AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2768-0487/135
Kanwhish Biotechnology Ltd., LifeBay Park, Wuzhong District, Suzhou City, China.
*Corresponding Author: Linglong Zou, Kanwhish Biotechnology Ltd., LifeBay Park, Wuzhong District, Suzhou City, China.
Citation: Zixian Li, Menglan Jiang, Linglong Zou, (2024), A Review of Analytical Methods for Adeno-Associated Virus Vectors’ Biodistribution and Shedding Studies, Journal of Clinical and Laboratory Research, 7(4); DOI:10.31579/2768-0487/135
Copyright: © 2024, Linglong Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 26 March 2024 | Accepted: 05 April 2024 | Published: 12 April 2024
Keywords: AAV; gene therapy; biodistribution; shedding; analytical methods
Adeno-associated virus (AAV)-based gene therapy (GT) products can effectively deliver transgenes to replace lost or defective genes, providing hope for patients. However, there is limited research on analytical methods for pre-clinical and clinical studies which could impede their development success. During the development of AAV-based GT products, it is crucial to define the biodistribution and shedding profiles following treatment. In this review, we summarize PK studies for the AAV-based GT products approved so far by U.S. Food and Drug Administration and European Medicines Agency. We then comparatively review the analytical methods employed to evaluate AAV vectors’ biodistribution and shedding of approved GT products. We further make recommendations on choice of methodologies for future GT product development based on the study data and the currently available regulatory guidance.
AAV: adeno-associated virus;
GT: gene therapy;
SMA: spinal muscular atrophy;
Adv: adenovirus;
CNS: central nervous system
PK: pharmacokinetic;
EMA: European Medicines Agency;
FDA: U.S. Food and Drug Administration;
NHPs: non-human primates;
SMN: survival motor neuron;
qPCR: quantitative PCR;
dPCR: digital PCR;
ISH: in situ hybridization;
ELISA: enzyme-linked immunosorbent assay;
IHC: immunohistochemistry;
IF: immunofluorescence.
There are millions of patients around the world who suffer from inherited diseases, and gene therapy mediated by AAV offers a promising treatment option for certain patients with such conditions [1]. Various AAV-based GT products are currently available on the market, including Luxturna, which treats retinal diseases, and Zolgensma, a treatment for SMA [2]. In clinical applications, AAV vectors present multiple benefits such as wide tropism, low immunogenicity, ease of production, non-pathogenic characteristics, rare integration into the host chromosome and persistent transgene expression [3].
AAV belongs to the Parvoviridae family. It was first discovered in 1965 as a contaminant of AdV preparations, and its replication relies on a helper virus like AdV. Wild-type AAV is a non-enveloped, single-stranded DNA virus with either a sense or anti-sense strand genome [4,5]. Although not associated with any diseases, its ability to infect various types of cells is noteworthy, and different serotypes of AAV exhibit varying tissue tropisms. When utilized as a GT product, it is possible to optimize the capsid to enhance its tropism towards a target tissue [6].
AAV-based GT products can deliver DNA sequences of interest (e.g., gene replacement) to target cells in order to treat inherited diseases caused by monogenic deficiencies or mutations. AAV vectors have the same capsid structure as wild-type AAVs, but their viral sequences are replaced by a transgene except for the inverted terminal repeats at the ends of the viral genomes [5].
Systemic delivery is a common route to administrate AAV vectors. AAV transduction can be greatly impacted by systemic clearance and its interaction with various proteins in circulation, such as pre-existing neutralizing antibodies [7]. In addition to systemic delivery, AAV vectors can also be administrated directly to target tissues using methods such as intramuscular, CNS delivery, or subretinal delivery [6]. In the course of AAV spreading and transduction (illustrated in Figure1), after vascular escape, AAV vectors first bind with cell surface receptors. The interaction between viral capsid proteins and cellular receptors triggers the internalization/entry of AAV vectors. Following viral intracellular trafficking, entry into nucleus and virion uncoating, the viral genome is released into the nucleus, where the single-stranded AAV genome is converted into double-stranded DNA (self-complementary AAV vectors do not undergo this process). Subsequently, the harbored transgene begins to transcribe into mRNA, which is further translated into a therapeutic protein. AAV genomes can be concatenated and circularized as episomes, persisting in transduced cells without integrating into host genomes [3]. However, the intracellular genomes and transgene expression of AAV may be diluted and even lost due to division and elimination of transduced cells [6, 8]. Meanwhile, AAV vectors can be shed from patients’ bodies in different ways. For instance, AAV2 can be excreted through various excreta and secreta, such as urine, feces, saliva, nasal and oral secretions, semen, and tears, depending on the route of administration [9]. Overall picture of AAV GT products’ spread within and out of patients’ bodies upon administration is shown in Figure 1.
1.AAV GT products can be administrated to patients through systemic delivery (e.g., intravenous injection) or local delivery (e.g., intramuscular injection);
2.In blood stream, AAV particles probably interact with various proteins in circulation (e.g., neutralizing antibodies);
3.AAV particles spread to various tissues (e.g., liver and muscle) and biological fluids (e.g., cerebral spinal fluid) after vascular escape;
4.AAV particles may or may not disseminate to blood stream from the target tissues upon local delivery;
5.Within tissues, AAV particles bind to specific cellular receptors on cell surface and enter into cells;
6.AAV particles are transported within cell and imported into nucleus;
7.After uncoating, AAV genomes are exposed;
8.AAV single-stranded genomes are converted into double-stranded genomes (except scAAVs);
9.Transgene mRNAs are transcribed from AAV genomes;
10.Transgene mRNAs are transported to cytoplasm;
11.Therapeutic proteins are translated from transgene mRNAs;
12.AAV particles can be shed from various excreta (e.g., feces) and secreta (e.g., saliva, urine and tear) depending on administration route.
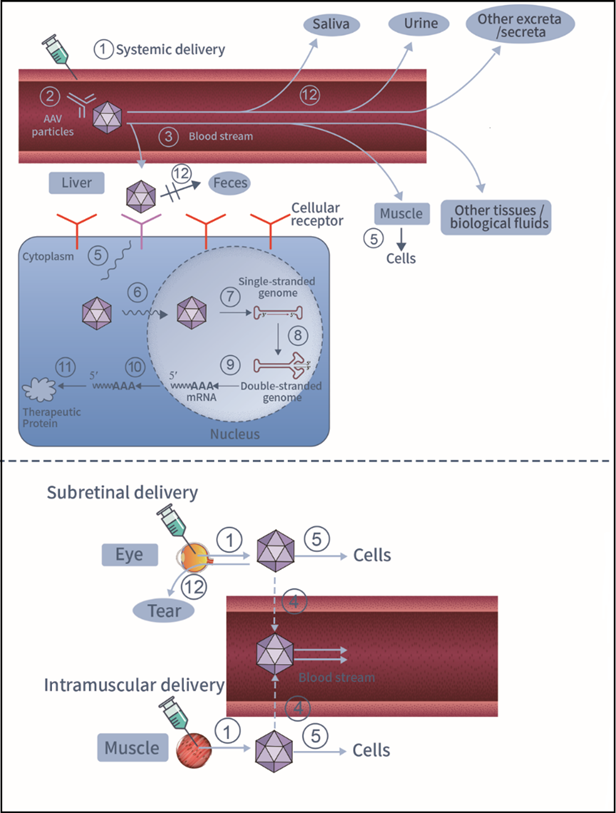
Figure 1: Overall picture of AAV GT products’ spreading and transduction upon systemic (top) or local (bottom) administration.
When conducting PK studies of a GT product, general scientific principles may apply. However, certain terms used in small molecular drug studies, such as absorption, distribution, metabolism, and excretion are not directly applicable [10]. The determination of the PK profile of a GT product is achieved through analyzing its biodistribution, which refers to how the product is distributed, how long it stays and how it is cleared from target and non-target tissues, as well as biofluids, following its administration. It is imperative to understand the biodistribution pattern of an AAV vector before any clinical trial is initiated [11, 12]. Biodistribution profiles from a preclinical study and/or an initial human trial help to interpret results of proof-of-concept studies, enhance safety assessments for GT products, and provide the required information for further designing other human trials (e.g., determining dose levels, dosing schedules and monitoring plans).
On the other hand, shedding refers to the release of a GT product from the dosed patient via one or all of the routes: excreta, secreta or skin (e.g., such as pustules, sores, or wounds). As all AAV-based GT products are derived from infectious viruses, shedding studies are necessary to evaluate the possibility of AAV transmission from treated to untreated individuals [13].
In this review, we present a detailed introduction of the analytical methods used in biodistribution and shedding studies of AAV-based GT products. In conjunction with pertinent regulatory guidance, we furnish comprehensive details regarding the applicability of each analytical method, experimental design considerations, challenges, and potential solutions for delineating the components of biodistribution investigations and shedding assays.
Overview of PK Studies for Approved AAV-based GT Products
Seven approved products are listed in Table 1. Their PK studies are briefly discussed in this section.
| Product name | Serotype | Disease | Agency | Year of approval | Reference |
| Glybera | AAV1 | lipoprotein lipase deficiency | EMA | 2012 | 14 |
| Luxturna | AAV2 | inherited retinal dystrophy | EMA (and FDA) | 2018 | 15 |
| Upstaza | AAV2 | aromatic L-amino acid decarboxylase deficiency | EMA | 2022 | 16 |
| Zolgensma | AAV9 | spinal muscular atrophy | FDA (and EMA) | 2019 | 17 |
| Elevidys | AAVrh74 | Duchenne muscular dystrophy | FDA | 2023 | 18 |
| Hemgenix | AAV5 | hemophilia B | FDA (and EMA) | 2022 | 19 |
| Roctavian | AAV5 | hemophilia A | EMA (and FDA) | 2022 | 20 |
Table 1: List of AAV-based GT products approved by FDA or EMA
Glybera: Following an intramuscular administration of Glybera in mice, AAV vectors were transiently detected in circulation. The viral genome was detected eight days after administration at higher levels in injected muscle and draining lymph nodes, but at much lower levels in non-target tissues, such as liver, lung and brain. After this time point, the measured residual viral genome levels remained high in target tissues, while they were progressively declining in non-target tissues. Comparable distribution patterns of AAV vectors were observed upon administration in cats and rabbits. In clinical trials, elevated levels of viral genome were observed to persist for up to 12 months upon administering Glybera in injected leg muscle, but viral genome was not found in non-injected muscle. The viral genomes were found at the highest level in the serum, while the levels decreased by one to two logs per week. Although initially detected, AAV vectors became undetectable in the saliva, urine and semen of patients after 12, 10 and 26 weeks, respectively [14].
Luxturna: Following a subretinal administration to NHPs’ eyes, AAV vectors were detected in intraocular fluids of injected eyes at the highest level, and in the optic nerve of the injected eye, optic chiasm, spleen, liver, stomach, and lymph nodes at lower levels for up to three months. In clinical studies, a low level of viral genome could be transiently detected in tear and serum samples from a portion of subjects after bilateral administration [15].
Upstaza: Similar to Glybera and Luxturna, Upstaza is also administered locally through intraputaminal administration. However, the AAV vector has not been demonstrated to distribute outside the CNS (target tissue) or shed in blood and urine after the CNS administration [16].
Zolgensma: It is a gene therapy prescribed for the treatment of SMA in children under the age of 2. The biodistribution of the AAV vector used in Zolgensma was evaluated in two patients who died 5.7 months and 1.7 months, respectively, following intravenous infusion. The viral genome was detected at the highest level in the liver, and also found in other organs such as the spleen, heart, pancreas, inguinal lymph node, skeletal muscles, peripheral nerves, kidney, lung, intestines, gonads, spinal cord, brain, and thymus. An immunostaining confirmed expression of the generalized SMN protein in spinal motor neurons, as well as in neuronal and glial cells of brain, heart, liver, skeletal muscles, and other tissues of the patients. Vector shedding was investigated over the course of 18 months. Like Glybera and Luxturna, the viral genome was found in saliva, urine, and feces, while the detected viral genome copies were decreased to undetectable levels in all the samples at different time points [17].
Other GT products: Elevidys, Hemgenix, and Roctavian are all administered via intravenous infusion. Like Zolgensma, AAV vectors were detected in samples such as serum/plasma, urine and saliva [18-20]. In Roctavian’s PK studies, patients’ plasma and semen samples were evaluated using an immunoprecipitation qPCR assay to detect capsid-wrapped viral genome, which could potentially be infectious, following conventional qPCR assay [20]. Encapsidated viral genome was detectable in plasma up to 10 weeks after administration, while its maximum time to clearance in semen was 12 weeks.
Analytical methods for PK studies of AAV-based GT products
Assessment of vector biodistribution
Samples collected in AAV vector biodistribution studies typically include tissues at the injection site (e.g., retina), as well as liver and gonad tissues, and biofluids such as blood and cerebrospinal fluid. The sampling time-points should be designed to cover the in vivo kinetics of AAV vectors, with additional time-points included to thoroughly capture the duration of the steady-state period or to estimate persistence. Vector biodistribution assessment typically involves detecting viral genome and transgene products [21]. In clinical trials, invasive techniques like biopsies and tissue collection may not always be practical or ethical to perform, resulting in limited number of clinical biodistribution studies [22]. Thus, biodistribution data about AAV vectors are primarily obtained from preclinical studies performed in animals [23].
Test systems of preclinical biodistribution studies
Several animal species are susceptible to AAV infection and have been employed in preclinical studies of AAV vectors, such as rats, mice, NHPs, dogs, cats, and pigs [24]. Rodents are widely used due to their ease of genetic manipulation, fast reproduction and lower cost. However, due to apparent physiological and anatomical differences among species, it is not always possible to extrapolate findings from rodent models to larger animals, humans, or even other rodent species. For example, AAV PHP.B vector demonstrates superior brain transduction efficiency in C57BL mice compared to AAV9, whereas this is not confirmed in other animal species [3]. Humanized mouse and xenograft models were employed to overcome interspecies discrepancies, but it is yet to be determined if the experimental results obtained from these models can be translated into human clinical trials [25].
Compared to rodents, large animal models often exhibit greater similarities to humans and can therefore lead to more accurate predictions of AAV vector biodistribution profile in humans. For instance, in a study of AAV-based GT product for osteoarthritis, researchers utilized an equine model to investigate PK profile of the AAV vector after intra-articular administration. The choice of the equine model was due to the similarity in size and function of equine forelimb joints to human knees, which are also prone to osteoarthritis due to their weight-bearing nature. The data obtained from the equine model regarding vector biodistribution, transgene (IL-1Ra) expression, and dose-effect were proved highly translatable for clinical trials [26]. However, tissue collection at multiple time-points may not always be feasible for a large animal species due to the limited number of animals used in a study. The restricted availability of large animals can be somewhat alleviated by collecting biofluid samples at multiple time points over an extended duration, or by conducting experiments that incorporate both large and small animals [11, 22].
In addition to animal models, organoids can also be used for biodistribution studies involving AAV vectors. Organoids are derived from stem cells and can self-organize into 3D cultures that resemble organs in terms of their physiology, cell composition and functionality to a certain extent. A significant advantage of using organoids derived from humans is that there are no inter-species differences [27]. However, host immune responses, that likely affect AAV vector’s transduction and biodistribution, cannot be accurately represented with organoids [22, 25].
Technical platforms for viral genome analysis
It is the genome of an AAV vector, but not its capsid, that carries the therapeutic transgene and can persist in transduced cells over an extended period. In addition, there is no reliable method available for measuring viral capsid levels in tissue samples. Thus, the viral genome is selected as a quantifiable surrogate for the whole vector in biodistribution studies [28]. qPCR is often utilized to detect the viral genome. For example, qPCR was used to analyze the biodistribution of an optimized scAAV9-SMN1 vector after intra-cerebroventricular administration in a SMA mouse model [29]. Similarly, qPCR was used in another study to investigate PK profile of a dual AAV vector for Usher syndrome type 1B in the retina of primates after subretinal administration [30]. Fluorescent dyes or probes can be incorporated into PCR reactions to monitor PCR process by tracing fluorescent signals after each cycle. By comparing the results with a standard curve, the copy number of viral genomes in a test sample can be determined. Linearized plasmids containing transgene or vector DNA are commonly used to prepare standard curves in AAV vector’s biodistribution studies [31].
PCR specificity and sensitivity heavily rely on the design of the primers, and multiple software applications can aid in primer design [32]. In addition to the typical considerations of amplicon size and GC content, it is crucial that qPCR amplicons are specifically tailored to AAV vectors. To ensure specificity, it is often recommended to design the sequence of an amplicon at the junction of the open reading frame of the transgene and its 3' or 5' untranslated region on the vector. This strategy prevents an overestimation of viral load caused by co-presence of empty capsids. To further improve assay specificity, the primer sets should be submitted to vigorous Primer-BlAST test against the host genomic DNAs, to eliminate any non-specific cross-reactions [29, 33].
As qPCR is a highly sensitive assay, the procedure should be performed in a sterile environment for ensuring its specificity [31]. Moreover, disposable or sterile DNA-free sets of reagents and containers should be used during sampling to minimize contamination risks. It is a good practice for sampling to first collect the organs that are less likely to contain viral vectors, such as the gonads, prior to those organs, such as the liver, that are more likely to contain a large quantity of viral vectors. Injection sites should be harvested at the very end as they contain the highest levels of the viral vectors. Furthermore, it is important to apply perfusion or rinse the organ samples thoroughly in phosphate buffered saline solution to decrease contamination from blood [34]. DNA extraction and purification methods should be optimized to reduce contamination as necessary. To monitor any unintended amplification, it is recommended to include a negative control (i.e., no-template controls, NTCs) in the assay [35].
Per FDA recommendations, a qPCR assay performed for a tissue sample must demonstrate a limit of quantitation of <50>
dPCR is a relatively new techniques, which is also used to quantify AAV vector’s copy number. For example, dPCR was used in a study to precisely quantify the viral load per cell in macaques’ CNS tissue samples following intraparenchymal injection or cerebrospinal fluid-mediated administration [38].
In a dPCR assay, a reaction mixture (sample) is partitioned into numerous individual compartments. The number of compartments with a positive versus negative reaction is tallied and a Poisson distribution model is used to calculate the copy number of the target sequence in the original sample [39]. This method offers an advantage over qPCR in that it does not require the use of a standard curve, which is sometimes hard to construct due to lack of well-characterized reference materials. In addition, a dPCR-based method displays superior sensitivity, repeatability, accuracy, and greater tolerance to PCR inhibitors [40]. However, compared to qPCR platform, dPCR requires more time for sample analysis and method development. Furthermore, it is more expensive to perform a dPCR versus a qPCR, and dPCR has a narrower dynamic range [28, 33].
While qPCR and dPCR methods have been widely used to study PK profiles of AAV-based GT products, there remains a lack of regulatory guidance on the validation and implementation of such methods for GT products. The Global CRO Council in Bioanalysis has made recommendations regarding the acceptance criteria for a qPCR or dPCR method validation and sample analysis [41].
What qPCR and dPCR assays do not show is the cellular or spatial context necessary for a thorough analysis of AAV vector’s biodistribution profile, such as the rates and types of infected cells, which can be supplemented by ISH [28, 42]. For example, a recent study introduced a new technique known as signal amplification by exchange reaction fluorescence in situ hybridization. ISH employs specific probes to localize and visualize target sequences in situ while preserving cell integrity. Together with image capture and analysis, this approach facilitated the visualization and measurement of individual AAV genomes in retinal cells following AAV subretinal delivery in mice [43]. In another case, upon intravenous bolus injection of AAV to Wistar Han rats and cynomolgus monkeys, the presence of viral genomes and transgene (SMN1) mRNA expression was confirmed in tissue samples via ISH to evaluate their relationships with toxicity findings [44]. Proper controls measures are critical in the development of ISH methods. Certain molecules commonly expressed by most mammalian cells, for example actin, can serve as positive controls to check for nucleic acid preservation. Furthermore, a negative control can be made by excluding the specific probe in the ISH experiment. In addition, the elimination of an ISH signal should occur after pretreating the tissues with an appropriate nuclease, which can serve as another control for specificity [42].
Southern blotting can also be used to detect AAV vectors, though its use is limited in AAV vectors’ biodistribution studies partially due to time-consuming and problematic procedures required to prevent contamination [23, 45]. Table 2 summarizes the advantages and challenges of all these viral genome detection techniques.
| Techniques | Applications | Advantages | Challenges | References |
| qPCR | Quantification of viral genome in tissues or biological fluids |
|
| 29-37 |
| dPCR |
| 38-41 | ||
| ISH | Localization and visualization of viral genome in tissues | Spatial and cellular context crucial for biodistribution assessment |
| 42, 43, 44 |
Table 2: Technical platforms for viral genome analysis.
Technical platforms for transgene product detection
Upon AAV vector’s entry into cells, a transgene must be transcribed into mRNA, and then translated into protein to exert its therapeutic function. The temporal and spatial pattern of transgene expression should, therefore, be investigated in biodistribution studies of an AAV vector [22]. Both qPCR and dPCR are commonly used for transgene mRNA detection, which require mRNAs to be reversely transcribed into cDNAs prior to PCR reactions [46, 47]. Compared to DNA samples, RNA samples are more prone to degradation, and RNase contamination poses a significant risk to RNA integrity. Thus, it is important to prevent RNase contamination from all sources, including reagents used in sampling, RNA extraction procedure, and other experimental procedures. Additionally, specific measures must be taken to maintain RNA integrity. For example, tissue samples were submerged entirely in RNA later reagent in sterile RNase-free tubes in a reported study [37]. In some cases, it is necessary for the primer set of a PCR assay to differentiate between transgene mRNA and its endogenous counterpart. In a study using a SMA mouse model, the primer set was specifically designed to detect SMN expression from the chicken-β-actin promoter of the AAV9 vector, and it did not amplify SMN originating from the SMN2 gene [48]. To ensure this required specificity, the primers must be submitted to a Primer-BLAST query against the host RNA database prior to use [31].
The detection of a transgene protein from complex mixtures of proteins extracted from tissues via Western blot is a widespread practice, where the target protein is separated from non-target ones via gel electrophoresis and probed by its specific antibody [45]. For example, SMN expression was analyzed via Western blot in the brain, spinal cord, and gastrocnemius muscle samples from wild-type, untreated SMA, and SMA mice treated with 2 types of AAV vectors. The results showed that SMN levels in the gastrocnemius muscles of both treated groups did not recover to wild-type levels, while the levels in the brain and spinal cord samples surpassed those of wild-type counterparts [47]. In another study, GAA expression in the liver was demonstrated using Western blot in NHPs after hepatic gene transfer with AAV vector, and GAA uptake by various tissues was also shown [49]. Efficient protein extraction from tissue samples is critical for accurate Western blot analyses. The BCA Protein Assay Kit is commonly used for determining protein concentration after extraction. It is also crucial to include an endogenous reference protein such as tubulin and GAPDH to aid in the assessment of Western blot results and the quantification of target protein.
ELISA is commonly used for quantifying a specific protein in liquid samples based on the reaction between the target protein and its primary antibody [50]. ELISA has been employed to determine the concentrations of circulating hFVIII or hFIX protein in the plasma of treated animals after intravenous infusion of AAV vector harboring hFVIII or hFIX gene [46, 51]. In another study, human p75ECD levels in the brain and serum samples of Alzheimer’s disease mice were analyzed with ELISA after AAV intramuscular administration [52].
To localize and visualize transgene expression in a tissue sample, one may utilize immunohistochemistry (IHC) or immunofluorescence (IF). Enzyme-linked antibody catalysis of the added substrate (in the case of IHC) or the use of fluorescence probe-conjugated antibody (in the case of IF) are commonly used in these techniques [45]. For example, assessed by IHC analysis of SMN expression in mice’s lumbar spinal cords, SMN expression in α-motor neurons of SMA mice was shown to recover after AAV intracerebroventricular administration [29]. Similarly, using IF analysis, another study confirmed the recovery of SMN expression in motor neurons of SMA mice after AAV prenatal administration [47]. However, if the transgene protein is secreted or transported axonally, IHC or IF may not be able to provide an accurate depiction of the cellular transduction pattern or the origin of the transgene protein after AAV delivery. It is preferable to detect transgene mRNA via ISH in this case [53].
When selecting primary antibodies for transgene proteins used in these techniques, it is necessary to verify and prevent any potential cross-reactivity with endogenous host proteins [46, 52]. Alternatively, a tag can be encoded in the transgene to differentiate between the transgene-derived protein and its endogenous counterpart [52, 54]. Table 3 summarizes the applications and challenges of all these techniques available for detecting transgene products.
| Techniques | Applications | Challenges | References |
| qPCR/dPCR | To quantify transgene mRNA in tissues | Difficulty in protection of RNA integrity | 37, 46-48 |
| ISH | To localize and visualize transgene mRNA in tissues | 53 | |
| Western blot | To identify and quantify transgene protein | 1.Protein extraction efficiency 2.Use of proper endogenous reference protein 3.Primary antibodies’ specificity | 47, 49 |
| ELISA | To determine transgene protein concentration | Primary antibodies’ specificity | 46, 50, 51, 52 |
| IHC/IF | To localize and visualize transgene protein in tissues | Primary antibodies’ specificity | 29, 47 |
Table 3: Technical platforms used for detection of transgene product.
Assessment of vector shedding
Shedding refers to the spread of the viral vector through patients’ secreta and excreta. Samples including urine, feces, and saliva are commonly collected for this assessment. While the spread of the viral vector in blood is necessary for its shedding, blood specimens are not typically collected for shedding assessment. The selection of sample type also depends on the route of AAV administration [13]. For example, if a viral vector is administrated via subretinal injection, it is recommended to collect tear samples from patients in addition to the commonly collected samples [55].
Regarding the sampling schedule, sampling should be more frequent in the first days after administration to examine a transient shedding profile. To ensure the reliability of the assessment, sampling should be performed until several consecutive samples are negative [56].
As in biodistribution studies, a qPCR technique is used to detect viral genome for assessing AAV vector shedding. Due to the replication deficiency of the AAV vector and its low potential to cause interpersonal transmission, qPCR is adequate as a primary assay to assess AAV vector shedding, especially when the viral genome copy number in samples decreases over time [13, 55]. For example, after intravenous injection of AAVLK03.hOTC for severe ornithine transcarbamylase deficiency, saliva, urine, and feces samples were collected from treated cynomolgus macaques and assessed for AAV shedding by qPCR. Viral genome was transiently detectable in all of those samples [57]. In a phase I/II study, patients received intra-articular injection of an AAV vector for inflammatory hand arthritis, and qPCR was used to detect the presence of viral genome in their saliva, urine, and feces samples [58].
It should be noted that the shedding assessment samples, such as feces, saliva, and nasal swabs, usually contain natural body flora, nucleases, and salts, which not only increase the risk of contamination but also inhibit PCR reactions. Thus, evaluation and assurance the specificity and sensitivity of the qPCR assay should be performed as done for biodistribution studies. If an interference is observed, sample dilution should be explored as the first solution to mitigate interference [13, 56].
As traditional qPCR cannot directly determine whether viral genomes in patient samples are infectious or not, the researchers have developed a novel immunoprecipitation qPCR assay as a solution. Using a highly specific monoclonal antibody against the AAV5 capsid, encapsidated viral genomes were captured, and then further purified from non-encapsidated ones by adding the nuclease, Benzonase. Post treatment, if viral genomes were still amplifiable in the subsequent qPCR, they should be wrapped by intact capsids and thus potentially infectious [20, 59]. In another study, researchers performed an infectious replication assay on PCR-positive samples to determine if infectious particles were present [60], which was complicated and probably unnecessary given a simpler method is available.
Biodistribution and shedding studies of an AAV-based GT product offer necessary data to support development and approval. Biodistribution results can establish if the viral vector successfully reaches targeted tissues and expresses the anticipated transgene protein. Furthermore, they aid in evaluating the potential connection between an unwanted effect and presence of the viral vector or transgene expression in specific cells or tissues. Additionally, studies on biodistribution offer valuable information for determining the first-in-human dosage [21], while viral shedding studies help researchers to address the safety concerns regarding the transmission potential of the vector from treated to untreated individuals [22]. To conduct meaningful biodistribution and shedding studies, analytical methods for these studies must be rationally designed and developed appropriately.
The techniques covered in this review typically involve sacrifice of animals and collection of tissue samples in order to assess the biodistribution profiles of AAV-based GT products. This can be problematic when animal availability is restricted, particularly in studies involving large animals. As a potential resolution, non-invasive in vivo imaging systems can be utilized to track AAV biodistribution patterns in live animals [45]. For example, researchers employed iodine-124 (I-124) radiolabeling of AAV capsid combined with positron emission tomography to analyze AAV biodistribution in organs of living NHPs following intravenous or intracisternal administration of the AAV vector for 1 to 72 hours [61]. In another study, a fluorescently biarsenical dye was used to label the core CCPGCC motif of tetracysteine sequence within AAV9 capsid, without compromising viral infectivity. The labelled AAV9 vector could be observed in anesthetized mice using intravital imaging after intravenous administration of the labeled virus [62]. To confirm successful infection and transduction in living animals' organs, fluorescent reporter or firefly luciferase imaging can be used to provide visual confirmation [47, 63]. As it is challenging to extrapolate biodistribution data from animal models to humans, coupled with difficulty in collecting biopsies or fluid samples in certain cases, in vivo imaging system offers a viable solution to obtaining valuable clinical data on AAV biodistribution in the future [22]. Radiolabeling and fluorescent dye tags on AAV particles, when combined with imaging techniques, may also provide a more convenient approach for detecting viruses in shedding studies.
Current biodistribution studies primarily focus on the transport of AAV vectors between tissues, while the "intracellular pharmacokinetics", that depicts how AAV particles are absorbed, deposited, and metabolized within cells warrants an increased attention in the future. Further understanding of the underlying mechanisms of AAV-mediated cell transduction and gene expression requires an improved application of molecular and cellular biological techniques [25, 64]. In addition to gene replacement, strategies like gene silencing are being developed for AAV-based GT products [5, 65]. The guidance for their pharmacological studies needs to be updated since they differ from the current approved products which are all based on gene replacement.
To better interpret and apply PK data of AAV vectors in clinical studies, several other factors must be examined beyond a rational use of analytical methods. For example, discrepancies among the results of biodistribution and shedding studies could be attributed to variations in the titration and characterization of the AAV vector, such as empty-to-full capsid ratio. Therefore, minimizing these variations is crucial [25, 66]. Host immunity to AAV can affect the biodistribution of AAV vectors, and should be evaluated in a biodistribution study [21]. Other factors, such as galectin 3 binding protein, C-reactive protein and a serotype’s blood clearance rate, can also influence vector biodistribution and should be taken into consideration during experimental design [7, 67]. Researchers are attempting to integrate all the PK pertinent factors into a novel physiologically based pharmacokinetic model, in order to address the interspecies discrepancies experienced while extrapolating AAV vectors’ preclinical PK data to clinical use [64].
In brief, this review outlines the major analytical methods used to support the study of biodistribution and shedding of AAV-based GT products. The attainment of reliable data relies not only on the careful consideration of the appropriate application of these analytical methods and potential caveats, but also on strict adherence to the guidance and requirements set forth by regulatory agencies, including recommended sampling procedures and data acceptance criteria. This review offers comprehensive details about two aspects that can assist researchers in designing and/or selecting methods for future studies. To facilitate the development and approval of AAV-based GT products, particularly the newly emerging ones, an improved understanding of biodistribution and shedding must be obtained through adequate use of the analytical methods discussed in this review.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
