Asp-85 is exchanged by the residues Asn, Gln, or Ala, agonist binding and signal transduction of the receptor becomes impaired. Mutations at the conserved tripeptide motif DRY (DRC in the case of the oxytocin receptor) result in an either inactive or a constitutively active oxytocin receptor. The cysteine residues in the first and second extracellular loops are highly conserved within the G protein-coupled receptors and are probably connected by a disulfide bridge. Two other well-conserved Cys residues reside within the COOH-terminal domain. Most likely, they are palmitoylated as demonstrated for the vasopressin (V2) receptor and other G protein-coupled receptors, and anchor the cytoplasmic tail in the lipid bilayer. However, for the vasopressin (V2) receptor as well as for the rat oxytocin receptor, elimination of palmitoylation sites by mutagenesis failed to produce significant alterations in receptor function. The oxytocin receptor has two (mouse, rat) or three (human, pig, sheep, rhesus monkey, bovine) potential N-glycosylation sites (N-X-S/T consensus motif) in its extracellular NH2-terminal domain. For the “core” oxytocin receptor, a molecular mass of; 40–45 kDa can be calculated on the basis of the amino acid sequence derived from the known cDNA sequences of several species. In photoaffinity labeling experiments using myometrial membranes obtained from guinea pigs during late pregnancy, a 68- to 80-kDa protein was specifically labeled by a photoreactive oxytocin antagonist. Deglycosylation of the photo labeled receptor with endoglycosidase F gave rise to a protein with 38–40 kDa. Similarly, in the same tissue, a 78-kDa protein was labeled by a photoreactive vasopressin analog. In contrast, in membranes from rat mammary gland and rabbit amnion cells, photoreactive oxytocin analogs are specifically incorporated into a 65-kDa binding protein. It is possible that the different molecular masses for the myometrial versus the mammary gland and amnion oxytocin receptor are due to differential glycosylation patterns. With the assumption of a mass of; 10 kDa for a typical glycosylation core, all of the potential glycosylation sites could be occupied by glycosylation moieties. Recombinant deglycosylation mutants of the human OT receptor have been created by site-directed mutagenesis by exchanging Asp for Asn in positions 8, 15, and 26. The deglycosylated receptors were highly expressed in HeLa cells and showed unaltered receptor binding characteristics. Thus, receptor glycosylation appears not to be necessary for proper expression and has no effect on the functional properties of the receptor [7].
One oxytocin receptor is only described, the gene for it is located on chromosome 3p24–26 in humans [8]. The oxytocin receptor gene encodes a G-protein–coupled receptor with a seven-transmembrane domain. The same oxytocin receptor is present in nervous tissue and other parts of the body as the uterus, GIT, and breast. oxytocin affects numerous different G protein-coupled receptors3). Oxytocin can bind to receptors that were identified in vitro, such as oxytocin receptors, but also to receptors of vasopressin (AVPR1A, AVPR1B, AVPR2) [9]. There are several other target receptors for oxytocin’s action as its action as an agonist on the pain-sensing transient receptor potential vanilloid-1receptor, where it attenuates pain sensation. Oxytocin also acts as a positive allosteric modulator of the m-opioid receptor (Figures 4 & 5) [10].
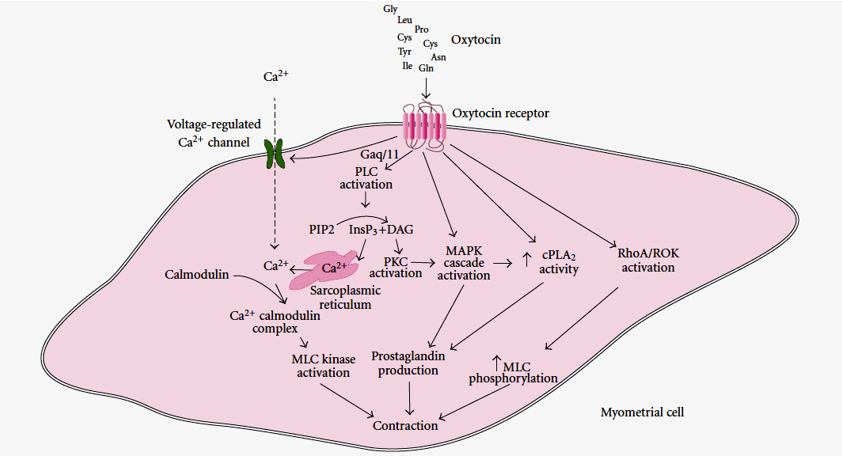
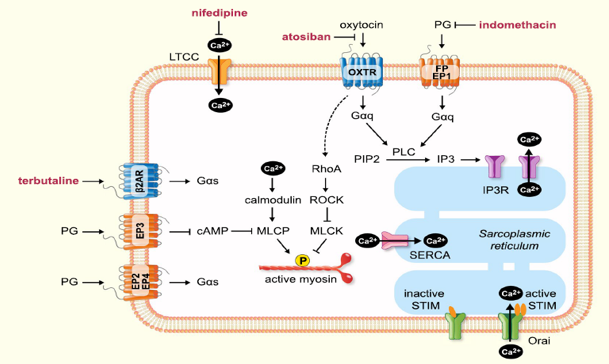
The binding of oxytocin to its receptors activates Gαq/11 and then phospholipase C, which hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) to InsP3 and diacylglycerol. inositol 1,4,5- triphosphate (InsP3) causes the release of Ca2+ from the sarcoplasmic reticulum, while DAG activates PKC. Gαq/11 also causes activation of voltage-regulated Ca2+ channels and Ca2+ entry into the cells. Ca2+ binds to calmodulin, and the Ca2+-calmodulin complex activates myosin light-chain (MLC) kinase, resulting in myometrial contraction. Both oxytocin receptors and protein kinases type C (PKC) activate the mitogen-activated protein kinase (MAPK) cascade, while oxytocin receptors and MAPK result in increased cPLA2 activity. Increased cPLA2 activity and MAPK activation result in prostaglandin production, which also contributes to the contractile effect. The activation of the RhoA associated protein kinase (Figures 6 & 7) [11].
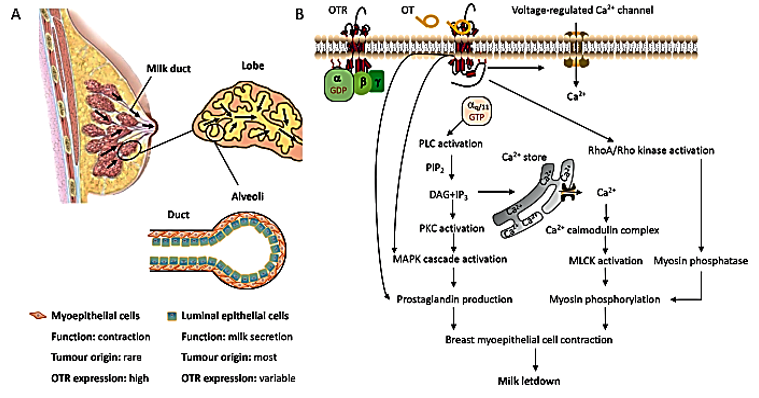
Physiological functions of oxytocin
Roughly 40% of paraventricular neurons which produce oxytocin project to central nervous structures such as the brain stem and pituitary gland, and 10% of these neurons connected to the brain stem have a role in the regulation of eating behavior via area postrema and nucleus tractus solitarius, the dorsal motor nucleus of the vagus nerve. The half-life time of oxytocin in the brain is triple as long as its half-life time the in the periphery, it is about 19 minutes in the brain and 6 minutes in the periphery, which means that, this neuropeptide acts as a relevant modulator of CNS function. Serotonin has been found to increase oxytocin concentrations while dopamine interactions with oxytocin can modulate the activity of the reward circuitry of the brain, this means that there is a relation between oxytocin and behavioral disorders such as depression, autism, and, eating disorders. Secretion of oxytocin in peripheral regions such as GIT submucosal and myenteric nerve plexus was also noticed, which augments its role in feeding and satiety behavior [14]. The effects of oxytocin on the uterus are well known. The sensitivity and density of oxytocin receptors increase during labor. After birth, the neonatal baby sucking of his mother's breast, causing the release of milk by stimulating hypothalamic neurons to produce oxytocin, causing contraction of myoepithelial cells of alveoli. Eating disorders are more dominant in females which speculates that because of the particular functional relevance of oxytocin in the female organism, changes in oxytocin pathways contribute to dysfunctional eating behavior patterns and that interventions aimed at these pathways hold a certain therapeutic action for this neuropeptide [14].
Social behavior
Animal studies on the effect of oxytocin on social behavior in a variety of mammals, including mice, rats, and sheep. These studies demonstrated oxytocin’s ability to induce the prosocial behavior of bonding, both pair-bonding and maternal bonding of mothers with their offspring. The pair-bonding behavior was measured by time spent in proximity of a partner versus a stranger, in which as the time spent with a partner was longer, means pair-bonding formation. Pair bonding can be induced in females. Injection of oxytocin in the intracerebroventricular region in the brain of
voles leads to the formation of pair-bonding among these animals, it can also be prevented by oxytocin receptor antagonists in the nucleus accumbens and prefrontal cortex of the brain. Maternal and offspring bonding has also been demonstrated also in voles. Individuals with social anxiety problems such as autism can struggle with forming and maintaining interpersonal relationships. The action of oxytocin in facilitating human bonding and social relation is well known, and future studies were needed for the possibility of treatment of social anxiety and autism by using oxytocin agonist drugs. Another aspect of social behavior the ability to recognize the individuals and differentiate between them, which is known as the social memory, was enhanced by central oxytocin administration in male rats, whereas using antagonists for oxytocin receptor blocks social memory in both male and female rats. The action of oxytocin affects social memory in multiple brain regions, including the ventral hippocampus, amygdala, olfactory bulb, and lateral septum. Studies on monkeys suggested the potential effects of oxytocin in promoting prosocial behaviors and enhancing social cognition in humans in spite of the complexity of social behavior in humans [15].
The role of melanocortin system and oxytocin neuropeptide in male sexual behavior
The dysfunction in sexual ability has effects on the quality of life in men. At least about 20% of men experience some form of dysfunction in their sexual forces during different periods of their life. The relation between age and decline in sexual function is well documented, this correlated to weight gaining and consequent cardiovascular diseases and diabetes with their complications. Although treatments with phosphodiesterase inhibitors drugs as sildenafil or other drugs for improving erection and sexual functions, responsiveness to these drugs are varying, and medications for other forms of sexual dysfunction are limited [16].
The targeting potential impairments of medications in brain circuits that underlie sexual function have newly recently garnered interest. For example, selective inhibitors for serotonin reuptake, which leads to an increase in serotonin in extracellular fluids, have been used in the treatment of rapid ejaculation. The agonists of dopamine as apomorphine were explored as sexual motivation and erectile dysfunction treatment. The melanocortin system has a role in erection function, and many drugs which targeted this system has been tested for erectile dysfunction treatment. Understanding the neurocircuitry mechanisms of sexual function may lead to more effective treatments [17].
Many clinical and preclinical studies support the involvement of the melanocortin system in sexual abilities and sexual actions. Melanocortin hormones, such as adrenocorticotropic hormone (ACTH), α-melanocyte-stimulating hormone (MSH), γ-MSH, and β-MSH, are produced from the proopiomelanocortin (POMC) polypeptide. These products bind to five different melanocortin receptors (MC1R, MC2R, MC3R, MC4R, MC5R) with different degrees of affinities. Since the MC3R and MC4R are the primary subtypes expressed by the brain, these two may mediate the central effects of melanocortin. MC4 receptors are G protein-coupled receptors that can couple with Gi/o, Gs, and Gq receptors and trigger a series of downstream pathways. The studies suggested that melanocortin receptors, MC4R, which have a role in satiety mediating, may mediate the sexual abilities and sexual actions. The administration of α-melanocyte-stimulating hormone, which is an agonist for MC3R and MC4R, has been found to lead to erection in mice and rats. At three to six months of age, MC4R null mice show decreased motivation to engage in sexual activity and take a longer time to reach ejaculation [18].
Male mice with insensitive POMC-producing neurons to insulin and leptin show reduced production in αMSH and MC4Rs expression. These mice showed a decrease in sexual motivation which was indicated by a reduction in mounting behavior during the initial sexual encounter; this phenotype is evident at four to six months of age, concurrent with increases in their weights and a decrease in insulin sensitivity [19].
POMC neurons project to nuclei that express Sim1, such as the supraoptic nucleus, paraventricular nucleus, and regions of the amygdala including the medial amygdala and basolateral amygdala. The study of Semple and Hill, [20] showed that genetically re-expressing MC4Rs only in Sim1 neurons of MC4RKO mice restores both intromission and ejaculation efficiency at age 6 months. The receptors MC4Rs in Sim1 neurons play a role in regulating feeding behavior; the weights of these mice were duplicated compared to control at the testing time. Therefore, it remains to be determined whether MC4Rs on Sim1 neurons directly regulate sexual function, or whether obesity is a necessary dysfunction factor [20].
Oxytocin neurons comprise a large portion of the Sim1 neuron population and are found in the supraoptic and paraventricular nuclei, downstream of POMC neurons. Oxytocin neurons have been heavily implicated in mediating sexual behavior in both humans and animals. These neurons are postulated to mediate the effects of MC4 receptor agonists on female sexual functions and partner preference. Semple et al., [17] hypothesized that oxytocin neurons may mediate MC4R-driven sexual behavior in male mice. They examined the ability of MC4Rs on oxytocin and Sim1 neurons in maintaining sexual function using mice, for 2-month-old, without obesity-related to age. their findings underline the feasibility of identifying specific neuronal targets for male sexual dysfunction treatment [17].
When the melanocortin 4 receptor (MC4R) is knocked out globally, male mice displayed low sexual desire, obesity, and difficulties in copulation; however, it is unclear whether these phenotypes are interdependent. To elucidate the neuronal circuitry involved in sexual dysfunction in MC4R knockouts, Semple et al., [17] in their study, re-expressed the MC4R in these mice exclusively on a subset of Sim1 neurons, namely oxytocin neurons (tbMC4Roxt mice) or Sim1 neurons (tbMC4RSim1 mice). The groups were compared to age-matched mice to observe the effects of obesity. In this experiment, young MC4R null mice had no deficits in erectile function or sexual motivation. However, MC4R null mice were found to have an increased latency to reach ejaculation compared to control mice, which was restored in both tbMC4RSim1 and tbMC4Roxt mice. These results indicate that the signaling of melanocortin via the MC4R on oxytocin neurons is important for normal ejaculation independent of the male’s metabolic health [17].
Oxytocin’s role in regulating erection and ejaculation has been studied in mice and rats. Oxytocinergic projections from the paraventricular nucleus to the hippocampus, medulla oblongata, and spinal cord facilitate penile erection. The results of Clement and Giuliano study, [21] revealed that MC4R signaling in oxytocin neurons permits ejaculation but argue against this pathway directly mediating erectile function. Systemic and intracerebroventricular (ICV) administration of oxytocin has been found to facilitate ejaculatory function, while intravenous (IV), ICV, and intrathecal administration of an antagonist of oxytocin receptor (GSK557296) have been cause inhibition in ejaculation. Similar studies have found a decreased latency to ejaculate in rabbits and rats after administration of oxytocin. The effect of oxytocin receptor ligands on the ejaculatory response may be due to the modulation of dopamine serotonin neurotransmission [21].
Genetic deletions of the peptide (MCH-KO) or its receptors (MCHR1-KO) result in mothering deficits similar to those seen in oxytocin receptor KO (OXTR-KO) mice. Both the melanin-concentrating hormone (MCH) and oxytocin lower the threshold for the initiation of maternal behavior but are not involved in its maintenance. Therefore, the oxytocin and MCH systems may interact to modulate maternal behavior. While only 4% of oxytocin neurons express MCHR1, OXTR mRNA is expressed in the majority of MCH neurons and only rarely in other neurons in the lateral hypothalamus (LH). Electrophysiological studies report that oxytocin interacts and selectively excites MCH neurons but not any other LH neurons [22].
The very selective action of oxytocin and the specific location of its receptors on MCH neurons suggests that these neurons may mediate or modulate some of the oxytocin actions on maternal behavior and emotion. The study by Sanathara et al. [23] showed that the MCH and oxytocin systems interact directly, nonetheless, whether oxytocin regulates maternal- or mating-regulated mood through oxytocin-MCH signaling is unknown. It is plausible to speculate that oxytocin may exert parts of its facilitating actions on mating and maternal responses through interacting with MCH neurons.
Measurement techniques of oxytocin
Oxytocin levels in bronchoalveolar lavage samples from asthmatic and healthy subjects were assayed in the study of Amrani et al., [24] using a competitive enzyme immunoassay, the method proceeds as follows: 100 μl of all standards and bronchoalveolar lavage samples were loaded in triplicate with 50 μl of blue conjugate antibody into each well, except the total activity and blank wells. The plate was sealed and incubated at 4°C for 24 hr. After incubation, wells were washed 3 times, and pNpp substrate solution was added and incubated at room temperature for 1 hr. Subsequently, the plates were read immediately at an optical density of 405 nm. The concentration of oxytocin in the samples was calculated based on the standard curve obtained with known concentrations of oxytocin.
Initial measurements of oxytocin by using radioimmunoassay and bioassays suggested that oxytocin concentration in blood is very low, 5 pg/ml, with small increases as pulses, 15 pg/ml, during lactation and uterine contractions [25]. Studies using immunoassay or mass spectrometry preceded by an extraction procedure continued to detect concentrations in the 5–30 pg/ml range. However, other methods, including mass spectrometry, have indicated that endogenous oxytocin may occur in blood at much higher concentrations greater than 500–1000 pg/ml. Studies on concentrations of oxytocin in tissues may give results of a particular understanding of the beneficial effects of this neuropeptide. For example, within the microenvironment of ovarian tumors, oxytocin levels were measured at 200 times higher than those in plasma. These high levels of oxytocin may reflect a response to the tumor with apparent benefits to reducing inflammation. But they also could be related to the absence within the tumor compared with the blood of peptide binding molecules, which, in turn, might affect the concentrations of oxytocin detected by antibody-based methods [26, 27].
However, many other works indicated that oxytocin readily engages in complexes at its disulfide bridge and that the vast majority of oxytocin in bio-samples evades detection using conventional approaches to measurement [28]. Importantly, these findings have been confirmed using mass spectrometry which is highly specific compared with immunoassay suggesting that earlier critiques of the high oxytocin concentrations detected by immunoassay may have been misguided. However, despite its advantages regarding specificity, mass spectrometry methods for measuring oxytocin are still in active development, and many researchers have faced substantial challenges in the reliable detection of oxytocin using this approach. Hence, it is important to keep in mind that although mass spectrometry is a very sophisticated approach, the verdict on the optimal measurement method is still being debated. Additional areas in which mass spectrometry measurement of oxytocin can be improved include the removal of interferents (e.g., phospholipids, which can suppress signals, resulting in vast underestimation of oxytocin concentrations), automation (including sample preparation) for higher throughput, and further development and maturation of high-sensitivity microfluidic separation systems [6].
Salivary measurements, possibly with fewer sources of variation, offer an alternative for the assessment of changes in oxytocin. Several studies have revealed reliable variations in oxytocin concentration, especially in rapid response to specific experiences, such as anticipation of breastfeeding, sexual stimulation, exercise, affiliative social contact, and psychologic stress [29, 30]. However, the relationships between concentrations of peptides in saliva, blood, and peptide concentrations in the brain nuclei are not yet well-understood [31]. The study of Yamamoto and Higashida [27] suggested that oxytocin is dynamically changing in biologic matrices, like blood. In addition, detecting the presence of binding molecules will be critical to accurate representations of the availability of these elusive molecules [27].
Measuring the Oxytocin Receptor
The measurement of oxytocin receptors is problematic because the pharmacological tools that are available for stimulating, identifying, or blocking receptors for oxytocin may not be sufficiently selective to allow easy manipulations or identification of these receptors in vivo. Cell culture or do not reflect the functional availability of these peptides, especially since the binding that occurs in vivo, including in tissues or blood, could be influenced by competing molecules that differ from one test to another, as in the case of microglial oxytocin receptors has been difficult to detect in vivo but appears to be significantly upregulated after immune challenges and stressors, especially when macrophages and microglia are challenged in vitro [27].
In the study Amrani et al. [24], Real-time PCR was performed to assess oxytocin receptor expression. In this study total, RNA was isolated, 1μg of total RNA was reverse transcribed at 37°C for 120 min followed by 25°C for 10 min. Forty ng of cDNA per reaction was used in the real-time PCR in the presence of Ampli Taq Gold DNA polymerase, the reaction was incubated for 2 min at 50°C followed by 10 min at 95°C. Then the reaction was run for 40 cycles at 15 sec, 95°C, and 1 min, 60°C per cycle. Assays-on-Demand™ primers and probes specific for oxytocin receptors were used in the PCR. The endogenous 18 S rRNA was measured and used to normalize all samples using the ΔΔCT method. Gene expression level of oxytocin receptor is expressed relative to 18 S and untreated samples in each stimulation study, respectively. At least 3 (often 6) replicates were run for each condition.
Drug discovery
Oxytocin plays a key role in adaptive processes associated with stress responses, tolerance reward, and memory. Through interactions with stress and brain reward systems, it is known to play a role in several psychiatric diseases, particularly those that involve altered social integration, such as addiction to drugs and alcohol. There is growing interest in the neuropeptide oxytocin as a potential therapeutic agent in the treatment of alcohol and drug abuse disorders. Accumulating preclinical evidence suggests that oxytocin administration affects the development of sensitization, tolerance, and withdrawal symptoms, and modulates numerous alcohol and drug-seeking and also alcohol and drug-taking behaviors. Further, there is some evidence to suggest that oxytocin may help to reverse neuroadaptations that occur as a result of the chronic taking of drugs and alcohol [32].
Early research in the drug discovery field indicated that administration of exogenous oxytocin can prevent the development of tolerance to opiates and ethanol, the induction of stereotyped, hyperactive behavior by drugs or ethanol, and the withdrawal symptoms associated with sudden abstinence from drugs and alcohol. Additionally, stimulation of endogenous oxytocin systems is underlying the prosocial and empathogenic effects of party drugs such as GHB (Fantasy) and MDMA (Ecstasy). Brain oxytocin systems exhibit profound neuroplasticity and undergo major neuroadaptations which resulted from drug use. Many drugs, including cocaine, cannabis, MDMA, GHB, opiates, and alcohol, cause long-term changes in markers of oxytocin function and this may be related to enduring deficits in social behavior in laboratory animals exposed to these drugs. Preclinical studies have illustrated a remarkable ability of exogenous oxytocin to inhibit stimulant and alcohol self-administration, alter associated drug-induced changes in glutamate, dopamine, and Fos expression in basal ganglia and cortical sites, and preventing of stress and priming-induced relapse to drug-seeking. Oxytocin, therefore, has fascinating potential to reverse the corrosive effects of long-term drugs abuse on social behavior and to inoculate against future vulnerability to addictive disorders. The clinical studies examining the effects of intranasal oxytocin in humans with drug abuse are eagerly awaited [33].
Oxytocin neuropeptide has a strong therapeutic potential for the treatment of a number of psychiatric disorders as social impairments in autism spectrum disorders based on animal studies that prove the critical role of oxytocin in the modulation of social cognition. The potential benefits of oxytocin, though, are limited by the biophysical properties of this neuropeptide. This necessitates the development and validation of animal models and behavioral paradigms of social cognition to identify the most efficacious methods of upregulating the oxytocin system. The development of more potent methods stimulating the oxytocin system could have profound effects on social cognitive processes, which could be harnessed clinically. Thus, while oxytocin may play a negligible role in the induction of social impairments of autism spectrum disorders it may still have a meaningful effect on the manifestation of this disorder. It is imperative that the fieldwork fill in the knowledge gaps in the relationship between the oxytocin system and human social cognition, including characterization of the human functional and disorder oxytocin system, the mechanism of action of intranasal oxytocin treatment, and the long-term efficacy of oxytocin administration to realize the clinical potential of oxytocin in the treatment of social impairments [34].
Oxytocin and arginine vasopressin receptors
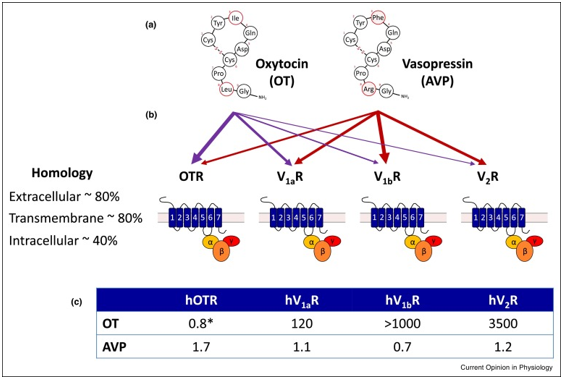
Arginine vasopressin (antidiuretic hormone) is another cyclic neuropeptide released from the posterior lobe of the pituitary gland. Its main functions are to retain water by kidneys and constriction of blood vessels to regulate blood pressure and body fluids volume and osmolarity. It is synthesized by magnocellular neurons in the hypothalamus beside neurons that produce oxytocin neuropeptide. In humans, the structure of oxytocin and vasopressin are very similar, each of them having 9 amino acids and a disulfide bond, and differing only in 2 amino acids (in positions 3 and 8). It also has a short half-life of about 20 minutes and is derived from a preprohormone. Like oxytocin, Arginine vasopressin also signals through G protein-coupled receptors (V1a, V1b, and V2) which have relatively high homology to oxytocin receptors.V1a receptors bind to Gαq/11 proteins and mediate effects via activation of PLC-β whilst V2 receptors bind to Gαs proteins which activate cAMP signaling.V1b receptors can activate several signaling pathways via different G-proteins, according to the concentration of vasopressin and level of receptor expression, e.g. PKC via Gαq/11 and cAMP via Gαs. The similarity in the structure of these two neuropeptides is about 80% homology and the sequence homology of the receptors is high, this characteristic of homology causes cross-reactivity between these peptides and receptors. That is, studies showed that vasopressin binds to its own receptor and to the oxytocin receptors, similarly, oxytocin binds mainly to its receptors and can also, to some extent, activate vasopressin receptors. Oxytocin and vasopressin receptors can also form functional homo- or heterodimers adding further complexity to this system. Vasopressin has been shown to induce contraction of the uterus in humans and other mammals. Uterus in humans has a high sensitivity to vasopressin than to oxytocin. The effect is thought to be mediated via the V1a receptors which, as well as the uterus, are also expressed in the smooth muscle of the blood vessels wall, adrenal cortex, liver, brain, and platelets. The V1b receptors are expressed in the anterior pituitary and adrenal medulla, whilst the V2 receptors are mainly located in the kidneys where it mediates arginine vasopressin antidiuretic action. As with the oxytocin receptors, there is some evidence to suggest that the density of V1a receptors is moderately increased near the end of gestation, and the expression of V1a receptors which suggests the biologically active role of AVP in the pregnant myometrium. The clinical observations that infusion of Arginine vasopressin is able to induce labour (Figure 8) [35].
Oxytocin metabolism, inhibitors, and termination
Oxytocin is administered parenterally and is fully bioavailable. It takes approximately 40 minutes for oxytocin to reach steady-state concentrations in the plasm after parenteral administration. Oxytocin is rapidly removed from the plasma by the liver and kidney. Oxytocinase activity increases throughout pregnancy and peaks in the plasma, placenta, and uterus near term. The placenta is a key source of oxytocin during gestation and produces increasing amounts of the enzyme in response to increasing levels of oxytocin produced by the mother. Oxytocinase activity is also expressed in mammary glands, the heart, the kidney, and the small intestine.Lower levels of activity can be found in the brain, spleen, liver, skeletal muscle, testes, and colon. The level of oxytocin degradation is negligible in non-pregnant women, men, and cord blood. The enzyme oxytocin is largely responsible for the metabolism and regulation of oxytocin levels in pregnancy; only a small percentage of the neurohormone is excreted in the urine unchanged. The plasma half-life of oxytocin ranges from 1-6 minutes. The half-life is decreased in late pregnancy and during lactation [37, 38].
In view of the proposed role of oxytocin in triggering parturition, there has been a long-standing effort to produce specific oxytocin antagonists as a potential means of inhibiting premature uterine contractions. Although several nonapeptide antagonists have been produced and tested, the most-studied oxytocin antagonist to date is the peptide antagonist atosiban, developed by Ferring. Studies attest to the effectiveness of oxytocin antagonists in inducing uterine quiescence in normal labor and in cases of naturally occurring or experimentally induced premature labor. The effectiveness of atosiban to delay premature labor has been demonstrated in many different species, including humans (Figure 9) [39].
It can be concluded that oxytocin is a peptide hormone that is synthesized in brain regions and other organs and posse receptors in many organs. It plays a role in social behavior, emotions love, and affection, the period after childbirth, and metabolic functions. Its potential effects in treating obesity. The half-life time of oxytocin in the brain is triple as long as its half-life time in the periphery. Oxytocin neurons may mediate MC4R-driven sexual behavior. Variations in oxytocin concentration, especially in rapid response to specific experiences. Oxytocin is rapidly removed from the plasma by the liver and kidney. The plasma half-life of oxytocin ranges from 1-6 minutes. The half-life is decreased in late pregnancy and during lactation.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell

Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. It was truly a rewarding experience to work with the journal “Clinical Cardiology and Cardiovascular Interventions”. The peer review process was insightful and encouraging, helping us refine our work to a higher standard. The editorial office offered exceptional support with prompt and thoughtful communication. I highly value the journal’s role in promoting scientific advancement and am honored to be part of it. Best regards, Meng-Jou Lee, MD, Department of Anesthesiology, National Taiwan University Hospital.

Dear Editorial Team, Journal-Clinical Cardiology and Cardiovascular Interventions, “Publishing my article with Clinical Cardiology and Cardiovascular Interventions has been a highly positive experience. The peer-review process was rigorous yet supportive, offering valuable feedback that strengthened my work. The editorial team demonstrated exceptional professionalism, prompt communication, and a genuine commitment to maintaining the highest scientific standards. I am very pleased with the publication quality and proud to be associated with such a reputable journal.” Warm regards, Dr. Mahmoud Kamal Moustafa Ahmed

Dear Maria Emerson, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews’, I appreciate the opportunity to publish my article with your journal. The editorial office provided clear communication during the submission and review process, and I found the overall experience professional and constructive. Best regards, Elena Salvatore.

Dear Mayra Duenas, Editorial Coordinator of ‘International Journal of Clinical Case Reports and Reviews Herewith I confirm an optimal peer review process and a great support of the editorial office of the present journal
Dear Editorial Team, Clinical Cardiology and Cardiovascular Interventions. I am really grateful for the peers review; their feedback gave me the opportunity to reflect on the message and impact of my work and to ameliorate the article. The editors did a great job in addition by encouraging me to continue with the process of publishing.

Dear Cecilia Lilly, Editorial Coordinator, Endocrinology and Disorders, Thank you so much for your quick response regarding reviewing and all process till publishing our manuscript entitled: Prevalence of Pre-Diabetes and its Associated Risk Factors Among Nile College Students, Sudan. Best regards, Dr Mamoun Magzoub.

International Journal of Clinical Case Reports and Reviews is a high quality journal that has a clear and concise submission process. The peer review process was comprehensive and constructive. Support from the editorial office was excellent, since the administrative staff were responsive. The journal provides a fast and timely publication timeline.

Dear Maria Emerson, Editorial Coordinator of International Journal of Clinical Case Reports and Reviews, What distinguishes International Journal of Clinical Case Report and Review is not only the scientific rigor of its publications, but the intellectual climate in which research is evaluated. The submission process is refreshingly free of unnecessary formal barriers and bureaucratic rituals that often complicate academic publishing without adding real value. The peer-review system is demanding yet constructive, guided by genuine scientific dialogue rather than hierarchical or authoritarian attitudes. Reviewers act as collaborators in improving the manuscript, not as gatekeepers imposing arbitrary standards. This journal offers a rare balance: high methodological standards combined with a respectful, transparent, and supportive editorial approach. In an era where publishing can feel more burdensome than research itself, this platform restores the original purpose of peer review — to refine ideas, not to obstruct them Prof. Perlat Kapisyzi, FCCP PULMONOLOGIST AND THORACIC IMAGING.

Dear Grace Pierce, International Journal of Clinical Case Reports and Reviews I appreciate the opportunity to review for Auctore Journal, as the overall editorial process was smooth, transparent and professionally managed. This journal maintains high scientific standards and ensures timely communications with authors, which is truly commendable. I would like to express my special thanks to editor Grace Pierce for his constant guidance, promt responses, and supportive coordination throughout the review process. I am also greatful to Eleanor Bailey from the finance department for her clear communication and efficient handling of all administrative matters. Overall, my experience with Auctore Journal has been highly positive and rewarding. Best regards, Sabita sinha

Dear Mayra Duenas, Editorial Coordinator of the journal IJCCR, I write here a little on my experience as an author submitting to the International Journal of Clinical Case Reports and Reviews (IJCCR). This was my first submission to IJCCR and my manuscript was inherently an outsider’s effort. It attempted to broadly identify and then make some sense of life’s under-appreciated mysteries. I initially had responded to a request for possible submissions. I then contacted IJCCR with a tentative topic for a manuscript. They quickly got back with an approval for the submission, but with a particular requirement that it be medically relevant. I then put together a manuscript and submitted it. After the usual back-and-forth over forms and formality, the manuscript was sent off for reviews. Within 2 weeks I got back 4 reviews which were both helpful and also surprising. Surprising in that the topic was somewhat foreign to medical literature. My subsequent updates in response to the reviewer comments went smoothly and in short order I had a series of proofs to evaluate. All in all, the whole publication process seemed outstanding. It was both helpful in terms of the paper’s content and also in terms of its efficient and friendly communications. Thank you all very much. Sincerely, Ted Christopher, Rochester, NY.
