AUCTORES
Globalize your Research
Case Report | DOI: https://doi.org/10.31579/2690-8808/230
1 Pediatrics Department at the Istishari Arab Hospital, Ramallah, Palestine
2 Dean Medical College and Health Science, Palestine Polytechnic University, Hebron, Palestine
3 Istishari Arab Hospital, Radiologic Technologist, Ramallah, Palestine
4 Department of Medical Imaging, Faculty of Allied Medical Health, Palestine Ahliya University, Bethlehem, Palestine.
*Corresponding Author: S Smerat. Istshari Arab Hospital, Radiologic Technologist, Ramallah, Palestine.
Citation: Linda Ataya, Abeer Omar, Raya Radi, Shireen Zahran, Majed Dwaik, (2024), A Case Report of Left Ventricular Endomyocardial Fibrosis., Journal of Clinical Case Reports and Studies, 5(10); DOI:10.31579/2690-8808/230
Copyright: © 2024, S Smerat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 29 October 2024 | Accepted: 06 November 2024 | Published: 14 November 2024
Keywords: restrictive cardiomyopathy; endomyocardial fibrosis; heart failure; cardiac surgery; hypereosinophilia; cardiac magnetic resonance
Endomyocardial fibrosis (EMF). is a prevalent restrictive cardiomyopathy worldwide, often linked to chronic eosinophilia and dietary, environmental, and infectious factors. Its hallmark is the fibrotic obliteration of the affected ventricle, leading to restrictive physiology. The disease affects 50% of cases, often affecting the apices of the right and left ventricles and causing atrioventricular valve dysfunction.
Endomyocardial fibrosis is characterized by fibrotic enlargement and thickening of the endocardium and myocardium of one or both ventricles. Massive fibrosis building of the ventricular endocardium causing architectural distortion, impaired and poor filling, and valvular insufficiency that defines the disease. Early diagnosing and treatment including surgical management and care in a suitable intensive cardiac support settings will enhance the survival rate especially in patients with advanced heart failure. Advancements in diagnostic imaging techniques and availability of new pharmacological choices and interfering with surgical managements as soon as possible whenever needed, defining the targeted and potential populations will support disease prevention and early diagnosis, all will contribute to lower the rates of morbidity and mortality
Endomyocardial fibrosis (EMF), is a dangerous and frequently underestimated cardiac condition, primarily affects areas in tropical and subtropical latitudes[1]. Even though EMF is the most common sign of restrictive cardiomyopathy, its exact cause is yet unknown[2]. Fibrotic tissue gradually accumulates within the heart's endocardial surface as a result of this condition, seriously impairing myocardial function[3]. This sneaky process mostly affects the ventricles and is closely linked to socioeconomic deprivation, persistent eosinophilia, parasite infections, and malnourishment[4].
Children and young adults are the most susceptible group to this ailment, and in the event that a curative solution is not available, the prognosis for individuals who suffer from it is dire[5].The disease progresses in two stages: an active phase characterized by episodic exacerbations, and a chronic phase terminating in significant heart failure[6]. In its latter stages, EMF can affect both ventricles, but isolated right-sided disease is fairly unusual[7]. The condition often causes restrictive heart failure, peripheral edema, and sometimes hydrocephalus[8]. While pharmaceutical therapy of heart failure remains the primary approach, early surgical procedures such as valve reconstruction or replacement have shown some efficacy in improving patient outcomes[9].
Endomyocardial fibrosis or “Endomyocardial sclerosis” was first defined in Uganda by a pathologist (Jack N. B. Davies), over 77 years ago (1947)[10]. Cases with high morbidity and mortality rates are mostly children and young adults specially females, originating from low socioeconomic communities in tropical Africa and subtropical countries such as Egypt, Nigeria, Brazil, Kerala in India and Sudan[11]. It is still very rare in Europe and North America; however, a few cases have been diagnosed in China and Japan. In Uganda, it accounted for 25% of cases reported by echocardiography, and in a random sample of the population in Mozambique it accounted for 20%. Although race, diet, poverty, eosinophilia, infection and malaria have all been shown to be highly associated with EMF[12].
Poverty, genetic vulnerability, parasite illnesses (particularly malaria, schistosomiasis, and filariasis), and nutritional deficiencies are thought to all have a role in the development of EMF [13]. It is theorized that eosinophils, mast cells, and fibroblasts interact in a complex way, with eosinophils releasing cytotoxic proteins that cause cardiac inflammation and fibrosis, ultimately leading to pathological constriction of the ventricular chambers[14]. Molecular pathways, notably those that regulate immune responses and collagen formation, exacerbate the illness, resulting in serious complications such as atrioventricular valve failure and acromegaly[15]. These degenerative changes, which can be detected via imaging modalities and histological analysis, are the most important indicators of this terrible disease[15].
A 55-year-old male was present at our emergency department with dyspnea, lower extremities edema, and weakness. He has been suffering from these symptoms for two years and has a previous medical history of schizophrenia.
An appointment has been set for echocardiogram, where it showed an increased thickness in the septum (18 mm) and free left ventricular wall (16 mm), obliteration of the left ventricular apex and inflow tract, and mitral valve regurgitation. Cardiac magnetic resonance imaging revealed thickening of the apical left ventricle wall; it also showed left ventricular apical obliteration and atrial enlargement.
And as for the delayed enhancement imaging, it showed endomyocardial enhancement including the left ventricular apex, mitral valve regurgitation caused by annulus dilation, and a thrombus at the left ventricular apex. The clinical features were more suggestive of endomyocardial fibrosis, and the final diagnosis was endomyocardial fibrosis.
Cardiac MRI revealed apical left ventricle wall thickening, left ventricular apical obliteration, atrial enlargement, thrombus at left ventricular apex, mitral valve regurgitation, and endomyocardial enhancement.
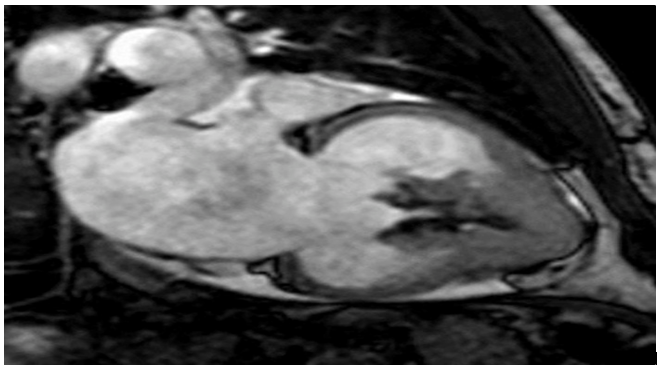
Figure1: enlargement of the corresponding atrium in conjunction with left ventricular apical obliteration.
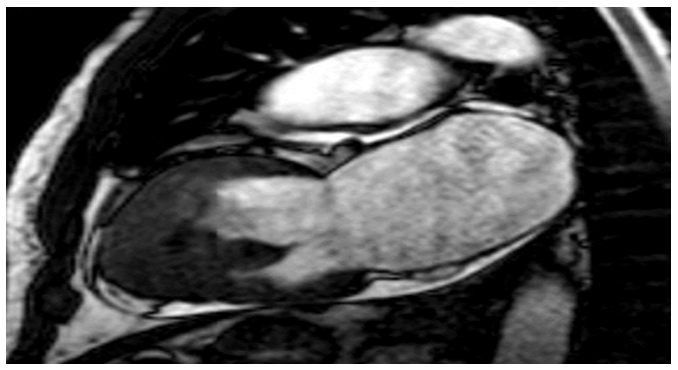
figure2: the apical region of the patients left ventricle walls exhibits endomyocardial fibrosis due to delayed enhancement.
At this time, left ventricle endomyocardial fibrosis (EMF), mitral valve regurgitation, thrombus at the left ventricular apex, and coronary artery insufficiency were identified. The patient underwent open heart surgery including mitral valve replacement, endocardial decortication, endomyocardiectomy, and double coronary artery bypass grafting. Following estuation, the patient was brought to the intensive care unit (CCU) and administered modest doses of norepinephrine and dobutamine (6μg/kg/min). On the fifth day, the administration of norepinephrine and dobutamine was ceased. CCU and hospital release occurred on the fifth- and eleventh-days following surgery, respectively, with beta-blocker and anticonvective treatment.
Tissue pieces retrieved after cardiac surgery revealed endocardial thickening with fibrous extension into the underlying myocardium (Fig. 1) and calcified portions of the left ventricle and septum endocardium (Fig.2). Histologically, the endomyocardium and mitral valve fragments showed fibrosis, hyalinization, and vascular tissue neoformation, as well as several sites of mononuclear inflammatory infiltration and calcification. The samples were consistent with the diagnostic suspicion (endomyocardial fibrosis).
A severe form of restrictive cardiomyopathy, with a poor natural prognosis unless treated before the occurrence of progressive complications[16, 17]. It is currently the most common type of restrictive cardiomyopathy globally, especially in regions like Asia, Africa, and South America [17]. EMF is chronic and progressive, characterized by fibrosis of the endocardium, structural changes in the myocardium, and increased ventricular stiffness, eventually leading to heart failure [17, 18].
Several hypotheses exist regarding the etiology of EMF, including viral infections, eosinophilia linked to allergies or autoimmune reactions, malnutrition, and exposure to toxins [19]. The link between EMF and eosinophilia is especially notable, with conditions such as Loeffler syndrome, involving eosinophil accumulation due to parasitic infections, potentially contributing to cardiac damage in endemic areas. Hypereosinophilia is found in 40% -50% of cases involving endomyocardial fibrosis, suggesting a possible connection between eosinophils and fibrotic cardiac damage [20, 21].
Early-stages in EMF typically presents with nonspecific symptoms such as fever, shortness of breath, and pan-carditis, often accompanied by eosinophilia [20, 22]. Collagen deposition, particularly type 1 collagen, and fibroblast proliferation lead to increased myocardial stiffness and diastolic ventricular dysfunction[23]. If the papillary muscles and tendinous cords are affected, this can result in atrioventricular valve regurgitation, ECG abnormalities such as atrial fibrillation, conduction issues, and ventricular arrhythmias are observed in approximately 30% of cases [18].
Diagnosis of EMF relies on major and minor criteria, including ventricular apex retraction, thrombi, and valve dysfunction [1]. Cardiac MRI, particularly with gadolinium contras plays a crucial role in differentiating EMF from other restrictive cardiomyopathies, as it provides detailed imaging of structural changes and fibrosis [1]. In our case, the clinical manifestations of the patient were in advanced stages of the disease.
These manifestations led to fibrosis of the left ventricular endomyocardium, resulted in dilated cardiomyopathy following mitral valve regurgitation [24-26], concurrent thrombosis, and endocardial calcification. The diagnosis of EMF was confirmed after surgery, surgical intervention is recommended for advanced cases, despite the high risk of postoperative mortality, however advanced imaging and eosinophil-targeting therapies, like Imatinib, gives a hope in improving EMF better prognosis. However, a better understanding of the disease and increased awareness among healthcare providers are essential for developing early diagnosis, effective treatment strategies to improve patient's outcomes [27].
By presenting a case of endomyocardial fibrosis, this case study aims to demonstrate its many features. In summary, advancements in imaging methods and heart disease therapy at a reference facility help patients with endomyocardial fibrosis receive an earlier diagnosis and have a higher chance of survival.