AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2578-8965/265
1 Department of Gynecology and Obstetrics, University Hospital Cologne, Germany.
2 Institute of Medical Statistics and Bioinformatics, University of Köln, Germany.
*Corresponding Author: Wolfram Jäger, Department of Gynecology and Obstetrics, University Hospital Cologne, Germany.
Citation: Wolfram Jäger, Nami Jamshidian, Andre Päffgen, Anna Hagemeier, Peter Mallmann, (2025), Urgency Urinary Incontinence Is a Consequence of The Decline in Estrogen Levels After Menopause, J. Obstetrics Gynecology and Reproductive Sciences, 9(3) DOI:10.31579/2578-8965/265
Copyright: © 2025, Wolfram Jäger. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 10 April 2025 | Accepted: 18 April 2025 | Published: 25 April 2025
Keywords: urgency urinary incontinence; OAB; CESA; VASA; hypo-estrogenic tissue remodeling; hormone replacement therapy
Background: Urinary incontinence is a chronically progredient condition that usually begins around in the early forties of life with urine leakage through coughing or sneezing (stress urinary incontinence, SUI). Several years later the patients realize that after the feeling of urge to void they may lose urine when waiting too long (urgency incontinence, UUI). In the beginning of UUI patients can still reach the toilet “dry” if they go immediately after the urge to void, however, within few years the time intervals shorten until loss of urine becomes uncontrollable. That has significant impact on the quality of private and social life.
The etiology of UUI is unknown. Therefore, there is no specific therapy for UUI and current treatments are aimed to reduce symptoms.
However, previous studies have shown that these patients with UUI could regain continence by the surgical tensioning and elevation of the vagina and the attached trigone of the bladder. That effect was achieved by the replacement of the uterosacral ligaments (USL) by laCESA or laVASA. An additional suburethral tape led to overall continence rates (CR) between 67% and 87% in these patients. Multivariate analyses revealed that the difference in the continence rates (CR) after laCESA and laVASA was dependent on the age of patients at surgery. Patients younger than 60 years had significantly higher CR than the older patients. The CR after a TOT; however, was independent of the age at surgery.
It has been hypothesized that this development of incontinence is caused by declining estrogen levels already before and after menopause. Therefore, this study was aimed to test the hypothesis that the increasing urinary incontinence from SUI to UUI was associated with menopause and the time interval after menopause.
Material and Methods: All patients were suffering from UUI. The diagnosis and the age at menopause were evaluated in the context of personal interviews. In all patients with UUI, the vagina was elevated and tightened in the longitudinal axis laparoscopically by substituting the uterosacral ligaments (USL) with PVDF-structures of defined length (laCESA, laVASA). If that did not lead to continence, a suburethral tape (TOT 8/4) was inserted some months later.
Results: Between 2010 and 2022, 326 patients with UUI underwent the surgical treatment. After previous SUI they had developed UUI after menopause. Patients who got estrogen or estrogen/gestagen combinations before menopause became incontinent after they had discontinued their hormonal treatment.
In total, between 56% and 87% of the patients became continent after surgery. The CR after tensioning the vagina by laCESA/laVASA were between 46% and 58% in 50-year-old patients decreasing to 16% and 20% in patients older than 70years. An additional TOT 8/4 led to continence in between 43% and 40% of the still incontinent patients irrespective of their age.
Conclusions: In all patients’ urinary incontinence started premenopausally as SUI and continued to UUI after estrogen deprivation, usually after menopause. This continuous worsening of UUI during the years after menopause was probably caused by the estrogen deficiency.
Since the USL, the vagina and the trigone of the bladder express estrogen receptors their physiological function is dependent or influenced by estrogen. Declining estrogen levels lead to a hypo-estrogenic loss of elasticity leading to laxity, particularly in the utero-sacral ligaments (USL) the vagina and the adherent trigone of the bladder. The decreasing CR of laCESA/laVASA with increasing age must be interpreted as a consequence of the loss of elasticity caused by the longer duration of estrogen deficiency especially in the trigone of the bladder. That supported the hypothesis of the NAMS and the ISWSSH that urgency urinary incontinence (UUI) is caused by estrogen deficiency. Patients with perimenopausal estrogen supplementation (HRT) only became incontinent when they stopped HRT. That observation suggested that early and continuous estrogen therapy may delay or even prevent urinary incontinence. As UI turned out to be an endocrine disorder the optimal estrogen, dosage, mode of application and the onset and duration of estrogen therapy should be investigated and defined by gynecological endocrinologists probably in co-operation with urogynecologists in the future.
| CR: | continence rate (percentage of all patients) | laVASA: | laparoscopic vagina-sacropexy |
| GSM: | “genito-urinary syndrome of menopause" | MUI: | stress and urgency (mixed) urinary incontinence |
| HRT: | hormone replacement therapy | NAMS: | North American Society of Menopause |
| ISWSSH: | International Society for the Study of Women’s Sexual Health | OAB: | overactive bladder (synonym for UUI) |
| laCESA: | laparoscopic cervico-sacropexy | PVDF: | polyvinylidene fluoride |
| laVASA: | laparoscopic vagina-sacropexy | PUL | pubo-urethral ligament |
| CR: | continence rate (percentage of all patients) | SUI: | stress urinary incontinence |
| GSM: | “genito-urinary syndrome of menopause" | TOT: | trans-obturator taped |
| HRT: | hormone replacement therapy | UI: | urinary incontinence |
| ISWSSH: | International Society for the Study of Women’s Sexual Health | UUI: | urgency urinary incontinence (UUI) |
| laCESA: | laparoscopic cervico-sacropexy | USL: | uterosacral ligament |
Urinary incontinence (UI) usually begins around in the early forties of life with urine leakage through coughing or sneezing (stress urinary incontinence, SUI). Most patients find this situation less annoying, as it happens seldom and the use of a simple slip pad suffices to maintain participation in social and private life without restrictions. (Figure 1).
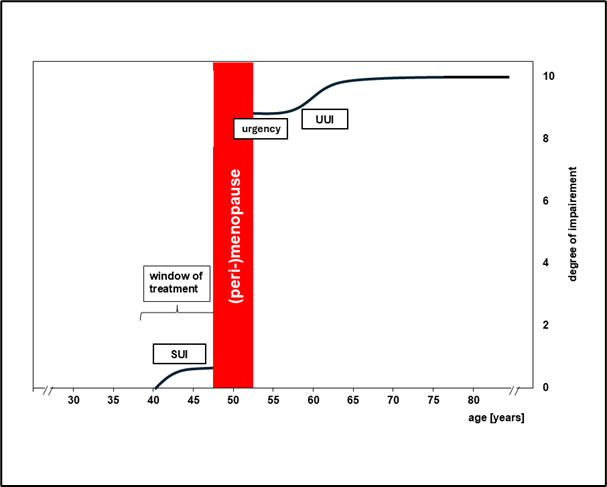
Figure 1: Subjective assessment of private and social limitations caused by different forms of incontinence according to patients’ age. (SUI: stress urinary incontinence; urgency: urgency without urine loss, UUI: urgency urinary incontinence) (The curve corresponds to the degree of limitation of the respective form of urinary incontinence: 0 = no limitation; 10 = maximum limitation)
Several years later the patients realize that if they wait too long after the feeling of urge to void, they may lose urine before reaching the toilet (urgency urinary incontinence, UUI).
Urgency urinary incontinence (UUI), however, imposes substantial limitations on both social and private activities. Initially, patients who experience the urge of a full bladder make the experience that they can still delay voiding and complete their current activities without fear of uncontrolled urine loss. However, within a few years, the time interval between the urge to void and loss of urine shortens and reaches the point when urine loss becomes completely uncontrolled [1, 2].
This uncontrollable loss of urine has significant impact on the quality of private and social life. This development is a severe experience for the affected women. Personal freedom to do what they want in public and private life is suddenly restricted by the bladder [3]. (Figure 1).
The etiology of urinary incontinence and the conditions underlying the transition from SUI to UUI are unknown. Historically, these two conditions were thought to have different etiologies. However, the chronological order of symptoms starting from SUI developing to UUI and finally to total incontinence in all patients indicate that these different forms of UI may have a common etiology. Furthermore, the chronological occurrence of the symptoms at defined decades of life indicated that the etiology of urinary incontinence is caused or associated with common biological processes at these ages.
The surveys of the North American Society of Menopause (NAMS) and the International Society for the Study of Women’s Sexual Health (ISSWSH) revealed that urgency and urgency urinary incontinence (UUI) typically begin after menopause. This association was so close that the NAMS and the ISSWSH have included "urgency" and "urgency urinary incontinence" in the syndrome "genito-urinary syndrome of menopause" (GSM) [4-6].
They assume that both symptoms of incontinence can be attributed to the decline of estrogens after menopause and are therefore a predominant endocrine disorder and not an urological problem [4-6].
Continence or incontinence is determined at the bladder outlet (meatus internus urethrae) which is located in the lower angle of the trigone of the bladder. The trigone of the bladder is the triangle between the two orifices of the ureters and the opening to the urethra (meatus internus). The sphincter urethrae and detrusor vesicae were considered as the crucial muscles for continence [7,8].
Petros demonstrated in the “Integral Theory” that the incontinence during coughing or sneezing (SUI) was not caused by a defective sphincter muscle but by a laxity of the pubo-urethral ligament (PUL) [9]. To increase the counter-pressure normally exerted by the PUL, a tape is placed under the urethra which provides resistance to the meatus urethrae internus during stress. [9]. The suburethral tape is so effective that it has been included in the guidelines for treatment of SUI [10]. However, the reason for this dysfunction leading to SUI and the occurrence in the early fourth decade of life remained unexplained.
As urgency urinary incontinence (UUI) is not dependent of increased abdominal pressure but follows the neurological sensation of an urge to void it was interpreted and treated as a neurological disorder of the detrusor muscle innervation [11-13].
The "urge sensation" (urgency) is triggered by stretch receptors in the bladder wall. In order to reduce the neurological stimulation of the detrusor muscle after urgency different approaches were developed. The pharmacological treatment only reduced the quantity and quality of the symptoms; however, the injection of Onabotulinumtoxin A blocked the detrusor muscle contractions after the stretch and urge sensation but did not lead to a permanent restoration of continence [14-17]. Therefore, significant doubts remained with regard to the neurological etiology of UUI.
A chance observation in operative oncological gynecology demonstrated that even total incontinence as the ultimate expression of UUI could be cured by surgery.
During a posterior exenteration in patients with cervical cancer invading the sigma colon the bladder was preserved. In order to stabilize the bladder suspension, the upper wall of the vagina under the bladder remained inside. For further stabilization of a full bladder the upper (abdominal) end of the vagina was fixed with a mesh to the promontory. During postoperative (cancer) follow-up examinations, patients reported that they had become continent after surgery, whereas they had been completely incontinent before the operation, and no treatment had helped them [18].
The interpretation of that surprising effect on restoring continence after posterior exenteration was that it must have been caused by the surgical tightening and elevation of the vagina. The urethra and the trigone of the bladder are the respective parts of the urinary system which are attached to the vagina and must have been affected. Therefore, the stretching and elevation of the vagina in the longitudinal axis must have increased the closing pressure at the meatus internus. Beside the longitudinal stretching of the trigone the elevation of the vagina took the trigone in a more vertical axis when standing [1] (Figure 2).
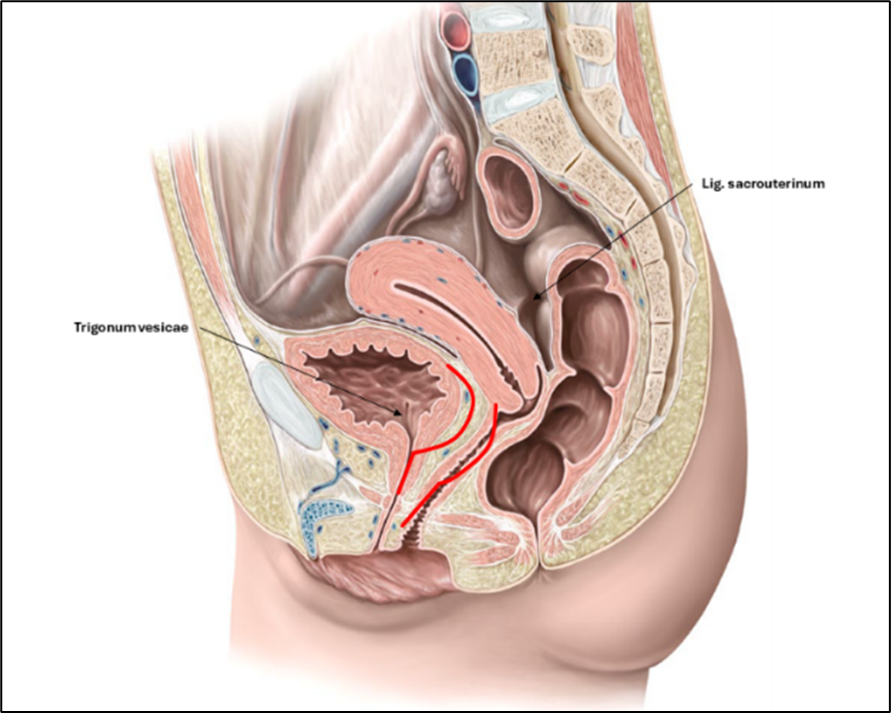
Figure 2: Hypothetical assumptions about how the anatomy of the trigonum vesicae and the anterior vaginal wall changes with the descent of the uterosacral ligament (USL) and the anterior vaginal wall with the trigone of the bladder and the area of the meatus internus urethrae (red lines).
Furthermore, the disappearance of urgency and urgency incontinence indicates that the trigone is of substantial importance in that respect. However, it could not be explained why the timespan between first urge to void and controlled micturition returned to normal immediately after surgery.
To offer this new treatment option to other patients with UUI, a new surgical procedure had to be developed which was based on the normal anatomy of the female pelvis. The vagina adheres to the cervix. Therefore, to tighten the vagina, the cervix had to be elevated. Because the cervix is held by the uterosacral ligaments (USL), it was decided to replace the USL by tapes [19-21].
In order not to interfere with “normal” anatomy and physiology it was decided to replace the USL by tapes with the identical length of the biological USL. To evaluate how continence could be achieved in patients with UUI the surgical procedures had to be standardized so that they could be performed identically in every patient.
Therefore, the most important aspects of that development were that only when all patients had the same symptoms of UUI and got the identical treatment the results could be compared between patients and analysed [22].
Due to the uniform dimensions of the bony pelvis in women, it was possible to develop USL PVDF-implants of identical length for all patients (Dynamesh-CESA: 9.3 cm, Dynamesh-VASA: 8.8 cm, Dahlhausen, Köln, Germany) [23]. The fixation points of the implants (structures) on the sacrum and the vagina or cervix were also precisely defined and marked on the structures. [23] Therefore the surgical performance of CESA and VASA (laparoscopical or by laparotomy) will always be identical [23].
The surgeries were developed for patients with a uterus (uterosacropexy, CESA) and already hysterectomized patients (vaginosacropexy, VASA) (Figure 3). In patients with a uterus, the corpus uteri was removed, and the structures were sewn to the physiological attachments at the cervical stump [24]. Since 2016, the abdominal surgeries were performed laparoscopically (laVASA, laCESA) [25, 26].
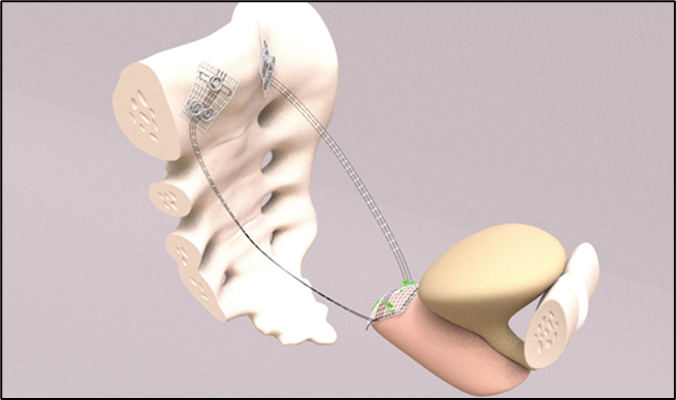
Figure 3: Schematic representation of the VASA structure in situ. Fixation of the structure to the vaginal stump and the "USL arms" to the promontory. The fixation sides are marked on the structure.
Between 2010 and 2022, 326 patients with UUI underwent surgical treatment. The overall CR after laCESA was 39% and after laVASA 33% [27]. The additional TOT led to continence in between 35% and 45% of the remaining incontinent patients [27]. In order to evaluate the reasons for the different outcome after surgery all relevant clinical data were statistically analysed. The multivariate analyses revealed that the only significant factor for getting continent after laCESA or laVASA was the age of the patients at the time of surgery. The statistical analyses proposed that the medium age at surgery was 60 years to separate patient’s outcome. The CR for patients younger than 60 years was 46% and for the older patients 28%. That difference was statistically significant [27].
The CR of an additional TOT 8/4 was between 40% and 43% - irrespective of the age of the patients [27].
The dependency of age on the outcome of surgical treatment of UUI had never been reported before. This observation supported the hypotheses of the NAMS and the ISSWSH underlying the “genito-urinary syndrome of menopause” (GSM) which stated that urgency and UUI are caused by the deprivation of estrogens after menopause.[4]. Therefore, it was decided that the results of surgery should be re-evaluated according to the time-interval after menopause.
Since 2010 all patients with UUI who failed conservative treatments were operated by laCESA or laVASA in a clinical Phase II study.
The clinical results were controlled in interviews and documented in questionnaires during follow-up examinations. According to the recommendations of the Institute of Medical Statistics the standard questionnaires for urinary incontinence were adopted in the way that every question could be answered by “yes” or “no” or a definitive number [27].
Urgency urinary incontinence (UUI) was defined as the condition when patients could no longer reach the toilet "dry" when experiencing the urge to urinate. Patients reported that upon the feeling of urge, they could not wait for the weather forecast during television news, as they would lose urine before reaching the toilet [27].
Based on the observation that all patients had SUI as the first symptom before UUI and that the etiology of SUI was a lax PUL, all patients should get a TOT.
However, it had already been noticed in a previous Clinical Phase 1 study that several patients, even patients with mixed urinary incontinence (MUI), became continent just after CESA or VASA and did not need a TOT anymore [18]. Therefore, it was decided to begin treatment with the abdominal part (laCESA, laVASA) and add the vaginal part (TOT 8/4) three months later if needed [18, 27-30].
Continence was defined as controlled micturition without any loss of urine irrespective of the time interval after the urgency sensation.
All patients were asked to determine the age at which they experienced the uncontrolled loss of urine for the first time (SUI) and when they realized that they lost urine short after the urgency sensation (UUI).
Besides the usual clinical examinations, the patients were also examined vaginally in standing position, because they were not incontinent in lying position. Examinations with a full bladder could not be performed therefore all data of the examinations were obtained in patients with a “not-maximally-filled” bladder.
The incontinence-questionnaire were explained to them and the answers documented. Beside the questions about losing urine under stress (coughing, sneezing getting up from sitting, walking) patients were also asked what they would do when they feel the urge to void during TV-news. Could they wait until the weather forecast or did they need to go immediately? Did they reach the toilet “dry” or did they lose urine on the way. The latter answer was an indication for UUI.
Additionally, to these urogynecological questions patients were asked about her age at menopause. Patients who were hysterectomized before menopause were asked about the age when they experienced hot flashes. They were further asked whether they had got peri- or postmenopausal hormone replacement therapy (HRT) and for how long.
Additionally, patients were interviewed about their family history of incontinence, especially about her mother.
All these data were recorded in a computer program at the day when they came to the clinic for the first time and during follow-up.
This study was focused on the descriptive analyses of the target variable “continence” as well as of the influencing variables “age at surgery”, “time-interval to menopause” and “kind of surgery (CESA or VASA)”. Age at surgery was subdivided in the timespans after menopause in patients unconnected samples.
To test significant differences of the categorial variables the Χ² test was applied for univariate analysis of significance. If the sample size (n) was applied Fisher´s exact test was applied. The level of significance α was defined at 0.05 therefore p<0>
For calculations the IBM SPSS Statistics Program (version 26) was used. All calculations were performed at the Institute for Medical Statistics and Bioinformatics, University of Köln, Germany [27].
The Clinical Trial was approved by the Scientific Committee of the University of Köln and the Ethic Committee of the University of Köln (approval number: TN 20-1267). The current analysis was approved on June 29th, 2022 (nr.20-2312) [27].
During the observation period 326 patients with UUI fulfilled the study criteria [27,29,30]. Since all patients of the study were postmenopausal the CR were analysed according to the time interval after menopause.
Incontinence began between the ages of 40 and 45 with urine loss during coughing and sneezing ("stress incontinence", SUI). Most patients experienced menopause at the age of 50 years. 24 patients were between 40 and 49 years. They became amenorrhoeic after chemotherapy for cancer treatment or surgical removal of the ovaries for different indications (endometriosis, ovarian cysts, family history of ovarian cancer).
After cessation of ovarian function, patients made the experience that after the urge to void they sometimes lost urine before reaching the toilet. After about 2 to 4 years, they experienced urine loss already upon standing up from a chair or on the way to the toilet. They never lost urine when sitting on the chair.
Overall, between 72% (laVASA) and 87% (laCESA) of patients under 60 years of age regained continence by the replacement of the USL and a suburethral tape (TOT 8/4).
In older patients (older than 60 years at surgery) continence rates (CR) ranged between 56% and 63% [27].
In the multivariate analysis patients´ age at surgery was the only factor with significant influence on the CR [27].
The proportion of patients who regained continence solely through elevation and tensioning of the vagina via laCESA or laVASA decreased significantly with increasing time since menopause (or stop of ovarian function). While between 46% and 58% of younger patients became continent, the corresponding proportion in older patients was between 16% and 20% (p<0.001) (Figure 4)
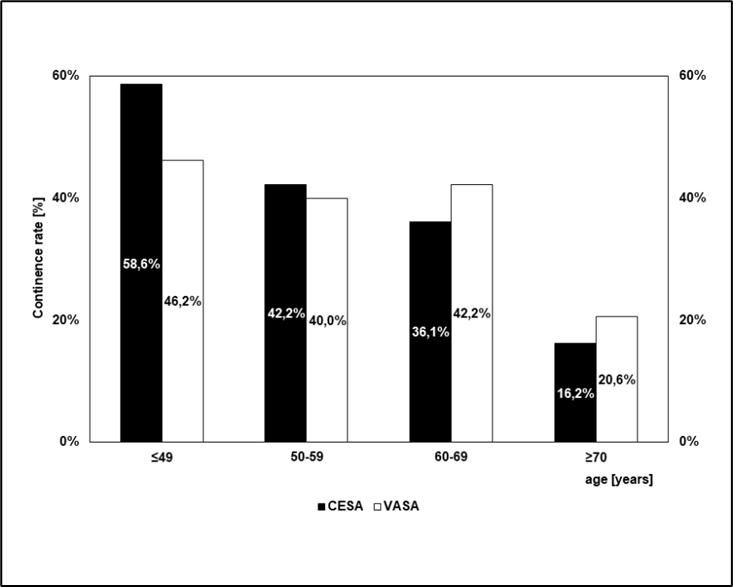
Figure 4: Continence rates after CESA or VASA depending on the age at the time of surgery.
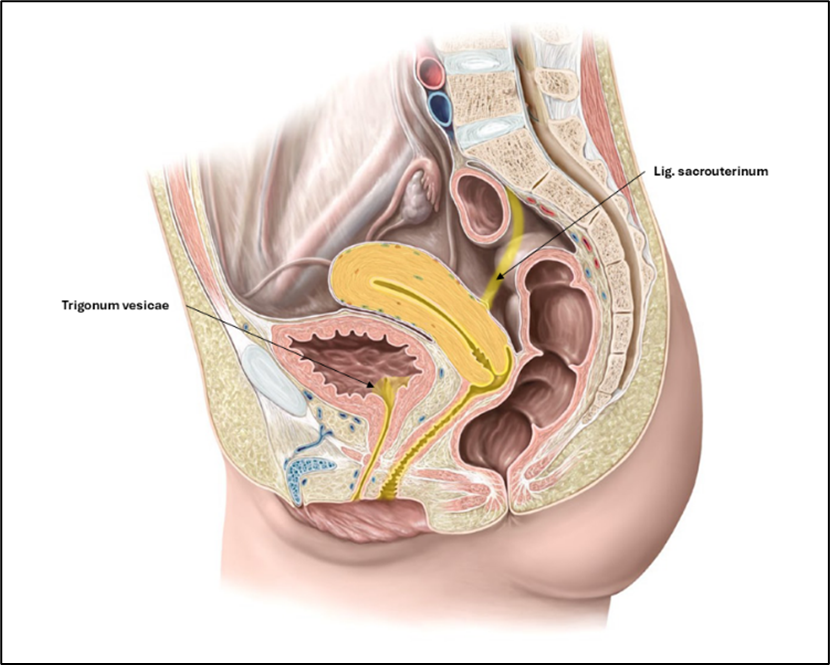
Figure 5: Distribution of estradiol receptors (ER) in the pelvis. ERs are primarily located in the uterosacral ligament, vaginal wall, endo-and myometrium, urethra, and trigonum vesicae.
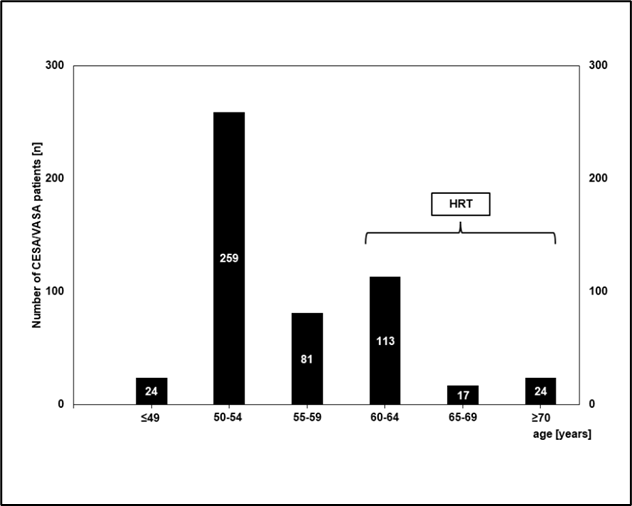
Figure 6: Age at which patients developed urge incontinence. The total number of patients in each age group is indicated in the respective columns.
Patient groups that received HRT are indicated by the bracket. (For this analysis, data from patients who received both a CESA or VASA and a TOT were also included in accordance with the study objective.)
The CR after the transobturator tape (TOT 8/4) was independent of patients’ age at surgery [27].
97% of patients reported that their mother also had UUI. As far as they knew that was probably at the same age when their own incontinence began.
The results of the Phase II study confirmed the observation of NAMS and ISSWSH that UUI is a disorder of menopause. All patients had experienced SUI as the first symptoms of UI.
That always started in their forties, however, the first symptoms of UUI appeared after menopause. The NAMS and the ISSWSH assumed that UUI is caused by the estrogen deficiency after menopause. Considering this hypothesis SUI and UUI are not two different etiological entities but probably the result of a continuous development (“pathophysiological continuum”).
The beginning of SUI in the forties of the patients does not necessarily disprove the estrogen dependency theory of UI. Urinary incontinence is a typical symptom of the premenstrual syndrome [31]. Incontinence in these women is definitively explained by hormonal changes during the menstrual cycle especially during the luteal phase of the cycle [31]
That gives a hint that urinary incontinence is not a urological disorder but a consequence of hormone imbalance. [4,5,31,32]
In principle, UUI could be cured by the replacement of the USL by laCESA or laVASA sometimes in addition with the replacement of the PUL by a suburethral tape (TOT 8/4).
The surgical elevation and tensioning of the vagina by laCESA or laVASA probably leads to the compression of the meatus [27]. However, the number of patients who regained continence after laCESA or laVASA decreased significantly with increasing age after menopause. That age dependency of CR was not observed after the suburethral tape (TOT 8/4).
Since the PVDF-USL structures (Dynamesh CESA/Dynamesh VASA) all had the same length and were always sutured to the same defined anatomical sides the differences in continence rates after laCESA or laVASA, e.g., between 50-years-old and 70-year-old patients, must have been due to structural changes of the vagina or the trigone of the bladder (or both).
The probable effects of estrogen deficiency on the urethra were not evaluated.
The continence restoring effects of a TOT 8/4, however, were independent of age after menopause. That was reasonable, considering that the TOT replaced the function of the PUL. However, if UI is based on estrogen deficiency than SUI should also have the same etiology. Since the Integral Theory has demonstrated that SUI is caused by a lax PUL it seems reasonable to assume that the PUL and the lower trigone are (one of) the first structures affected by estrogen deficiency [9]
While a suburethral tape can cure SUI, this method alone is not capable of curing UUI. Considering the 17% CR in 70-year-old patients after laCESA or laVASA, but the additional 40% of patients who became continent at this age following the TOT, a clinical Phase I study was started in patients older than 70 years with UUI with the initial placement of the TOT 8/4. However, that study had to be discontinued after 10 patients, as none of the older patients became continent after the TOT alone. This observation strongly indicates that the effect of the suburethral tape is restricted to the area below the meatus internus. It demonstrated that UUI is a problem of the trigone including the meatus internus which are not directly touched by a TOT. The results implicate that a suburethral tape (e.g. TOT) can probably only be effective for treatment of SUI as long as the trigone and the vagina are not submitted to the hypoestrogenic tissue remodelling.
Therefore, urgency and the accompanying incontinence must be triggered in the area of the trigone and the adherent vaginal wall. The beginning of UUI after menopause implicates that the decline of estrogens must affect these structures. Immunohistochemical studies supported that assumption. It has been shown that estrogen receptors (ER) are expressed in the uterus, the endometrium, the USL and the vaginal epithelium. Beside these structures, however, ER were also found in the trigone of the bladder and in the epithelial lining of the urethra [33-35] (Figure 5).
ER in these anatomical structures indicate that estrogens must be important for the physiological function of these structures [36].
It is probably the ability of estrogens to form elastic hyaline structures. Elasticity is the ability to stretch and to contract. That elasticity is of crucial importance for the uterus, the USL and the vagina during pregnancy. [4,5,37].
Beside the increase in uterine size during pregnancy the USL must increase their elasticity in order to let the cervix descend in the pelvis. Extremely important is the increasing elasticity of the vagina. The elastic capacity of the vagina is enormous considering that until delivery the circumference of the vagina must stretch to approximately 38 cm (97 inches) for the passage of the child [38]. When the child during birth reaches the lower vagina where the bladder trigone is adherent, not only the vaginal wall must stretch but also the trigone of the bladder in order to prevent tearing of the bladder during the passage of the child's head. Finally, the urethra must also be able to stretch longitudinally during the child's exit out of the birth canal.
During pregnancy, parallel to the child´s growth the concentrations of estrogens increase massively [35]. These increasing amounts of estrogen needed for uterine growth and the other mentioned anatomical structures until birth cannot be produced by the ovaries and therefore becomes a significant task of the placenta. The increase in estrogen levels during pregnancy, particularly in the last trimester, supports the assumption that estrogens are crucial for the aforementioned increase in tissue elasticity [39, 40].
During the puerperal phase all these structures have to return to their normal size and elasticity.
This phase is accompanied by decreasing estrogen levels after removal of the placenta until the elasticity returns to normal.
It can be assumed that the declining levels of estrogens around or after menopause will lead to a loss of elasticity in all of these estrogen-sensitive tissues. This can lead to a reduced tension of the tissues (laxity) or a shrinkage. These effects of estrogen deprivation are established knowledge in vulvovaginal atrophy as well as in pelvic organ prolapse but only recently the decline of estrogens was associated with the development of urinary incontinence [3-5]. With the increasing period of estrogen deficiency after menopause the elasticity of the trigone will continuously decrease leading to a laxity of the trigone which does not allow further stretching when the bladder fills. Therefore, the time interval between the sensation of urgency and urine discharge also shortens continuously. The additional effect of the laxity of the USL will lead to a reduced suspension of the vagina bringing the meatus internus in a more horizontal axis especially when standing up in an upright position. [Figure 2]
Taking into consideration the observations of the study, UUI and probably also SUI – and therefore urinary incontinence - are based on declining estrogen levels. That was at least in part the hypothesis of the NAMS and the ISWSSH [4,5]
That leads to the hypothesis that the laxity of the PUL is also a consequence of decreasing estrogens before menopause. That seems to contradict the estrogen deficiency theory, but urinary incontinence is a classical symptom of the premenopausal syndrome which is definitively caused by hormonal changes [31]
That would offer a hypothesis why SUI usually appears around the forties of life and not at any other time of life.
The exact prevalence of UUI is not known. If the described pathogenesis is correct, UUI would be expected in every woman after menopause. However, only about 30% of women in postmenopause are affected, possibly slightly more assuming some underreporting [3,10, 41,42]. The result of the survey within the study that 97% of patients reported the mother was also incontinent suggests a genetic predisposition. Recent genetic studies support that assumption [42,43].
The decision of the NAMS and ISSWSH to include the symptoms "urgency" and "urgency urinary incontinence" in the Genito-urinary Syndrome of Menopause (GSM) to express that both symptoms of incontinence can be attributed to the decline of estrogens after menopause were fully supported by the results of the study [2, 44-49]. Based on these ideas, treatment should consist of substituting ovarian steroids [50–53]. However, it has not been determined when to start HRT and how long it should be continued.
The first analyses of several studies of "hormone replacement therapy" (HRT) in postmenopausal women, which included information about the incontinence status of patients during HRT contradicted the effectiveness of estrogens to influence urinary incontinence. [53]. These studies demonstrated that UUI cannot be influenced or improved by HRT which suggests that the hypoestrogenic laxity of the trigone is not reversible anymore by hormones after menopause [53]. These patients will need surgical treatment.
Nevertheless, the study provided evidence that such an approach appears sensible and additionally offers a promising perspective. Most patients (67%) developed the urge symptoms between the ages of 50 and 56 (Figure 6). However, 33% of patients developed UUI between the ages of 57 and 60 years. These patients reported being treated perimenopausally with various estrogen preparations due to a "dry vagina" or hot flashes. When they discontinued these treatments for their own reasons (fear of breast cancer) after a few years, UUI occurred shortly thereafter.
An interesting perspective emerged from the 41 patients who developed incontinence around the age of 70 (Figure 6). These patients reported being ovariectomized between the ages of 45 and 50 for various indications. They were given various oral estrogen/progestogen combinations and advised to continue this medication until the age of 65 or 70. When these patients discontinued therapy at the recommended age, they became incontinent within 1 to 2 years.
The observation that patients remained continent as long as they continued estrogen substitution suggests that the onset of incontinence can be delayed or even prevented by substituting ovarian steroids. This substitution should then be lifelong, as incontinence otherwise begins delayed in older age. Treatment should start early, before estrogen deficiency leads to hypoestrogenic tissue remodeling. If urinary incontinence starts to bother the patients a return to continence by estrogens or progestogens is not possible anymore, according to our current knowledge. [46].
There is a familial disposition to incontinence.
It has been shown that urinary incontinence has a familial predisposition [53-55].
The familial predisposition to incontinence is a clinical important observation. This suggests that daughters of a mother with UUI have a significantly increased risk of becoming urgency urinary incontinent after menopause as well. Early substitution of estrogens to prevent incontinence would mean starting at an earlier age in the daughter than the mother became her first symptoms of urinary incontinence (SUI). If such "prophylactic" substitution is rejected by the daughter, this decision should be reconsidered at her first symptoms of SUI (urine loss when coughing). It is then known that the patient is on her way to UUI. Therefore, HRT should be started at that time. If the patient accepts to get a suburethral tape in that situation it would be interesting to evaluate the effects of HRT on further development of continence/incontinence.
Starting HRT when the patient has already developed urgency symptoms (” when I feel I should go – I must go immediately!”) does not improve incontinence as respective HRT studies have shown [54].
Hormonal substitution for the prevention of incontinence has not yet been performed. Based on current observations, estrogens should be used [56,57] The usual aspects of conducting HRT must be considered. Uncertainties remain regarding dosage, the mode of application and ultimately the choice of ovarian steroids. This should be investigated in randomized trials which take several years. In the meantime, until the results of these studies are available, patients should nevertheless be offered a local (vaginal) treatment with estrogens. The biological effects of estrogen treatment can be controlled by vaginal smears during regular patient check-ups [57]. Clinical symptoms of UUI can be documented in simple questions (“what do you do after an urge sensation during the news when you want to see the weather forecast?”).
The prevention of incontinence would be invaluable. A possible "incontinence prophylaxis" would be a new extension of gynecological-endocrinological therapy. Thus, urinary incontinence extends from the reactive-surgical treatment spectrum of gynecology into the preventive-hormonal spectrum of gynecological endocrinology. Several clinical studies will be necessary in the future. Overall, these insights present promising perspectives and tasks for the fields of gynecological urology and especially of gynecological endocrinology in future.
Urinary incontinence (UI) is a genetically predisposed disorder. It follows a symptomatically and chronological sequence starting with stress urinary incontinence (SUI) leading to urgency urinary incontinence (UUI) after menopause.
That pathogenesis is hypothetically caused by the decline of estrogen levels during the pre-, peri- and postmenopausal phases of life. Urinary incontinence is based on the uncontrolled loss of urine through the meatus internus of the bladder. The meatus is the lower angle of the trigone of the bladder, the area between the orifices of the ureters and the meatus internus of the urethra. The trigone is the only part of the bladder which expresses estrogen receptors (ER), indicating that estrogen is needed for the physiological function of the trigone. This physiological function is elasticity in order to stretch that part of the bladder during filling and to contract at the meatus when stress is exerted.
With decreasing estrogen levels, the elasticity diminishes what leads to laxity or shrinkage of the estrogen responsive tissues.
Furthermore, when the bladder fills, the trigone continuously loses its elasticity leading to an ever-shorter interval between urgency and urine loss.
The clinical consequence is uncontrolled loss of urine after the urge sensation (UUI).
UUI can surgically be cured by the standardized replacement of the lax USL by laCESA or laVASA and the replacement of the PUL by a TOT 8/4. However, the continence rates decrease significantly after menopause during each decade thereafter. That indicates that the postmenopausal hypoestrogenic tissue remodelling probably at the trigone cannot be reversed by these surgical procedures anymore.
It was an important observation that patients who had a previous hormone replacement therapy (HRT) starting before menopause developed UUI only when they stopped HRT. Therefore, early estrogen substitution can probably prevent the tissue remodelling leading to UUI.
HRT should start at the same age as SUI started in the mother. HRT should be continued lifelong. The methods of application, preparation, or dosage of the estrogen should be studied in future clinical trials by gynecological endocrinologists.
The authors thank Dr. Ludwig, who, together with Dr. Morgenstern, developed and performed the laparoscopic surgical techniques (laCESA, laVASA). The authors also thank Ms. E. Neumann for data documentation and her always open ears for all the wishes and concerns of the patients.
W. Jäger states that there is no conflict of interest regarding the claims made in the manuscript. He receives licensing fees for the structures.
All our studies had been approved by the Ethics Committee of the University of Köln.