AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2690-4861/471
1Clinical Neurophysiology and Instituto de Investigación Biomédica, Hospital Universitario de La Prin-cesa, C/Diego de León 62, 28006 Madrid, Spain.
2Innovation in Neurosurgery, Department of Surgery, Autonomous University of Madrid, 28049 Madrid, Spain.
3Neurosurgery and Instituto de Investigación Biomédica, Hospital Universitario de La Princesa, C/Diego de León 62, 28006 Madrid, Spain.
*Corresponding Author: Jesús Pastor, Clinical Neurophysiology and Instituto de Investigación Biomédica, Hospital Universitario de La Prin-cesa, C/Diego de León 62, 28006 Madrid, Spain.
Citation: Pastor J., Lorena V Zelaya., Rafael G. Sola., Paloma Pulido, (2024), Challenge of Clinical Diagnosis and Treatment for Proteus Syndrome: A Case Report, International Journal of Clinical Case Reports and Reviews, 18(2); DOI:10.31579/2690-4861/471
Copyright: © 2024, Jesús Pastor. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 14 May 2024 | Accepted: 27 May 2024 | Published: 28 June 2024
Keywords: The performance of the presurgical test (preSurg) for temporal lobe epilepsy (TLE) was evaluated via video-electroencephalography (VEEG), electroencephalography (EEG), 99mTc-HmPAO single-photon emission tomography (SPECT)
The performance of the presurgical test (preSurg) for temporal lobe epilepsy (TLE) was evaluated via video-electroencephalography (VEEG), electroencephalography (EEG), 99mTc-HmPAO single-photon emission tomography (SPECT) and 1.5 T magnetic resonance imaging (MRI) in a group of 112 men (37.0 ± 1.1 years) and 106 women (39.7 ± 1.1 years) operated on for TLE. The epileptic zone (EZ) was adequately identified to determine whether the patient reached an Engel I grade (EI) at least one-year postop. Accuracy was evaluated by the coefficient α, ranging from 3 (when result = EZ) to 2 (result in the same hemisphere as the EZ), 1 (noninformative result) or 0 (EZ in the contralateral hemisphere). The simplicity of diagnosis was defined as the number of preSurg surgeries needed to identify the EZ. EI was obtained in 85.8% of patients, even though 42.2% had noninformative MRI results. For preSurg α was higher for vEEG, followed by MRI and lower for EEG. The accuracy (combination of sensitivity and specificity) was calculated as follows: VEEG (0.797) > MRI (0.518) > EEG (0.446) > SPECT (0.360). The likelihood positive ratio was more significant, and the likelihood negative ratio was lower for VEEG. Both results indicated the highest discriminatory ability for this study. The most relevant factor for the regression model, in order to predict a good function post-surgical outcome were, from more to less relevant VEEG, MRI and EEG, and the factors were not significantly related to SPECT. EZs in EI patients with low simplicity were identified mainly by VEEG. A very good postoperative outcome can be obtained even in TLE patients with no lesions on MRI. This is a relevant idea, because, even patients without apparent lesions in imaging, should be referred to a specialized center to presurgical evaluation. The VEEG is the most reliable preSurg test and may be the only reliable test for patients with very low simplicity.
Temporal lobe epilepsy (TLE) is the most common type of focal epilepsy. Fortunately, drug-resistant patients have good outcomes after surgery [1]. Accurate localization of the epileptogenic zone (EZ) is a prerequisite for successful surgical treatment of patients with pharmacoresistant focal epilepsy [2]. Identifying that region requires careful evaluation via several presurgical tests (preSurg) in highly specialized centers [3]. Among these methods, long-term scalp video-electroencephalography (VEEG) monitoring is mandatory for recording interictal EEG features and seizures, including bioelectrical patterns and semiology, neuropsychological assessments and magnetic resonance imaging (MRI) that are specifically related to epileptic evaluation. Other tests can include interictal 18-fluoro-deoxyglucose ([18F]-FDG) positron emission tomography (PET) or 99mHmPAO single-photon emission computed tomography (SPECT) [4–8]. Approximately 30–90% of epileptic patients with concordant electroclinical data may have seizure freedom [9-12]
The EZ is the region in which resection or disconnection results in the disappearance of seizures [2,13]. Therefore, it is an operational definition and does not allow for positive identification before surgery. Consequently, no gold-standard method can be used for a statistical analysis of preSurg because it is currently impossible to assess the degree of certainty in cases of non-Engel I (and dubiously II, too). This means we can only be sure about the presurgical accuracy in Engel I patients.
Nevertheless, it seems highly relevant to assess the contribution of preSurg to the treatment of drug-resistant epileptic patients. Nonetheless, there is no canonical definition for which preSurg surgery must be included in the presurgical evaluation, except for the mandatory use of VEEG and MRI. Similarly, patient selection for surgical treatment depends strongly on the experience of the clinical team [6]. However, there is a significant proportion of epileptic patients with no evident anatomical lesions on MRI or even discordant preSurg who could benefit from surgery. However, the percentage of these patients remains to be determined. In this sense, we must remember that in recent years, the concept of network epilepsy has developed and is not necessarily associated with morphological lesions according to imaging studies [14-18].
In this work, we assessed two complementary goals: i) evaluation of the accuracy of the preSurg protocols of EEG, SPECT, MRI and scalp VEEG in determining the location of the EZ and ii) the evaluation of agreement between tests in temporal lobe epilepsy (TLE) patients operated on in the last 20 years in a national reference unit for the treatment of epilepsy. The first goal depends on the specific capacity of the tests to identify the EZ, and the second goal is related to the intrinsic difficulty of diagnosis for every patient. Therefore, regarding the difficulty in the diagnosis, we have termed simplicity the degree of agreement of different preSurg, e.g., a higher simplicity implies that more preSurg correctly identified the EZ.
We were not interested in the surgical details, anatomy or other pathological or therapeutic considerations.
2.1. Patients
This study retrospectively evaluated 112 men and 106 women who underwent surgery for TLE at the National Reference Unit for the Treatment of Refractory Epilepsy, University Hospital La Princesa (Spain), from 2001 to 2021. The experimental procedure was approved by the medical ethical review board of the Hospital Universitario de La Princesa and was deemed “care as usual”. Under these circumstances, written informed consent was not needed. Most of the patients were treated with at least two antiepileptic drugs (AEDs) and had a history of epilepsy longer than two years.
The presurgical evaluation was performed according to the protocol of Hospital La Princesa and has been described in detail elsewhere [5]. Briefly, all the patients diagnosed with drug-resistant epilepsy and sent for evaluation for surgery to the National Reference Unit for the Treatment of Refractory Epilepsy were carefully evaluated by a neurologist and then underwent a standardised presurgical work-up including EEG, neuropsychology, SPECT, and MRI using a specific protocol for epilepsy and EEG. Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue. The neuropsychological evaluation of special memory reserve and language lateralization was considered in detail. When doubts about possible language/cognitive deficiencies after surgery remain, we performed the Wada test to identify language dominance and memory reserve unequivocally.
All patients were evaluated with a 19-channel scalp EEG (EEG32U, NeuroWorks, XLTEK®, Oakville, ON, Canada) following the international 10–20 system. Additionally, we employed interictal single-photon emission computed tomography (SPECT, Starcam 3200, General Electric®, Fairfield, CT, USA) using 99mTc-HmPAO and magnetic resonance imaging (MRI, General Electric®, Fairfield, CT, USA) 1.5 T with specific epilepsy study and video-electroencephalography (VEEG; EMU64, NeuroWorks, XLTEK®, Oakville, ON, Canada) using 19 scalp electrodes according to the international 10–20 system plus additional electrodes in T1/T2, T9/T10 and P5/P8 (for a total of 25 electrodes). VEEG monitoring was performed until a sufficient number of seizures was obtained (1 to 3). Usually, this period prolonged 3-5 days. No activation manoeuvres (such as sleep deprivation or hyperventilation) were used, although systematic anti-epileptic drug tapering was done. Usually, a third of the anti-epileptic drug dosage per day was removed during the first three days of recording. Sometimes, foramen ovale or depth electrodes were used after VEEG. However, in this paper, we considered only the information obtained from the scalp. Patients who underwent surgery after the use of intracranial electrodes were not included.
All preSurg were performed by different highly specialized staff (clinical neurophysiologists, nuclear medicine specialists and radiologists) without knowledge of the results from the remaining studies. Only during the final clinical meeting were the results publicly discussed, and if needed, ambiguous results could be reinterpreted according to the rest of preSurg. However, in this work, we selected the former results (before the clinical meeting). All the unit members had more than ten years of professional experience.
Postsurgical outcomes were assessed through Engel’s scale [6]. Patients were evaluated at three, six and twelve months after surgery. The evaluation of the Engel scale at any time involved considering the presence/absence of ES between the previous assessment (or the immediate postop period) and the current evaluation. Considering that the EZ is an operational definition, only in patients with an Engel grade I (EI) can we be sure of the anatomical location of the EZ. This is a very restrictive classification because we classified non-Engel I patients (nEIs) with early postsurgical seizures despite the absence of seizures for many years.
Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue.
2.2. Performance assessment of presurgical tests
The sampling space (Ω) for any epileptic patient has 8 possibilities, i.e., four lobes (frontal, temporal, parietal and occipital) from the left and right hemispheres, namely,  . Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms
. Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms  . However, if the patient has nEI, we know that
. However, if the patient has nEI, we know that  , Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ (
, Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ ( ) was included in the vector
) was included in the vector  . Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
. Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
The same formalism can be used to codify the results of preSurg. For example, if we have the following results for SPECT = hypoperfusion in the right temporal lobe, EEG = no presence of irritative activity, VEEG = left temporal lobe epilepsy and MRI = left temporal lobe sclerosis, we can codify these results in vectorial form as  ,
,  ,
,  and
and  .
.
We considered the following diagnosis from preSurg for localization of the OpZ. We had any of the following possibilities on MRI: hippocampal sclerosis/atrophy, cortical dysplasia, low-grade tumours, cavernoma, cortical development disorder or vascular malformation; on VEEG (in descending order of relevance): ictal patterns and clinical semiology; presence of irritative activity > 75% in the same lobe; and presence of irritative activity during rapid eye movement sleep; on EEG: irritative activity, including spikes, sharp waves, temporal intermittent rhythmic delta activity or any combination of these; or on SPECT: hypoperfusion.
Using a formalism in terms of vectors allowed us to implement an algorithm to compute the performance assessment from all the preSurgs. The accuracy of the preSurg in locating the EZ was assessed using a coefficient (α) defined in this way: if the test identified the EZ, then we assigned a value of 3; if the test identified the hemisphere (e.g., the test indicated more lobes than OpZ in the same hemisphere), we assigned a value of 2; if the test could not discriminate between the two hemispheres (e.g., normal MRI), we assigned a value of 1; and if the test indicated the contralateral hemisphere, we assigned a value of 0. In the case of nEI, if the test revealed a region outside of the OpZ, we assigned a value of 1; however, if the test demonstrated the OpZ, we assigned a value of 0, the same as the contralateral localization for EI.
We used α to evaluate the degree of difficulty in diagnosing a patient (i.e., the opposite concept of simplicity) or of a group of patients utilizing the concept of simplicity. We calculated simplicity by computing the mean of α from all the preSurg, and in this way, we obtained a value that reflected the degree of agreement between all the preSurg values and the OpZ. According to this definition , the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
, the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
We also evaluated the performance of the preSurg classification using a confusion matrix, obtaining sensitivity (S), specificity (Sp) and several related measures [19]. To do that, we computed the confusion matrices according to these definitions:2.1. Patients
This study retrospectively evaluated 112 men and 106 women who underwent surgery for TLE at the National Reference Unit for the Treatment of Refractory Epilepsy, University Hospital La Princesa (Spain), from 2001 to 2021. The experimental procedure was approved by the medical ethical review board of the Hospital Universitario de La Princesa and was deemed “care as usual”. Under these circumstances, written informed consent was not needed. Most of the patients were treated with at least two antiepileptic drugs (AEDs) and had a history of epilepsy longer than two years.
The presurgical evaluation was performed according to the protocol of Hospital La Princesa and has been described in detail elsewhere [5]. Briefly, all the patients diagnosed with drug-resistant epilepsy and sent for evaluation for surgery to the National Reference Unit for the Treatment of Refractory Epilepsy were carefully evaluated by a neurologist and then underwent a standardised presurgical work-up including EEG, neuropsychology, SPECT, and MRI using a specific protocol for epilepsy and EEG. Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue. The neuropsychological evaluation of special memory reserve and language lateralization was considered in detail. When doubts about possible language/cognitive deficiencies after surgery remain, we performed the Wada test to identify language dominance and memory reserve unequivocally.
All patients were evaluated with a 19-channel scalp EEG (EEG32U, NeuroWorks, XLTEK®, Oakville, ON, Canada) following the international 10–20 system. Additionally, we employed interictal single-photon emission computed tomography (SPECT, Starcam 3200, General Electric®, Fairfield, CT, USA) using 99mTc-HmPAO and magnetic resonance imaging (MRI, General Electric®, Fairfield, CT, USA) 1.5 T with specific epilepsy study and video-electroencephalography (VEEG; EMU64, NeuroWorks, XLTEK®, Oakville, ON, Canada) using 19 scalp electrodes according to the international 10–20 system plus additional electrodes in T1/T2, T9/T10 and P5/P8 (for a total of 25 electrodes). VEEG monitoring was performed until a sufficient number of seizures was obtained (1 to 3). Usually, this period prolonged 3-5 days. No activation manoeuvres (such as sleep deprivation or hyperventilation) were used, although systematic anti-epileptic drug tapering was done. Usually, a third of the anti-epileptic drug dosage per day was removed during the first three days of recording. Sometimes, foramen ovale or depth electrodes were used after VEEG. However, in this paper, we considered only the information obtained from the scalp. Patients who underwent surgery after the use of intracranial electrodes were not included.
All preSurg were performed by different highly specialized staff (clinical neurophysiologists, nuclear medicine specialists and radiologists) without knowledge of the results from the remaining studies. Only during the final clinical meeting were the results publicly discussed, and if needed, ambiguous results could be reinterpreted according to the rest of preSurg. However, in this work, we selected the former results (before the clinical meeting). All the unit members had more than ten years of professional experience.
Postsurgical outcomes were assessed through Engel’s scale [6]. Patients were evaluated at three, six and twelve months after surgery. The evaluation of the Engel scale at any time involved considering the presence/absence of ES between the previous assessment (or the immediate postop period) and the current evaluation. Considering that the EZ is an operational definition, only in patients with an Engel grade I (EI) can we be sure of the anatomical location of the EZ. This is a very restrictive classification because we classified non-Engel I patients (nEIs) with early postsurgical seizures despite the absence of seizures for many years.
Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue.
2.2. Performance assessment of presurgical tests
The sampling space (Ω) for any epileptic patient has 8 possibilities, i.e., four lobes (frontal, temporal, parietal and occipital) from the left and right hemispheres, namely,  . Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms
. Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms  . However, if the patient has nEI, we know that
. However, if the patient has nEI, we know that  , Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ (
, Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ ( ) was included in the vector
) was included in the vector  . Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
. Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
The same formalism can be used to codify the results of preSurg. For example, if we have the following results for SPECT = hypoperfusion in the right temporal lobe, EEG = no presence of irritative activity, VEEG = left temporal lobe epilepsy and MRI = left temporal lobe sclerosis, we can codify these results in vectorial form as  ,
,  ,
,  and
and  .
.
We considered the following diagnosis from preSurg for localization of the OpZ. We had any of the following possibilities on MRI: hippocampal sclerosis/atrophy, cortical dysplasia, low-grade tumours, cavernoma, cortical development disorder or vascular malformation; on VEEG (in descending order of relevance): ictal patterns and clinical semiology; presence of irritative activity > 75% in the same lobe; and presence of irritative activity during rapid eye movement sleep; on EEG: irritative activity, including spikes, sharp waves, temporal intermittent rhythmic delta activity or any combination of these; or on SPECT: hypoperfusion.
Using a formalism in terms of vectors allowed us to implement an algorithm to compute the performance assessment from all the preSurgs. The accuracy of the preSurg in locating the EZ was assessed using a coefficient (α) defined in this way: if the test identified the EZ, then we assigned a value of 3; if the test identified the hemisphere (e.g., the test indicated more lobes than OpZ in the same hemisphere), we assigned a value of 2; if the test could not discriminate between the two hemispheres (e.g., normal MRI), we assigned a value of 1; and if the test indicated the contralateral hemisphere, we assigned a value of 0. In the case of nEI, if the test revealed a region outside of the OpZ, we assigned a value of 1; however, if the test demonstrated the OpZ, we assigned a value of 0, the same as the contralateral localization for EI.
We used α to evaluate the degree of difficulty in diagnosing a patient (i.e., the opposite concept of simplicity) or of a group of patients utilizing the concept of simplicity. We calculated simplicity by computing the mean of α from all the preSurg, and in this way, we obtained a value that reflected the degree of agreement between all the preSurg values and the OpZ. According to this definition , the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
, the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
We also evaluated the performance of the preSurg classification using a confusion matrix, obtaining sensitivity (S), specificity (Sp) and several related measures [19]. To do that, we computed the confusion matrices according to these definitions:2.1. Patients
This study retrospectively evaluated 112 men and 106 women who underwent surgery for TLE at the National Reference Unit for the Treatment of Refractory Epilepsy, University Hospital La Princesa (Spain), from 2001 to 2021. The experimental procedure was approved by the medical ethical review board of the Hospital Universitario de La Princesa and was deemed “care as usual”. Under these circumstances, written informed consent was not needed. Most of the patients were treated with at least two antiepileptic drugs (AEDs) and had a history of epilepsy longer than two years.
The presurgical evaluation was performed according to the protocol of Hospital La Princesa and has been described in detail elsewhere [5]. Briefly, all the patients diagnosed with drug-resistant epilepsy and sent for evaluation for surgery to the National Reference Unit for the Treatment of Refractory Epilepsy were carefully evaluated by a neurologist and then underwent a standardised presurgical work-up including EEG, neuropsychology, SPECT, and MRI using a specific protocol for epilepsy and EEG. Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue. The neuropsychological evaluation of special memory reserve and language lateralization was considered in detail. When doubts about possible language/cognitive deficiencies after surgery remain, we performed the Wada test to identify language dominance and memory reserve unequivocally.
All patients were evaluated with a 19-channel scalp EEG (EEG32U, NeuroWorks, XLTEK®, Oakville, ON, Canada) following the international 10–20 system. Additionally, we employed interictal single-photon emission computed tomography (SPECT, Starcam 3200, General Electric®, Fairfield, CT, USA) using 99mTc-HmPAO and magnetic resonance imaging (MRI, General Electric®, Fairfield, CT, USA) 1.5 T with specific epilepsy study and video-electroencephalography (VEEG; EMU64, NeuroWorks, XLTEK®, Oakville, ON, Canada) using 19 scalp electrodes according to the international 10–20 system plus additional electrodes in T1/T2, T9/T10 and P5/P8 (for a total of 25 electrodes). VEEG monitoring was performed until a sufficient number of seizures was obtained (1 to 3). Usually, this period prolonged 3-5 days. No activation manoeuvres (such as sleep deprivation or hyperventilation) were used, although systematic anti-epileptic drug tapering was done. Usually, a third of the anti-epileptic drug dosage per day was removed during the first three days of recording. Sometimes, foramen ovale or depth electrodes were used after VEEG. However, in this paper, we considered only the information obtained from the scalp. Patients who underwent surgery after the use of intracranial electrodes were not included.
All preSurg were performed by different highly specialized staff (clinical neurophysiologists, nuclear medicine specialists and radiologists) without knowledge of the results from the remaining studies. Only during the final clinical meeting were the results publicly discussed, and if needed, ambiguous results could be reinterpreted according to the rest of preSurg. However, in this work, we selected the former results (before the clinical meeting). All the unit members had more than ten years of professional experience.
Postsurgical outcomes were assessed through Engel’s scale [6]. Patients were evaluated at three, six and twelve months after surgery. The evaluation of the Engel scale at any time involved considering the presence/absence of ES between the previous assessment (or the immediate postop period) and the current evaluation. Considering that the EZ is an operational definition, only in patients with an Engel grade I (EI) can we be sure of the anatomical location of the EZ. This is a very restrictive classification because we classified non-Engel I patients (nEIs) with early postsurgical seizures despite the absence of seizures for many years.
Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue.
2.2. Performance assessment of presurgical tests
The sampling space (Ω) for any epileptic patient has 8 possibilities, i.e., four lobes (frontal, temporal, parietal and occipital) from the left and right hemispheres, namely,  . Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms
. Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms  . However, if the patient has nEI, we know that
. However, if the patient has nEI, we know that  , Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ (
, Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ ( ) was included in the vector
) was included in the vector  . Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
. Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
The same formalism can be used to codify the results of preSurg. For example, if we have the following results for SPECT = hypoperfusion in the right temporal lobe, EEG = no presence of irritative activity, VEEG = left temporal lobe epilepsy and MRI = left temporal lobe sclerosis, we can codify these results in vectorial form as  ,
,  ,
,  and
and  .
.
We considered the following diagnosis from preSurg for localization of the OpZ. We had any of the following possibilities on MRI: hippocampal sclerosis/atrophy, cortical dysplasia, low-grade tumours, cavernoma, cortical development disorder or vascular malformation; on VEEG (in descending order of relevance): ictal patterns and clinical semiology; presence of irritative activity > 75% in the same lobe; and presence of irritative activity during rapid eye movement sleep; on EEG: irritative activity, including spikes, sharp waves, temporal intermittent rhythmic delta activity or any combination of these; or on SPECT: hypoperfusion.
Using a formalism in terms of vectors allowed us to implement an algorithm to compute the performance assessment from all the preSurgs. The accuracy of the preSurg in locating the EZ was assessed using a coefficient (α) defined in this way: if the test identified the EZ, then we assigned a value of 3; if the test identified the hemisphere (e.g., the test indicated more lobes than OpZ in the same hemisphere), we assigned a value of 2; if the test could not discriminate between the two hemispheres (e.g., normal MRI), we assigned a value of 1; and if the test indicated the contralateral hemisphere, we assigned a value of 0. In the case of nEI, if the test revealed a region outside of the OpZ, we assigned a value of 1; however, if the test demonstrated the OpZ, we assigned a value of 0, the same as the contralateral localization for EI.
We used α to evaluate the degree of difficulty in diagnosing a patient (i.e., the opposite concept of simplicity) or of a group of patients utilizing the concept of simplicity. We calculated simplicity by computing the mean of α from all the preSurg, and in this way, we obtained a value that reflected the degree of agreement between all the preSurg values and the OpZ. According to this definition , the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
, the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
We also evaluated the performance of the preSurg classification using a confusion matrix, obtaining sensitivity (S), specificity (Sp) and several related measures [19]. To do that, we computed the confusion matrices according to these definitions:2.1. Patients
This study retrospectively evaluated 112 men and 106 women who underwent surgery for TLE at the National Reference Unit for the Treatment of Refractory Epilepsy, University Hospital La Princesa (Spain), from 2001 to 2021. The experimental procedure was approved by the medical ethical review board of the Hospital Universitario de La Princesa and was deemed “care as usual”. Under these circumstances, written informed consent was not needed. Most of the patients were treated with at least two antiepileptic drugs (AEDs) and had a history of epilepsy longer than two years.
The presurgical evaluation was performed according to the protocol of Hospital La Princesa and has been described in detail elsewhere [5]. Briefly, all the patients diagnosed with drug-resistant epilepsy and sent for evaluation for surgery to the National Reference Unit for the Treatment of Refractory Epilepsy were carefully evaluated by a neurologist and then underwent a standardised presurgical work-up including EEG, neuropsychology, SPECT, and MRI using a specific protocol for epilepsy and EEG. Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue. The neuropsychological evaluation of special memory reserve and language lateralization was considered in detail. When doubts about possible language/cognitive deficiencies after surgery remain, we performed the Wada test to identify language dominance and memory reserve unequivocally.
All patients were evaluated with a 19-channel scalp EEG (EEG32U, NeuroWorks, XLTEK®, Oakville, ON, Canada) following the international 10–20 system. Additionally, we employed interictal single-photon emission computed tomography (SPECT, Starcam 3200, General Electric®, Fairfield, CT, USA) using 99mTc-HmPAO and magnetic resonance imaging (MRI, General Electric®, Fairfield, CT, USA) 1.5 T with specific epilepsy study and video-electroencephalography (VEEG; EMU64, NeuroWorks, XLTEK®, Oakville, ON, Canada) using 19 scalp electrodes according to the international 10–20 system plus additional electrodes in T1/T2, T9/T10 and P5/P8 (for a total of 25 electrodes). VEEG monitoring was performed until a sufficient number of seizures was obtained (1 to 3). Usually, this period prolonged 3-5 days. No activation manoeuvres (such as sleep deprivation or hyperventilation) were used, although systematic anti-epileptic drug tapering was done. Usually, a third of the anti-epileptic drug dosage per day was removed during the first three days of recording. Sometimes, foramen ovale or depth electrodes were used after VEEG. However, in this paper, we considered only the information obtained from the scalp. Patients who underwent surgery after the use of intracranial electrodes were not included.
All preSurg were performed by different highly specialized staff (clinical neurophysiologists, nuclear medicine specialists and radiologists) without knowledge of the results from the remaining studies. Only during the final clinical meeting were the results publicly discussed, and if needed, ambiguous results could be reinterpreted according to the rest of preSurg. However, in this work, we selected the former results (before the clinical meeting). All the unit members had more than ten years of professional experience.
Postsurgical outcomes were assessed through Engel’s scale [6]. Patients were evaluated at three, six and twelve months after surgery. The evaluation of the Engel scale at any time involved considering the presence/absence of ES between the previous assessment (or the immediate postop period) and the current evaluation. Considering that the EZ is an operational definition, only in patients with an Engel grade I (EI) can we be sure of the anatomical location of the EZ. This is a very restrictive classification because we classified non-Engel I patients (nEIs) with early postsurgical seizures despite the absence of seizures for many years.
Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue.
2.2. Performance assessment of presurgical tests
The sampling space (Ω) for any epileptic patient has 8 possibilities, i.e., four lobes (frontal, temporal, parietal and occipital) from the left and right hemispheres, namely,  . Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms
. Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms  . However, if the patient has nEI, we know that
. However, if the patient has nEI, we know that  , Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ (
, Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ ( ) was included in the vector
) was included in the vector  . Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
. Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
The same formalism can be used to codify the results of preSurg. For example, if we have the following results for SPECT = hypoperfusion in the right temporal lobe, EEG = no presence of irritative activity, VEEG = left temporal lobe epilepsy and MRI = left temporal lobe sclerosis, we can codify these results in vectorial form as  ,
,  ,
,  and
and  .
.
We considered the following diagnosis from preSurg for localization of the OpZ. We had any of the following possibilities on MRI: hippocampal sclerosis/atrophy, cortical dysplasia, low-grade tumours, cavernoma, cortical development disorder or vascular malformation; on VEEG (in descending order of relevance): ictal patterns and clinical semiology; presence of irritative activity > 75% in the same lobe; and presence of irritative activity during rapid eye movement sleep; on EEG: irritative activity, including spikes, sharp waves, temporal intermittent rhythmic delta activity or any combination of these; or on SPECT: hypoperfusion.
Using a formalism in terms of vectors allowed us to implement an algorithm to compute the performance assessment from all the preSurgs. The accuracy of the preSurg in locating the EZ was assessed using a coefficient (α) defined in this way: if the test identified the EZ, then we assigned a value of 3; if the test identified the hemisphere (e.g., the test indicated more lobes than OpZ in the same hemisphere), we assigned a value of 2; if the test could not discriminate between the two hemispheres (e.g., normal MRI), we assigned a value of 1; and if the test indicated the contralateral hemisphere, we assigned a value of 0. In the case of nEI, if the test revealed a region outside of the OpZ, we assigned a value of 1; however, if the test demonstrated the OpZ, we assigned a value of 0, the same as the contralateral localization for EI.
We used α to evaluate the degree of difficulty in diagnosing a patient (i.e., the opposite concept of simplicity) or of a group of patients utilizing the concept of simplicity. We calculated simplicity by computing the mean of α from all the preSurg, and in this way, we obtained a value that reflected the degree of agreement between all the preSurg values and the OpZ. According to this definition , the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
, the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
We also evaluated the performance of the preSurg classification using a confusion matrix, obtaining sensitivity (S), specificity (Sp) and several related measures [19]. To do that, we computed the confusion matrices according to these definitions:2.1. Patients
This study retrospectively evaluated 112 men and 106 women who underwent surgery for TLE at the National Reference Unit for the Treatment of Refractory Epilepsy, University Hospital La Princesa (Spain), from 2001 to 2021. The experimental procedure was approved by the medical ethical review board of the Hospital Universitario de La Princesa and was deemed “care as usual”. Under these circumstances, written informed consent was not needed. Most of the patients were treated with at least two antiepileptic drugs (AEDs) and had a history of epilepsy longer than two years.
The presurgical evaluation was performed according to the protocol of Hospital La Princesa and has been described in detail elsewhere [5]. Briefly, all the patients diagnosed with drug-resistant epilepsy and sent for evaluation for surgery to the National Reference Unit for the Treatment of Refractory Epilepsy were carefully evaluated by a neurologist and then underwent a standardised presurgical work-up including EEG, neuropsychology, SPECT, and MRI using a specific protocol for epilepsy and EEG. Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue. The neuropsychological evaluation of special memory reserve and language lateralization was considered in detail. When doubts about possible language/cognitive deficiencies after surgery remain, we performed the Wada test to identify language dominance and memory reserve unequivocally.
All patients were evaluated with a 19-channel scalp EEG (EEG32U, NeuroWorks, XLTEK®, Oakville, ON, Canada) following the international 10–20 system. Additionally, we employed interictal single-photon emission computed tomography (SPECT, Starcam 3200, General Electric®, Fairfield, CT, USA) using 99mTc-HmPAO and magnetic resonance imaging (MRI, General Electric®, Fairfield, CT, USA) 1.5 T with specific epilepsy study and video-electroencephalography (VEEG; EMU64, NeuroWorks, XLTEK®, Oakville, ON, Canada) using 19 scalp electrodes according to the international 10–20 system plus additional electrodes in T1/T2, T9/T10 and P5/P8 (for a total of 25 electrodes). VEEG monitoring was performed until a sufficient number of seizures was obtained (1 to 3). Usually, this period prolonged 3-5 days. No activation manoeuvres (such as sleep deprivation or hyperventilation) were used, although systematic anti-epileptic drug tapering was done. Usually, a third of the anti-epileptic drug dosage per day was removed during the first three days of recording. Sometimes, foramen ovale or depth electrodes were used after VEEG. However, in this paper, we considered only the information obtained from the scalp. Patients who underwent surgery after the use of intracranial electrodes were not included.
All preSurg were performed by different highly specialized staff (clinical neurophysiologists, nuclear medicine specialists and radiologists) without knowledge of the results from the remaining studies. Only during the final clinical meeting were the results publicly discussed, and if needed, ambiguous results could be reinterpreted according to the rest of preSurg. However, in this work, we selected the former results (before the clinical meeting). All the unit members had more than ten years of professional experience.
Postsurgical outcomes were assessed through Engel’s scale [6]. Patients were evaluated at three, six and twelve months after surgery. The evaluation of the Engel scale at any time involved considering the presence/absence of ES between the previous assessment (or the immediate postop period) and the current evaluation. Considering that the EZ is an operational definition, only in patients with an Engel grade I (EI) can we be sure of the anatomical location of the EZ. This is a very restrictive classification because we classified non-Engel I patients (nEIs) with early postsurgical seizures despite the absence of seizures for many years.
Most of the patients (200/218) underwent electrocorticography (ECoG)-tailored anterior medial temporal resection, which was our systematic approach to temporal lobe epilepsy surgery. Five patients underwent only lateral cortectomy (because of the presence of well-localized lesions), and only three patients underwent amygdalo-hippocampectomy. All eight of these patients were in the EI group; therefore, the type of surgery could not influence the results. Consequently, the kind of surgery cannot be considered as a variable. We did not use a different surgical approach because we considered that the ECoG guidance would be good enough to eliminate the epileptic tissue.
2.2. Performance assessment of presurgical tests
The sampling space (Ω) for any epileptic patient has 8 possibilities, i.e., four lobes (frontal, temporal, parietal and occipital) from the left and right hemispheres, namely,  . Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms
. Therefore, the operation zone (OpZ) must be one of these options. Any lobe can be represented by an 8th-dimensional vector, where 1 indicates a specific lobe and the rest are 0. For example, the OpZ of a patient with intervention in the left temporal lobe can be shown by OpZ= [0,1,0,0,0,0,0,0]. Considering that the EZ is an operational definition, not a positive concept, its identification can be performed only in terms of the procedures used for evaluation (in this case, the absence of seizures after the excision/disconnection of a brain region). Therefore, we cannot precisely know a priori its placement. However, we have an objective determination, which is the OpZ. Hence, if the patient has EI, we assume that the EZ is in the OpZ, as in topographical terms  . However, if the patient has nEI, we know that
. However, if the patient has nEI, we know that  , Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ (
, Unfortunately, we have no way of knowing which other lobe it can be located in. In the example considered (nEI in a patient operated on from the left temporal lobe), the putative EZ ( ) was included in the vector
) was included in the vector  . Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
. Obviously, this formalism does not indicate that the EZ would be in fact located in all the lobes except the left temporal lobe; rather, it only shows our lack of knowledge.
The same formalism can be used to codify the results of preSurg. For example, if we have the following results for SPECT = hypoperfusion in the right temporal lobe, EEG = no presence of irritative activity, VEEG = left temporal lobe epilepsy and MRI = left temporal lobe sclerosis, we can codify these results in vectorial form as  ,
,  ,
,  and
and  .
.
We considered the following diagnosis from preSurg for localization of the OpZ. We had any of the following possibilities on MRI: hippocampal sclerosis/atrophy, cortical dysplasia, low-grade tumours, cavernoma, cortical development disorder or vascular malformation; on VEEG (in descending order of relevance): ictal patterns and clinical semiology; presence of irritative activity > 75% in the same lobe; and presence of irritative activity during rapid eye movement sleep; on EEG: irritative activity, including spikes, sharp waves, temporal intermittent rhythmic delta activity or any combination of these; or on SPECT: hypoperfusion.
Using a formalism in terms of vectors allowed us to implement an algorithm to compute the performance assessment from all the preSurgs. The accuracy of the preSurg in locating the EZ was assessed using a coefficient (α) defined in this way: if the test identified the EZ, then we assigned a value of 3; if the test identified the hemisphere (e.g., the test indicated more lobes than OpZ in the same hemisphere), we assigned a value of 2; if the test could not discriminate between the two hemispheres (e.g., normal MRI), we assigned a value of 1; and if the test indicated the contralateral hemisphere, we assigned a value of 0. In the case of nEI, if the test revealed a region outside of the OpZ, we assigned a value of 1; however, if the test demonstrated the OpZ, we assigned a value of 0, the same as the contralateral localization for EI.
We used α to evaluate the degree of difficulty in diagnosing a patient (i.e., the opposite concept of simplicity) or of a group of patients utilizing the concept of simplicity. We calculated simplicity by computing the mean of α from all the preSurg, and in this way, we obtained a value that reflected the degree of agreement between all the preSurg values and the OpZ. According to this definition , the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
, the maximum value indicates perfect identification in all the patients or of all the preSurg in a given patient. We assumed that a patient whose preSurg test results coincided with the EZ had a more straightforward diagnosis than a patient with EI when only one or two preSurg tests correctly indicated the EZ.
We also evaluated the performance of the preSurg classification using a confusion matrix, obtaining sensitivity (S), specificity (Sp) and several related measures [19]. To do that, we computed the confusion matrices according to these definitions:
True positive (TP): patient EI + preSurg localizing (α=3)
False-negative (FN): patient EI + preSurg not localizing ( )
)
True negative (TN): patient, nEI + preSurg not in OpZ (α=1)
False-positive (FP): patient, nEI + preSurg in OpZ (α=0)
With these expressions, we can define


The use of these confusion matrices allows us to obtain several accuracy measurements to characterize the performance of a given preSurg and compare them, defined according to these expressions.
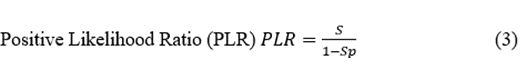
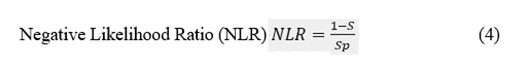
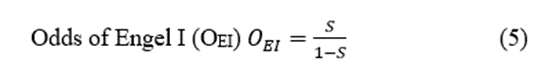
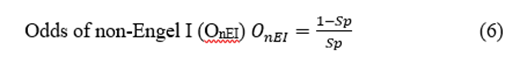
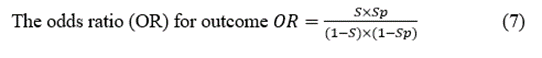
Predictive Positive Value (PPV)  (8)
(8)
Predictive negative value (PNV)  (9)
(9)
PD (predominance) is the prevalence estimator (in this case, EI).
Finally, as a comprehensive measure of precision, we used accuracy (AC), defined as
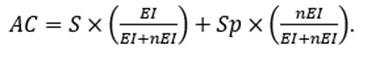
These definitions and their equivalences are summarized in Table 1.
| Outcome | Result of preSurg | α | True/False classification | Example from EEG* |
| EI | Indicates a lobe that coincides with the EZ | 3 | TP | Left temporal sharp waves |
| Indicates the hemisphere where EZ is located | 2 | FN | Left fronto-temporal sharp waves | |
| Indicates a non-informative result | 1 | FN | Physiological or generalized spike-wave | |
| Indicates the contralateral hemisphere | 0 | FN | Right sharp waves | |
| nEI | Indicates the same OpZ | 0 | FP | Left temporal sharp waves |
| Indicates a region different from OpZ | 1 | TN | Left frontal sharp waves |
Table 1: Performance of preSurg for different variables.
EI = Engel’s I grade; FN = false negative; EZ = epileptic zone; FP = false positive; nEI = non-Engel’s I grade; OpZ = operated zone; TN = true negative; TP = true positive; *Suppose a patient operated from the left temporal lobe.
2.3. Multiple binary logistic model
We constructed a multiple binary logistic regression model to evaluate the contribution of preSurg to obtaining an EI outcome. To do that, we used the coefficients (α) from the different preSurg variables and the outcome of Engel’s scale (1 for EI and 0 for nEI) as the dependent variable. We evaluated the goodness of fit using the Hosmer–Lemeshow statistic and the significance of the variables by the Wald statistic [20-21].
A detailed description of the model is given in Appendix A.
2.4. Statistics
We used the relative frequencies as probabilities; consequently, we could use the formula for conditional probability [22] to evaluate the likelihood of occurrence of two simultaneous events. For two events, namely A and B, the conditional probability ( )) is given by the expression
)) is given by the expression
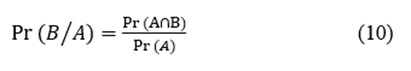
where  are the probabilities of events A and B simultaneously and
are the probabilities of events A and B simultaneously and  is the probability of event A.
is the probability of event A.
Statistical comparisons between groups were performed using Student’s t test or ANOVA for normally distributed data. Normality was evaluated using the Kolmogorov–Smirnov test. The Mann–Whitney rank sum test or ANOVA on ranks was used when normality failed. In the last case, either the Tukey or Holms–Sidak test was used for all pairwise post hoc comparisons of the mean ranks of treatment groups. The Chi-square test ( ) was used to assess the differences between groups. SigmaStat® 3.5 software (SigmaStat, Point Richmond, CA, USA) and MATLAB® were used for statistical analysis.
) was used to assess the differences between groups. SigmaStat® 3.5 software (SigmaStat, Point Richmond, CA, USA) and MATLAB® were used for statistical analysis.
The significance level was set at p = 0.05. The results are shown as the mean ± SEM, except where otherwise indicated.
3.1. Clinical results
In this paper, we analysed all the patients diagnosed with temporal lobe epilepsy who underwent surgery to control seizures; therefore, some patients who underwent palliative care, indicated to diminish the frequency or severity of seizures, were omitted. The proportions of male and female patients were similar (Table 2), and their clinical features were identical, except for the distribution of the number of AEDs, which differed. In this cohort, 111 patients underwent surgery on the left temporal lobe, and 107 underwent surgery on the right.
| Variable | Men | Women | p |
| N | 112 | 106 | |
| Age (years) | 37.0 ± 1.1 | 39.7 ± 1.1 | 0.077* |
| Start epilepsy (years) | 13.9 ± 1.1 | 14.1 ± 1.0 | 0.521** |
| Time of epilepsy (years) | 23.1 ± 1.2 | 25.6 ± 1.2 | 0.159* |
| AED | 3.1 ± 0.2 | 2.8 ± 0.1 | 0.055** |
| One | 3.6 | 3.6 | < 0> |
| Two | 10.7 | 32.1 | |
| Three | 57.1 | 50.0 | |
| Four | 25.0 | 14.3 | |
| Five | 3.6 | 0.0 | |
| Frequency | |||
| Daily | 16.8 | 18.5 | 0.500*** |
| Weekly | 51.3 | 54.6 | |
| Monthly | 31.9 | 26.9 |
Table 2: Clinical and demographic features.
*Student-t test; **Mann-Whitney Rank Sum test; ***Chi-squared test.
In this group of patients, we obtained constant EI during the first year in 187/218 (85.8%) and nEI in 31/218 (14.2%). At the second post-operative year, five patients decayed from EI, remaining at this stage 181/218 (83.0%) and 37/218 (17.0%) in nEI.
No modification of AED treatment was accomplished in the first postoperative year.
In the EI group, the most frequent histological finding was hippocampal sclerosis (35.0%), followed by gliosis (26.3%), and no alterations were found in third place (14.6%). In the case of nEI patients, the most common finding was no alterations (31.6%), followed by gliosis (21.1%) and hippocampal sclerosis (15.8%). Pie charts showing the distribution of pathology in both groups can be found in Figure 1.
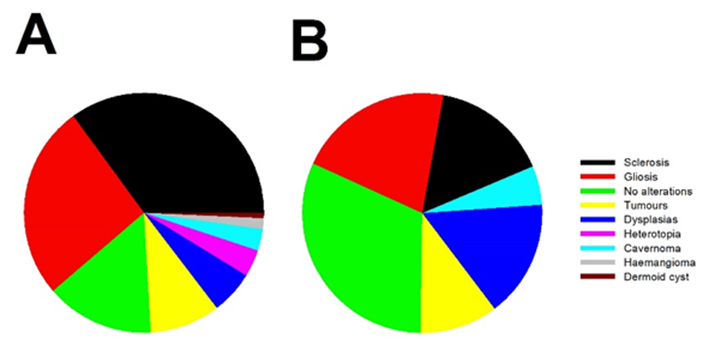
Figure 1: Pie charts showing the percentage of pathological findings from surgical specimens for a) EI and b) nEI patients.
3.2. Evaluation of presurgical accuracy in localization of the EZ.
We used the α coefficient in EI patients to evaluate the overall accuracy of the preSurg. Figure 2A shows that VEEG had a more significant percentage at α = 3 (92.0%), which implies lobar identification of the EZ. However, globally, all the preSurg values had a bimodal distribution, with the maximum occurring at α = 3 (lobar identification) and the second
occurring at α = 1 (hemispheric identification), as shown in Figure 2B, in which we overlapped the distributions of α for all the preSurg values.
The high percentage of α = 1 (not-localizing) results for SPECT, EEG, and MRI was shocking, mainly for MRI, and it is an index of patients' complexity (see below).

Figure 2: Accuracy in the identification of EZ in Engel’s grade I patients. (a) 3D bar graph showing the distribution of coefficients for the different preSurg; (b) 2D bar graph showing overlapped distributions of the different tests and the averages from all the test (black lines). Colors are the same for both graphs: dark red = SPECT, orange = EEG; yellow = MRI and green = VEEG.
Figure 2: Accuracy in the identification of EZ in Engel’s grade I patients. (a) 3D bar graph showing the distribution of coefficients for the different preSurg; (b) 2D bar graph showing overlapped distributions of the different tests and the averages from all the test (black lines). Colors are the same for both graphs: dark red = SPECT, orange = EEG; yellow = MRI and green = VEEG.
| Pre-surgical test | Outcome | Localization | Not localization | Total |
| EI | 0.386* (0.379-0.393) | 0.614 | 171 | |
| SPECT | nEI | 0.808 | 0.192** (0.179-0.206) | 26 |
| Total | 85 | 112 | 197 | |
| EI | 0.475* (0.467-0.482) | 0.525 | 158 | |
| EEG | nEI | 0.731 | 0.269** (0.253-0.285) | 26 |
| Total | 94 | 90 | 184 | |
| EI | 0.578* (0.571-0.584) | 0.422 | 187 | |
| MRI | nEI | 0.839 | 0.161** (0.150-0.173) | 31 |
| Total | 134 | 84 | 218 | |
| EI | 0.914* (0.911-0.918) | 0.086 | 187 | |
| VEEG | nEI | 0.935 | 0.065** (0.057-0.072) | 31 |
| Total | 200 | 18 | 218 |
Table 3: Matrix of confusion for all the preSurg. Variables are shown as probabilities and totals in absolute frequencies. Inside brackets are shown 95% confidence intervals.
* Sensitivity; ** specificity.
Table 3 shows that the S value for VEEG was the highest, practically double that for MRI. The lowest value was for SPECT. In the case of Sp, the highest value was for EEG, and the lowest was for VEEG. To describe a classification as a whole, we used AC information, which ranged from lowest to highest 0.360, 0.446, 0.518 and 0.797 for SPECT, EEG, MRI and VEEG, respectively. Therefore, despite the small Sp values for all the preSurg, especially for VEEG, the best classification was attained by VEEG.
The discriminatory ability of a preSurg can be expressed as a function of the likelihood ratio (LR), which can be either positive (LRP) or negative (LRN). LR reflects the degree of evidence of a presurgical location in favour of the presence of the condition (e.g., EI) relative to the absence of the condition (e.g., nEI). Both the LRP and LRN can help compare different preSurg values, which can be graphically plotted in a graph formed by 1-Sp on the x-axis and S on the y-axis (Figure 3). We plotted a straight line passing through those values for VEEG: (0.935, 0.914) and point (0,0). Then, we plotted a second line passing through the same point for VEEG and point (1,1). The graph is divided into 4 regions, and the region located below both lines represents the worst performance in confirming the presence of the condition and absence [23].
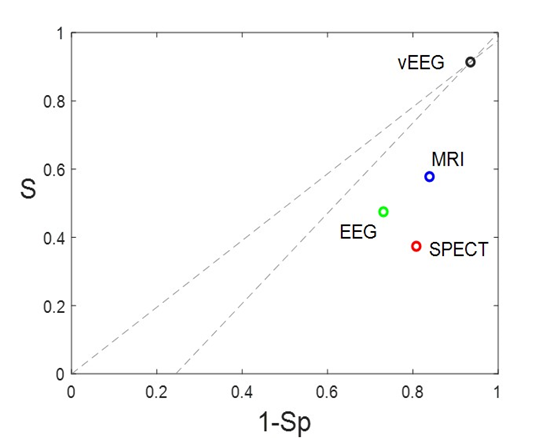
Figure 3: Different regions are defined by the VEEG values of 1-Sp, and S. Gray dashed lines going through this point and extreme points (0,0) and (1,1). Both lines have the expressions  and
and  . Points for the rest of preSurg are also shown, and all of them are located below both of the lines.
. Points for the rest of preSurg are also shown, and all of them are located below both of the lines.
Therefore, SPECT and EEG, like MRI, performed worse than VEEG in localizing the EZ.
However, thus far, we have assessed the localisation capacity, considering that we know that patients have either EI or nEI. Nevertheless, the most crucial feature of a presurgical test is the capacity to predict an outcome of EI after the specific result of that test. This information can be obtained by Bayes’ theorem for the PPV and PNV (Equations 8 and 9). Ranking from the most to least expected, the PPVs of the subsequent tests (between brackets) were as follows: VEEG (0.855) > MRI (0.806) > EEG (0.798) > SPECT (0.759). Therefore, all the tests predicted with p > 0.75 the
probability of obtaining an EI, although again, the highest value was for the VEEG.
The PNV indicates the probability of an nEI after a nonlocalizing result in the test, and the preSurg can be ordered from high to low: VEEG (0.111) < EEG>
Finally, we constructed a multiple binary logistic model to assess the contribution of the different preSurg to the outcome (see Appendix A). The null distance was d0 = 177.46. For only one variable, we could order the distances in ascending order: VEEG (34.9) < MRI xss=removed xss=removed xss=removed xss=removed>

where P represents the outcome, P = 1 EI, and P = 0 nEI.
The Hosmer–Lemeshow Statistic was 0.247, indicating that the model fit the data well. We have added the model's features to Table 1A in the Appendix.
| Variable | Coefficient (± SEM) | Wald statistic | Odds Ratio | Confidence interval |
| Constant | -4.425 ± 1.385 | 10.207 | 0.012 | 0.001-0.181 |
| VEEG | 3.106 ± 1.224 | 6.440 | 22.329 | 2.028-245.859 |
| MRI | 2.558 ± 1.364 | 3.516 | 12.914 | 0.891-187.250 |
| EEG | 1.905 ±1.419 | 1.803 | 6.718 | 0.417-108.335 |
Table 1A: Definition of accuracy for preSurg for different variables.
3.3. Evaluation of simplicity of diagnosis
We defined the simplicity of a patient's diagnosis as the degree of agreement between the preSurg the EZ, which was calculated as the average of the coefficients of lateralization. The range was [0,3], where 0 means no preSurg test identified the EZ and 3 means that all the preSurg tests did so. Let us have an example from a patient with left temporal mesial sclerosis, left temporal sharp waves at EEG, left temporal hypoperfusion at SPECT and a left theta pattern with ipsilateral automatisms and contralateral dystonia. The simplicity of this specific case is  . However, this is not always the case.
. However, this is not always the case.
The distribution of patient characteristics within our EI group is shown in Figure 4. We fitted the data to a logistic function using the least-squares method to obtain the expression.
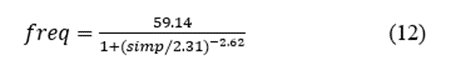
where freq and simp are the frequency and simplicity, respectively. This function fits the data very well (r2 = 0.9251).
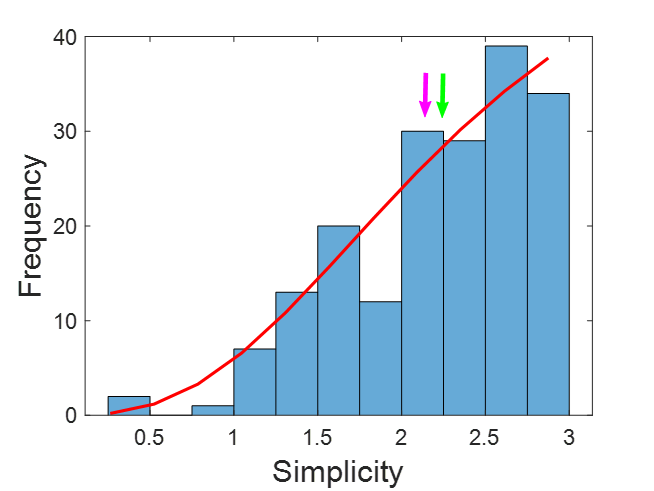
Figure 4: Distribution of simplicity for all the EI patients. Green arrow = median; Purple arrow = mean. The red line represents the logistic function fitted. Bin = 0.25.
The figure shows that the mean and median are the next most common. In fact, half of the data are between 2.25 and 3.0, representing 25% of the range. Nevertheless, the remaining half was distributed in the lower 75% range, from 0.2 to 2.25. In this case of low simplicity, the agreement of preSurg was very low.
We assessed the contribution of every preSurg to both the low and high simplicity groups and computed the percentage of localizing results in both groups, as shown in Figure 5.
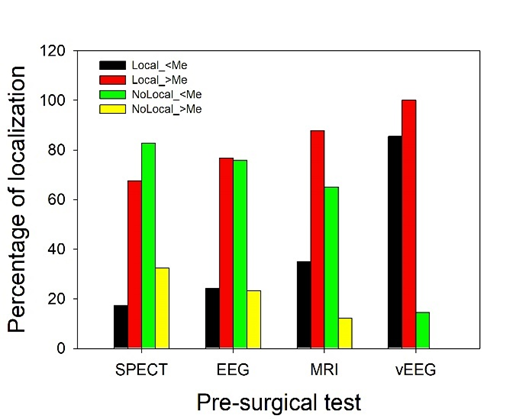
In patients with low simplicity, most of the MRI (65.0%), EEG (75.9%) and SPECT (82.6%) results were not localizing, but in the case of VEEG, 85.4% of patients had a localizing result. Therefore, practically all these patients underwent surgery using the VEEG information. In high-simplicity patients, on the contrary, the information obtained for localization from all the preSurg was greater than 67%, with 87.8% for MRI and 100.0% for VEEG.
We assessed the probability of adequate localization in patients with EI for different combinations of preSurg. Following the definition of probability [22], we have assumed the equivalence between relative frequencies and probabilities. These results are shown in Table 4.
| Event | Probability |
| Pr(EI) | 0.858 |
| Pr(nEI) | 0.142 |
 | 0.386 |
 | 0.475 |
 | 0.578 |
 | 0.920 |
 | 0.208 |
 | 0.265 |
 | 0.388 |
 | 0.302 |
 | 0.465 |
 | 0.535 |
 | 0.212 |
 | 0.158 |
 | 0.269 |
 | 0.297 |
 | 0.158 |
Table 4: Conditional probability for different combinations of Surg.
For individual tests, we ranked the preSurg from highest to lowest probability of localization: Pr(VEEG Ç EI) > Pr(MRI Ç EI) > Pr(EEG Ç EI) > Pr(SPECT Ç EI). The probability was greater for VEEG, in fact, 59.2% greater than for MRI. Interestingly, the probability of MRI results being slightly more significant than the chance ratio was because many patients did not present any specific lesions associated with epilepsy. Nevertheless, it must be remembered that these patients had EI, meaning that not performing an MRI alone did not exclude an excellent postsurgical outcome. For the two presurgical test combinations, we listed the results as follows: Pr(VEEG Ç MRI Ç EI) > Pr(VEEG Ç EEG Ç EI) > Pr(VEEG Ç SPECT Ç EI) > Pr(EEG Ç MRI Ç EI) > Pr(MRI Ç SPECT Ç EI) > Pr(EEG Ç SPECT Ç EI). All the pairs, including VEEG, better localized the EZ. Similar results were observed for the combination of the three tests. Finally, the combination of the four tests was localized to the EZ with a low probability (0.158).
In this work, we showed that VEEG was the preSurg method with the best ability to identify the EZ in TLE patients. Additionally, a very good outcome (85.8% of EI) could be attained even when a significant percentage of patients showed no localizing results on MRI (42.2% in EI). This is a very interesting finding because epileptic patients must be surgically evaluated even when no localizing information about the EZ can be obtained via MRI.
The first aspect we must consider is the unsatisfactory definition of EZ [2,13]. This operational definition cannot guarantee a positive definition of the EZ in most patients and depends on the postsurgical outcome. This situation has worsened with the appearance of the concept of network epilepsy and its variant of different threshold EZs [24] because, in this kind of pathophysiology, a true EZ could not be found [14, 15,18]. In fact, although presurgical evaluations and surgeries have recently decreased in some TLE patients at most centres, the number of nonlesional patients, patients requiring intracranial recordings, and patients requiring neocortical resection has increased [25]. It is essential to be conscious that the absence of structural lesions in imaging studies must not limit the referral of a patient to a specialized unit for presurgical evaluation.
Another important aspect to consider is the retrospective nature of our study. Obviously, we are conscious that selection bias cannot be avoided, especially for patients rejected for surgery. This is a common problem of single-center studies, where results strongly depend on the team's experience. However, this bias does not invalidate the results obtained, although we acknowledge that its value can be different for other teams.
Currently, imaging studies of a significant number of TLE patients who underwent surgery due to refractory seizures revealed structural lesions [26-31]. However, patients with nonlesional TLE can be candidates for standard surgery and have good postoperative outcomes comparable to those of patients with lesional TLE, although invasive recording is usually needed in these patients [32].
The main flaw of our approach is the highly defective definition of variables related to nEI patients. We have assumed that an nEI outcome is due to the presence of the EZ in a brain area other than the OpZ; however, this is not necessarily true. The EZ may have been adequately identified, but the resection was insufficient, or the patient suffered from network epilepsy and a new EZ substituted for the former EZ or subsequent pathology (e.g., infection or bleeding) following the surgery, biasing the outcome. The main problem is that we cannot unmask the actual reason. Therefore, the definitions of TN and FP and the coefficients given to patients in the nEI group are more dubious than those given to patients in the EI group. Fortunately, this definition does not excessively affect our results because the percentage of nEI patients was only 14.2%. In this sense, we obtained similar statistical results for the whole cohort and the nEI group, confirming this idea.
The value of the PPV is strongly dependent on the PD, and in cases where this value is low, the PPV can be underestimated. However, in our case, the PD was 0.858, which means that we could be confident in this probability. In the case of the PNV, the main problem was not the PD value but the difficulty in identifying actual FN cases. As mentioned above, this problem cannot be satisfactorily resolved until an EZ can be defined better.
It has been proposed that VEEG is not imperative in patients with unilateral mesial TLE hippocampal sclerosis who have compatible semiology with unilateral interictal epileptiform discharges (IEDs) ipsilateral to hippocampal sclerosis [31]. This finding agrees with our finding for uncomplicated patients because, in these cases, the preSurg test results will coincide; therefore, redundant information was obtained. A completely different problem emerges for complex patients, in which no matches are obtained for the preSurg test; in these patients, the most informative and mandatory preSurg test is VEEG. In fact, we observed that scalp VEEG was sufficient to identify the EZ. Indeed, hippocampal sclerosis on MRI, although probabilistically relevant as a predictor of a good outcome, cannot be identified with certainty by the EZ because all patients (or at least a probability near one) with hippocampal sclerosis should obtain an EI outcome, which is not valid, with percentages of EI between 67 and 82% [31,33]. Moreover, hippocampal sclerosis can be the main factor for predicting disease outcome, not the VEEG result [34].
Globally, the percentage of adults with EI postsurgical outcomes is between 60 and 90% [35-38]. In our series, we obtained 85.8%. This result is in the upper part of the interval. However, more importantly, a high percentage of patients in our study did not have any localizing lesions on MRI (up to 42.2%) despite the postsurgical outcome being EI. This is not the first time that surgery has been performed in nonlesional patients. In fact, more than a decade ago, patients with medically intractable epilepsy and normal MRI findings appeared to benefit from epilepsy surgery [39].
In our work, a very interesting finding was the close predictive value of EEG compared with MRI for most of the analyses performed (for α, PPV, PNV or probability), although some values obtained from confusion matrices were lower for EEG. This fact is more relevant when considering that EEG has access to neither intracranial data (as MRI or SPECT do) nor ictal events (as VEEG does. Therefore, this topic continues to be a very important topic in presurgical evaluation [6,25]. However, the low values obtained for interictal SPECT and the exclusion of these data from the logistic model indicate that this test is unnecessary to evaluate TLM patients systematically. We have not evaluated the utility of peri-ictal SPECT, although etomidate-activated SPECT can be used to localize the EZ very precisely during either interictal activity [40,41] or the ictal period.
It is commonly assumed that the agreement of preSurg will be correlated with functional outcome. Therefore, high congruence implies a better result than low agreement. This is a very reasonable hypothesis, but it is vital to keep in mind that not all the intrinsic information obtained from preSurg is similar, and on the other hand, patients will have intrinsic complexity, which means that presurgical evaluation needs to be highly individualized.
Finally, we would like to comment on an unexpected finding, as the presence of gliosis is the second most frequent histopathological finding in our series. Usually, gliosis is considered a non-specific finding in specimen tissue from epileptic patients, except for those cases suffering from neurocysticercosis [42,43], although it has been proposed that astrocyte activation could be associated with the appearance of epilepsy [44]. In fact, there is increasing evidence that astrocyte activation by albumin could be related to some epilepsies, especially those with blood-brain barrier dysfunction [45-47].
Obviously, we are aware of the limitations of the work. This is a single center study and, furthermore, techniques such as 1.5 T MRI or PET are not used. Therefore, the conclusions cannot necessarily be extrapolated to all centers. However, this may affect generalization to other hospitals but in no way invalidates the results. On the contrary, the fact of obtaining good functional results without these techniques makes it especially important for centers with limited access to the latest technological developments.
As a final summary, epilepsy patients cannot be excluded from presurgical evaluation because of the absence of lesions on imaging. Temporal lobe epilepsy is likely more complex than hippocampal sclerosis because it includes more pathophysiological aetiologies, and we must remember that epilepsy is primarily an illness affecting bioelectrical excitability [48-50]. Therefore, preSurg, which evaluates bioelectrical activity, mainly during seizures, is more directly related to pathophysiology than morphological, metabolic or vascular perfusion.
EZs in temporal lobe epilepsy patients can sometimes be very difficult to identify because preSurg do not overlap within the same anatomical region. Even in these complicated cases, presurgical evaluation should be performed because the outcome is not necessarily poor. In fact, the information obtained from VEEG can be enough to accurately identify the EZ and increase the probability of success in a group of patients with a high percentage of noninformative imaging studies. Scalp EEG is a very informative technique, although interictal SPECT can be considered unnecessary as a systematic presurgical test.
This work was financed by a grant from the Instituto de Salud Carlos III (ISCIII) PI23/00393 and was partially supported by FEDER (Fonds Europeen de Developpment Economique et Regional).
For every model, we computed the likelihood (L), which is defined as
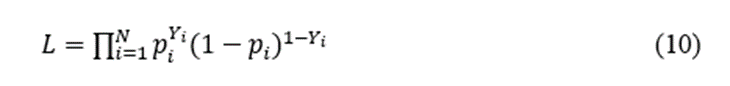
where pi is the probability of EI/nEI assigned by the model and Y1 = EI and Y0 = nEI. L is a measure of how good the model fits the real data. A better classification of all the patients by the model would give rise to L = 1. Usually, the numerical values are very small because it is preferable to convert a more useful variable, called deviance (d), defined as

Therefore, the lower d is, the better the prediction of a model. For every pair of us, we can obtain the following statistics: the likelihood ratio (LR), which follows a chi-squared  ) distribution, and N0-N degrees of freedom (d.o.f.). Then, LR has the form
) distribution, and N0-N degrees of freedom (d.o.f.). Then, LR has the form
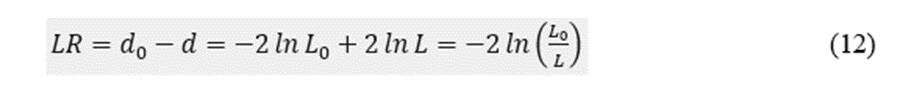
The algorithm for building a better regression model follows the next steps (Silva, Barroso 2004[20]):
Initially, we computed the “null model” (L0) and obtained the null deviance (d0). Then, we computed a simple binary regression model for every preSurg, obtaining the deviances for everyone, i.e., dSPECT, dEEG, dMRI and dVEEG. Obviously, each of these parameters was smaller than d0. Then, we computed 〖LR〗_i;i=SPECT,EEG,MRI,vEEG following Equation 12 and identified the highest. Let us suppose 〖LR〗_k=-2 ln(L_0/L_k )
We evaluated the significance of the LR by means of χ^2. If 〖LR〗_k was greater than 3.84 (95 percentile for χ^2 one d.o.f.), then the variable k was incorporated into the model.
We computed two-variable models, obtaining L_ki, where i = preSurg-{k}. Obviously, we had three possibilities. We identified the lowest one, named L_kl. We evaluated 〖LR〗_kl=-2 ln(L_k/L_kl ), and if it was greater than 3.84, we incorporated the variable l into the model.
We repeated this procedure until the four preSurg were incorporated or after a new incorporation did not result in significant results.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.
