AUCTORES
Globalize your Research
Case Report | DOI: https://doi.org/10.31579/2690-8808/247
1 Physician, specialist in Medical Oncology, Department of Medical Oncology, Regional Institute of Neoplastic Diseases (IREN) – Center, Concepción, Peru
2 Physician, specialist in Radiology, Department of Diagnostic Imaging, Regional Institute of Neoplastic Diseases (IREN) – Center, Concepción, Peru.
3 Physician, subspecialist in Pediatric Oncology, Pediatric and Adolescent Oncology Unit, Edgardo Rebagliati Martins National Hospital, Lima, Peru
*Corresponding Author: Zegarra-Cárdenas, J.A, Department of Medical Oncology, Regional Institute of Neoplastic Diseases (IREN) – Center, Concepción, Peru.
Citation: Zegarra-Cárdenas, J.A., Ugarte-Castillo, F.A., Paredes-Guerra, G., (2025), Trastuzumab-Induced Interstitial Lung Disease: A Case Report, J, Clinical Case Reports and Studies, 6(3); DOI:10.31579/2690-8808/247
Copyright: ©, 2025, Zegarra-Cárdenas, J.A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 15 February 2025 | Accepted: 24 February 2025 | Published: 06 March 2025
Keywords: breast cancer; trastuzumab; diffuse interstitial lung disease; case report
We present the case of an adult woman, with a history of controlled hypothyroidism and osteoporosis, who comes to the institute with a clinical picture of a tumor in the right breast. Chest tomography showed bronchiolectasis; metastatic subcarinal adenopathy; tumor in the right breast and infiltrative axillary lymphadenopathy; a breast biopsy was performed with results compatible with pure HER2 subtype NOS carcinoma. It was classified as oligometastatic disease, so she began neoadjuvant therapy with adriamycin/cyclophosphamide for 4 courses and trastuzumab/paclitaxel for 4 courses. Then, she underwent conservation surgery, obtaining a complete pathological response, and continued with trastuzumab until progression. On the fifth course, she developed cough and progressive dyspnea; The imaging study revealed severe diffuse interstitial lung disease that was refractory to treatment, leading to the patient's death. It is suggested to take into account that the use of trastuzumab can trigger diffuse interstitial lung disease that can be fatal.
Breast cancer is the most common cancer and the leading cause of cancer death in women worldwide [1]. Approximately 15-20% of patients carry amplification and/or overexpression of the ERBB2 gene, associated with aggressive biology, poor survival outcomes and diminished responses to standard therapy [2]. The development of anti-HER2 therapies has improved patient survival, with trastuzumab generally being well tolerated, however, there is a proportion of patients who develop adverse events, such as cardiac dysfunction and anaphylactic reactions [3].
Drug-induced interstitial lung disease (DIILD) accounts for 3-5% of interstitial lung diseases (ILD), with antineoplastic therapy being the main cause [4]. Trastuzumab-associated DIILD is a very rare complication, with an approximate incidence of 0.5%, and a mortality rate of around 20% [5]. DIILD occurs when exposure to a drug produces inflammation and, sometimes, pulmonary fibrosis; it is diagnosed based on: clinical, pathological and radiological findings consistent with ILD; temporal relationship between the onset of symptoms and exposure to the drug; absence of other more probable causes such as infections, pulmonary edema, radiation-induced lung injury; and, improvement with the discontinuation of the probable causative agent with or without corticosteroid therapy [4]. Although it is true that the presentation is very rare and the diagnosis is by exclusion, it must always be considered when antineoplastic therapy and monoclonal antibodies are used. We present the case of a patient with breast cancer with a single mediastinal metastasis (oligometastatic) luminal B subtype with HER2 overexpression, who received neoadjuvant therapy and had a complete pathological response after surgery. After five courses of trastuzumab, she developed severe ILD that led to death.
A 65-year-old female, postmenopausal, natural from Huancayo, with a history of hypothyroidism under treatment with levothyroxine and osteoporosis, was evaluated at another institution for a 3-month-old tumor in the right breast. She brought a mammogram reported as BIRADS-4b and a biopsy result that reported a ductal carcinoma type NOS; therefore, she came to our institution for Breast and Soft Tissue Surgery in September 2022.
Clinical findings
Physical examination revealed a 2 cm mobile right axillary adenopathy and a 4 cm tumor in the right breast at the level of the external quadrants, with nipple retraction.
Calendar
The patient was diagnosed with clinical stage IV right breast cancer due to a single (oligometastatic) mediastinal (subcarinal) adenopathy, Luminal B subtype with HER2 overexpression in October 2022 (Figure 1). She received neoadjuvant therapy from 10/19/2022 to 04/05/2023, and was evaluated with tomographic studies in April 2023, which showed incipient interstitial lung disease and complete clinical response (Figure 2). She underwent right breast quadrantectomy + sentinel node biopsy dual method: lymphography + blue patent at another institution on 04/24/2023, showing a complete pathological response. Five courses of trastuzumab were administered from 05/25/2023 to 08/17/2023 and external radiotherapy to the residual breast from 06/19/23 to 07/24/23.
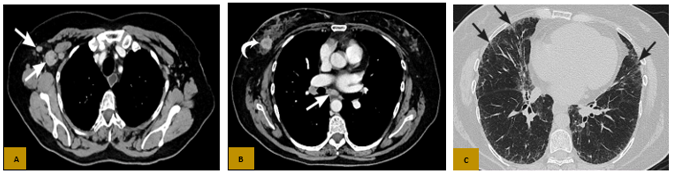
Figure 1. Axial CT with contrast in the mediastinal window (A and B) shows a tumor in the right breast with axillary and subcarinal lymphadenopathy (white arrows in A and B); and the parenchymal window (C) showed nonspecific diffuse subpleural parenchymal reticulations (black arrows).
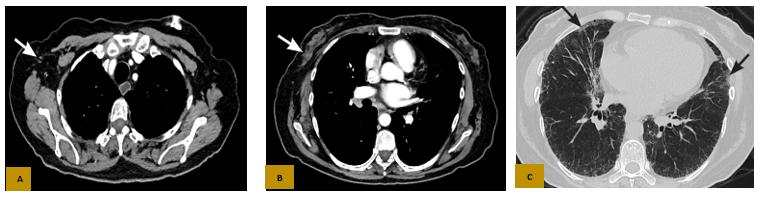
Figure 2: Axial CT with contrast in the mediastinal window (A and B) shows complete imaging response in the chest wall (white arrow in B), no axillary or mediastinal lymphadenopathy (white arrow in A), and the parenchymal window (C) shows incipient signs of Non-Specific Interstitial Lung Disease (NSIL) predominantly in the middle lobe and left lower lobe (black arrows).
Diagnostic Evaluation
The initial Multislice Spiral Tomography of the Chest, Abdomen and Pelvis (Figure 1) reported nonspecific diffuse subpleural reticulations; metastatic subcarinal adenopathy of 12 mm; neoformative mass in the right breast region of 20x19mm, associated with metastatic ipsilateral axillary adenopathy of 19x20mm; in addition, the institutional slide review was performed, which reported an infiltrating ductal carcinoma, non special type (NST), Histological Grade 3, with immunohistochemistry Estrogen Receptor (ER) Positive (2+/3+, 95%), Progesterone Receptor (PR) Negative (0+/3+, 0%), HER2 Positive (3+), Ki67 60%. She was evaluated by Cardiology for the start of chemotherapy, her echocardiography showed an Ejection Fraction (LVEF) of 62%. A qualitative SARS-COV-2 Antigen Detection Test was performed before treatment, which was negative.
Therapeutic Intervention
The patient received chemotherapy with Adriamycin 60mg/m2 - Cyclophosphamide 600mg/m2 scheme every 21 days for 4 courses (10/19/2022 - 12/22/2022) and continued with Trastuzumab 8mg/kg scheme the first course, then 6mg/kg for 3 more courses, every 21 days together with Paclitaxel 80mg/m2, for 12 weeks (01/12/2023 - 04/05/2023), with complete clinical response. Once the systemic treatment was completed, she underwent right breast quadrantectomy + sentinel node biopsy dual method: lymphography + blue patent at another institution on 04/24/2023, whose institutional slide review showed no malignant neoplasia in the tumor or in the sentinel node, showing a Complete pathological response (RCB: 0). The case was discussed at the Medical Board of the Multidisciplinary Committee on Breast and Soft Tissue Tumors, where it was concluded that trastuzumab associated with tamoxifen (due to osteoporosis) would continue until disease
progression, and external radiotherapy to the residual right breast. Therefore, five courses of trastuzumab were administered, the first course at a dose of 8 mg/kg, then 6 mg/kg for the next 4 courses every 21 days (05/25/2023 to 08/17/2023) and, External Radiotherapy at a dose of 5000 cGy in 25 sessions to the level of the right breast and ipsilateral axillary and supraclavicular region and 5625 cGy to the surgical bed in integrated IMRT BOOST, from 06/19/2023 to 07/24/2023.
After the fourth course of trastuzumab, the patient was evaluated by the Pulmonology Department for a clinical picture of cough and mild dyspnea. She was prescribed inhalation therapy for 14 days, with clinical improvement.
Seven days into the fifth course of trastuzumab, the patient presented with nonproductive cough, chest pain, and dyspnea. Physical examination revealed fine basal crackles in the right hemithorax. A chest TEM without contrast was requested, which showed thickening of the interlobular and subpleural intralobular interstitium with honeycombing areas, related to diffuse chronic interstitial disease (Figure 3). Based on these results, the patient was diagnosed with Diffuse Interstitial Pneumonitis Grade 2, and was prescribed prednisone 1 mg/kg/day for 14 days, and treatment with trastuzumab was discontinued. A bronchofibroscopy was performed, and cultures of common germs, fungi, bacilloscopy tests, and BK culture in bronchoalveolar lavage (BAL) were requested, which were negative. In addition, cytopathology tests (Pap smear - PAP) and a cell block in BAL were requested, as well as a transbronchial biopsy, the results of which showed a cellular smear made up of macrophages and lymphocytes, and the biopsy showed a pulmonary epithelium and stroma with mild chronic inflammatory infiltrate.
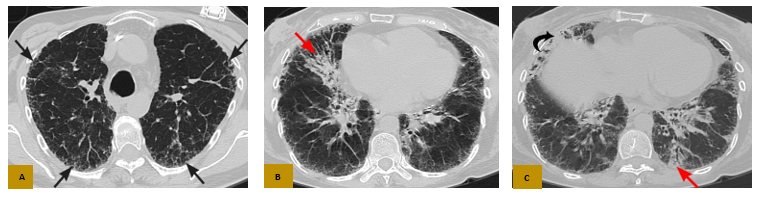
Figure 3: Axial CT in parenchymal window (A, B and C), shows thickening of the interlobular and intralobular interstitium at the subpleural level (black arrows in A) with some areas of incipient consolidations (red arrows in B and C) as well as honeycombing (black arrow in C), in relation to chronic diffuse interstitial lung disease.
At the end of September 2023, a multidisciplinary medical board meeting was held, which determined that the patient had grade 2 pneumonitis associated with trastuzumab, so she would continue treatment with prednisone 1 mg/kg/day for six weeks, and then restart treatment with trastuzumab. After three weeks of treatment with corticosteroids, she was admitted to the emergency room due to severe dyspnea, productive cough with greenish expectoration, oxygen saturation of 77%, and tachycardia. Auxiliary tests were requested, which showed leukocytosis without left shift and a C-reactive protein value of 132. A chest TEM without contrast was performed on 10/19/23 (Figure 4) which showed a marked subpleural interstitial thickening in both lung fields, with multiple bronchiectasis
predominantly in the LDR, in addition to ground glass areas, configuring a crazy paving pattern, classified as a severe superinfected Unspecified Interstitial Lung Disease (UNILD). She was re-evaluated by Pulmonology who indicated treatment with methylprednisolone 1 mg/kg/day every 8 hours for 48 hours, meropenem 1 g IV every 8 hours and oseltamivir 75 mg every 12 hours, for 5 days, in addition to oxygen therapy by Venturi mask with FiO2 50%. She was hospitalized in the Medical Oncology Service, and after 2 days of hospitalization, she presented clinical deterioration and increased oxygen requirement, so she was transferred to the Intensive Care Unit. Patient on mechanical ventilator, with hemodynamic instability, and poor ventilatory pattern, died on 10/24/23 due to respiratory arrest.
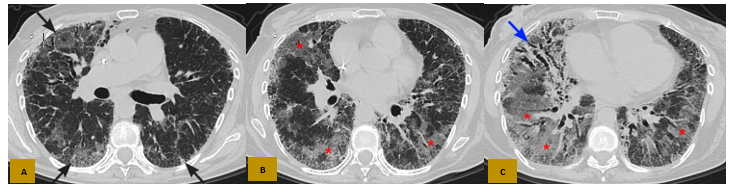
Figure 4: Axial CT in parenchymal window (A, B and C), shows subpleural interstitial thickening in both lung fields with basal predominance (black arrows in A), with multiple bronchiectasis predominantly in the right lower lobe (light blue arrow in C) and extensive ground-glass areas that form a crazy paving pattern (asterisks in B and C).
Trastuzumab is the standard treatment for HER2-enriched and luminal breast cancers with HER2 overexpression. There are many studies evaluating its safety and efficacy, but few report diffuse interstitial pneumonitis as an adverse reaction [6]. In the NSABP B-31 study, there were 1015 patients treated with trastuzumab-containing regimens, of which, four patients with interstitial pneumonitis were reported [7]. The few cases reported worldwide did not indicate a correlation between patient history, dosing regimen, or symptom onset, and all were evaluated extensively, with no association with other causes of ILD being evident. Within these reports, those described by Radzikowska et al [8], Bettini et al [9], Costa et al [10], Sugaya et al [11] and Errisiruz et al [12], closely resemble the case we documented, in relation to the time of presentation, the clinical picture and the resolution of the symptoms.
It is known that, among the highest risk factors for DIILD, we have previous treatment with multiple chemotherapy or radiotherapy regimens, history of radiation pneumonitis, lung cancer, lung metastases, male sex, and age over 60 years. DIILD has been classically reported with bleomycin and mTOR inhibitors, and, to a lesser extent, with taxanes, gemcitabine, and anti-HER2 therapy [13]. In our case, the confluence of the various risk factors (chemotherapy, anti-HER2 therapy, and radiotherapy) and the incipient NSIP detected in previous studies, could lead to a more serious association between interstitial lung disease and exposure to trastuzumab. The pathogenesis of DIILD is not well known, but cytotoxic and immune-mediated mechanisms may be involved, alone or in combination, depending on the drug; Direct damage to pneumocytes or alveolar endothelial cells, cell-mediated lung injury, oxidative stress, and systemic release of cytokines may contribute to DIILD [14].
It is important to mention that, as trastuzumab is a widely used treatment in patients with breast cancer in early and advanced stages, documenting adverse reactions has a scope for public health due to the after-effects that remain after treatment, generating restrictive respiratory functional alterations, especially in elderly patients, with previous diffuse interstitial diseases, and with pulmonary metastases.
Limitations of this report include limited experience in the management of trastuzumab-associated interstitial lung disease, due to the small number of cases, the low degree of suspicion, and the limited information provided, mainly in case reports; therefore, it is important to identify and monitor patients at higher risk of ILD, which is crucial for timely intervention.
In this study, although drug-related diffuse interstitial pneumonitis is a diagnosis of exclusion, it has a high morbidity and mortality rate, so it is important to detect it early in order to establish timely treatment, especially in cases of breast cancer, a disease with a high incidence and mortality in our population, who receive monoclonal antibodies as neoadjuvant, adjuvant or first-line treatment. In daily practice, it is recommended to be exhaustive with the possible complications caused by monoclonal antibodies, in order to take optimal care measures.
Ethical criteria: Informed consent was obtained from the patient's family member and institutional permission was obtained for the case report to be prepared by the Innovation and Research Subunit of the Regional Institute of Neoplastic Diseases – Center. This report follows the recommendations of the CARE guidelines for publication.