AUCTORES
Globalize your Research
Review ariticle | DOI: https://doi.org/10.31579/2690-1897/255
Department of Pharmacology, School of pharmacy, RK Universit Rajkot, India.
*Corresponding Author: Chinmyee saha, Department of Pharmacology, School of pharmacy, RK Universit Rajkot, India.
Citation: Chinmyee Saha, Ishita Zalavadiya, (2025), Necrosis and Gangrene – A Haunting Tale of Decay, J, Surgical Case Reports and Images, 8(5); DOI:10.31579/2690-1897/255
Copyright: © 2025, Chinmyee Saha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 06 May 2025 | Accepted: 21 May 2025 | Published: 29 May 2025
Keywords: necrosis; inflammatory disorders; cell death; gangrene; treatment types; risk factors
Necrosis is a crucial pathological process implicated in various disease states, including stroke, organ failure, and inflammatory disorders. It refers to the premature cell death characterized by mitochondrial dysfunction, altered membrane permeability, and uncontrolled release of cellular contents. This article provides an overview of the molecular mechanisms underlying necrosis, sign & symptoms, causes, emphasizing the role of different diseases in necrosis formation & different types of necrosis. Additionally, the detrimental consequences of necrotic cell death on tissue homeostasis, triggering a robust inflammatory response resulting in tissue damage, will be discussed with details about treatment. Moreover, new insights into the role of autophagy in modulating necrotic cell death and potential therapeutic interventions will be explored. Furthermore, the article highlights an overview of gangrene, including its causes, symptoms, and treatment options. It explores the different types of gangrene, such as dry gangrene, wet gangrene, and gas gangrene, highlighting their unique characteristics and risk factors. Additionally, the article discusses the importance of early detection and prompt medical intervention in preventing complications and saving lives. Lastly, it emphasizes the role of preventative measures, such as practicing good hygiene and managing underlying health conditions, in reducing the risk of developing gangrene. This article aims to enhance medical professionals' understanding of necrosis and gangrene, enabling accurate diagnosis and optimal management. By unravelling the underlying mechanisms and highlighting the diverse manifestations, this comprehensive overview aids in the identification and treatment of these potentially life-threatening conditions.
Necrosis and gangrene are both terms used to describe a type of tissue death in the body. While they are related, they have certain distinctions. Necrosis is a broad term used to refer to the unnatural death of cells, tissues, or organs in the body. It may occur due to various reasons such as injury, infection, lack of blood supply, toxins, or certain medical conditions. Necrosis can affect any part of the body and can range in severity from a small localized area to widespread tissue death. The affected tissue becomes non-functional, loses its structural integrity, and cannot be repaired or regenerated. Gangrene, on the other hand, specifically refers to the death of body tissue caused by a lack of blood supply. It often occurs when an injury or infection disrupts or blocks the blood flow to an area of the body. Gangrene can be categorized into dry gangrene, when the affected tissue becomes dry and shrunken, and wet gangrene, when there is added bacterial infection resulting in a foul odor, pus, and fluid accumulation. If left untreated, gangrene can spread rapidly, leading to serious complications and even death. Both necrosis and gangrene can lead to significant health problems and require immediate medical attention. Surgical intervention, such as removing the dead tissue, is often necessary to prevent the spread of infection and preserve the overall health of the patient. In severe cases, amputation may be required to save the patient's life. In conclusion, necrosis is a general term for the unnatural death of tissue, while gangrene is a specific type of tissue death caused by a lack of blood supply. Understanding the causes, symptoms, and treatment options for necrosis and gangrene is crucial in effectively managing these conditions and preventing further complications.
Necrosis is a type of cell damage that leads to autolysis, causing cells in live tissue to die prematurely, followed by tissue disintegration and an inflammatory response [1]
Lytic Enzyme-mediated Cell Digestion:
This process results in the destruction of both the cell membrane and all other components of the cell [1].
Protein Denaturation:
Acidic pH causes the nucleus to undergo alterations such as chromatin clumping (pyknosis), nucleus disintegration (karyolysis), and fragmentation of the nucleus (karyorrhexis) [1].
Pain, redness, swelling, blisters, fever, chills, and foul-smelling liquid dripping from incisions are signs of a serious skin wound.
Necrosis symptoms differ according to the part of the body where the affected tissue has formed.
For instance, the following are signs of renal necrosis:
Pain in the flanks or back,Urine that is bloody, hazy, or dark, Excessive or painful urination, Frequently or heavily urinating at night
When necrosis stems from a wound, symptoms can include:
Severe pain, high fever, chills, elevated heart rate, numbness, redness, blisters, popping sound, foul-smelling liquid, trouble thinking, and excessive perspiration are signs of a severe infection.
Necrosis occurs when tissues infected lack blood and oxygen, resulting from factors like blood clots, wounds, infections, long-term illnesses, and poisons that reduce blood supply.
6.1Injury
Necrosis occurs when tissues infected lack blood and oxygen, resulting from factors like blood clots, wounds, infections, long-term illnesses, and poisons that reduce blood supply [2]
Other wounds that may result in necrosis include as follows:
Burns caused by electricity, Fractures of the bones, Brain damage from trauma, Burning chemicals, Exposure to radiation
6.2 Infarction
Infarction-related necrosis, often caused by blood clots like deep vein thrombosis, results in tissue death due to insufficient blood flow to the affected area [3]
6.3 Infection
Necrosis, caused by various illnesses, can result from even small infected cuts or scrapes, with Streptococcus group A being the most prevalent necrotizing infection [4]
The most prevalent locations for necrotic tissue produced by infection are the genitalia and the extremities, particularly the hands and feet [5]
Necrosis can also be caused by the following viruses:
HIV, or the human immunodeficiency virus, Type 1 herpes simplex virus (HSV-1), West Nile virus,The virus linked to vaccinations (smallpox) [6]
Disease
Necrosis is a common symptom in autoimmune diseases, with systemic lupus erythematosus (SLE) being the most common. People using corticosteroids to treat SLE are at higher risk due to their gradual bone weakening. Necrosis can also be caused by various illnesses that damage blood vessels and restrict blood flow to bones and tissues [7].
They include:
Alcoholism, Sickle cell disease, decompression disease, chronic kidney failure, Cushing's disease, Gaucher disease, toxins, and chemical agents trigger necrosis, while certain disorders cause fat cell accumulation [8].
Arsenic exposure, levamisole-laced cocaine, brown recluse spider bite venom, and bites from various creatures can trigger kidney necrosis, while contaminated groundwater, rat poison, and venom from spiders can also cause necrosis[9,10,11].
7.1 Ischemia:
Ischemia is a condition characterized by restricted blood supply to tissues, resulting in a shortage of oxygen and glucose for cellular metabolism.
7.2 Physical Agents:
Physical agents like heat or radiation can damage cells by coagulating their contents, while impaired nutrient supply can deprive them of essential materials for survival.
7.3 Chemical Agents:
Necrosis can be caused by internal factors such as trophoneuratic disorders, nerve cell injury, pancreatic enzymes, toxins, and pathogens [12]. Age, alcohol abuse, open wounds, trauma, and long-term use of corticosteroids increase the risk of necrosis. Alcohol is cytotoxic, and heavy alcohol use can kill liver cells, triggering necrosis[13,14]. Necrosis is a common complication of autoimmune diseases like lupus, making it a significant risk factor. [8]
Other conditions that increase the risk of necrosis include:
• Diabetes mellitus • Vascular disease • Chronic renal (kidney) failure • HIV [14]
a) Coagulative Necrosis, b) Liquefactive Necrosis, c) Caseous Necrosis, d) Fat Necrosis, e) Fibrinoid Necrosis [14]
8.1 Coagulative Necrosis:
Coagulative necrosis is a type of necrosis where cell boundaries are preserved but cellular details are lost. Severe ischemia causes denaturation or coagulation of cell membrane proteins, denatures enzymes, prevents proteolysis, requires leukocytes, digests dead cells, and removes debris through phagocytosis. This type occurs in infracts like the heart, kidney, and adrenal glands. [14]
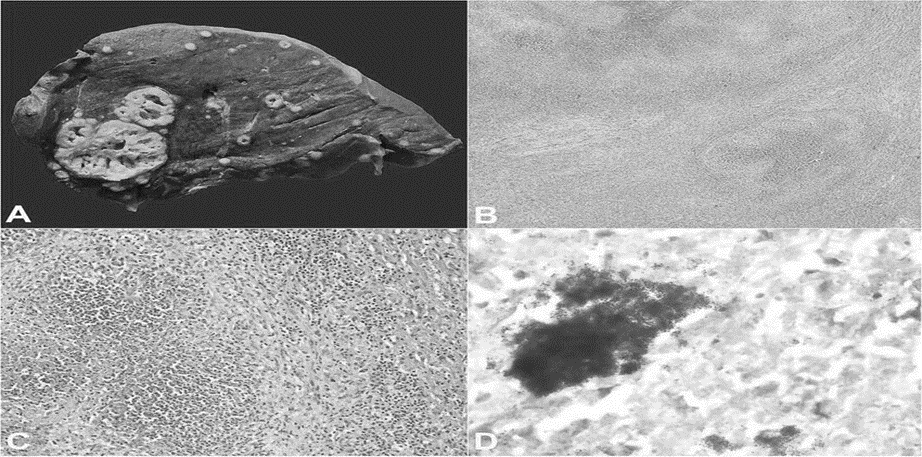
Figure 1: Coagulative necrosis of Spleen [58]
8.2 Liquefactive Necrosis:
Liquefaction necrosis is a process where cells are destroyed by powerful enzymes, often seen in bacterial or fungal infections. This occurs when autolysis and heterolysis prevail over protein denaturation. The tissue is digested by leukocytes, turning it into a liquid viscous mass. If initiated by acute inflammation, the material is called pus. Examples include abscesses, brain abscess, and empyema gall bladder. [14]
Ø Example is Abscess (Collection of pus in tissue or on hollow viscus lined by pyogenic membrane), Ischemic necrosis of Brain Ø Example of Abscess- Breast abscess, Liver abscess, Brain abscess, Empyema gall bladder (pus accumulated in hollow viscus in gall bladder Ø If ischemia occurs in brain it forms liquefaction necrosis, because cell membrane of brain contains more lipid than protein, that’s why liquefaction necrosis occur. [14]
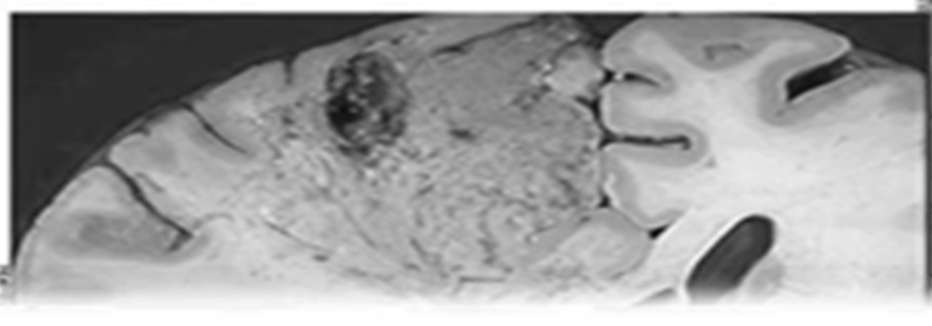
Figure 2: Liquefactive Necrosis of Brain [57]
8.3 Caseous Necrosis:
Caseous necrosis is a type of lung necrosis caused by tuberculous infection, resembling cheese due to its lipopolysaccharide structure. Fat necrosis can be categorized into two types: traumatic fat necrosis, which occurs after trauma, and enzymatic fat necrosis, which occurs due to acute pancreatitis or pancreatic injury. Traumatic fat necrosis occurs in subcutaneous tissue, extremities, or female breasts, while enzymatic fat necrosis occurs in pancreatic fat, producing glycerin and fatty acids that form calcium soap at the site of fat necrosis. [14]
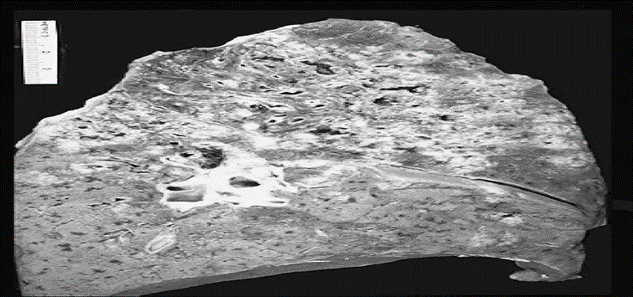
Figure 3: Caseous Necrosis of Lung [57]
8.4 Fat Necrosis:
Fat necrosis can be categorized into two types: trauma-induced and enzymatic.
8.4.1 Traumatic Fat Necrosis:
Ø It is focal type of necrosis of fat, following trauma Ø Site of Traumatic fat necrosis: It may be – Fat of subcutaneous tissue of -Abdomen, Extremities, Female breast Ø Fat is come out of the cell from fat cell as neutral fat following rupture of the cell, due to trauma & this fat as droplet is engulfed by macrophages [14]
8.4.2 Enzymatic fat necrosis:
ØNecrosis / hydrolysis of fat by enzyme lipaseØCause of Enzymatic fat necrosis -Acute Pancreatitis, Pancreatic InjuryØThe cell containing lipase, cell membrane become disrupted/weakØIn pancreatic Injury , the cell membrane undergoing injury, from injured cell membrane of pancreatic cell, pancreatic lipase out of the cell, causes hydrolysis of intra cellular fat in different areas within abdomen-Pancreatic fat, Peri-pancreatic fat, Omental fat,ØHydrolysis of intracellular fat, then release or production of – Glycerin & Fatty acid,ØThese fatty acids combine with calcium & form calcium soap at site of fat necrosis,ØThis calcium soap precipitate as chalky material, Ø Which enable the surgeon & the pathologist to identify the lesion [14]
8.5 Fibrinoid Necrosis:
Fibrinoid Necrosis is a unique form of necrosis, visible through light microscopy, characterized by the deposit of antigen and antibody complexes in artery walls, resulting in a bright pink, amorphous appearance, often seen in immunologic cell injury, hypertension, and peptic ulcers.[14]
Necroptosis, a nonapoptotic form of cell death, occurs following the activation of the tumor necrosis receptor (TNFR1) by TNFα, despite TNFα being considered an inducer of apoptosis. Other cellular receptors trigger necroptosis, including death receptors, Toll-like receptors, and cytosolic nucleic acid sensors. These pathways induce type I interferon (IFN-I) and TNFα production [15,16] , promoting necroptosis in an autocrine feedback loop. However, additional inhibition of the proteolytic enzyme Caspase-8 by microbes or pharmacological agents triggers the necroptotic pathway. Active RIPK1 is recruited within an oligomeric complex that includes FADD, caspase-8, and caspase-10 [17,18]. In the absence of caspase-8 activity, RIPK1 recruits and phosphorylates RIPK3, forming a complex called the ripoptosome [19,20]. The RIPK1/RIPK3 complex recruits and phosphorylates MLKL, thus forming the necrosome [21,22]. The RIPK1/RIPK3/MLKL pathway induces cell death through mitochondrial destabilization via a phosphoglycerate mutase family member 5 (PGAM5)- and dynamin-related protein 1 (DRP1)-dependent pathway [23]. However, mice with genetic deficiency of Pgam5 or Drp1 display no alterations in TNF-induced necroptosis. MLKL oligomerization and membrane translocation depend on a specific inositol phosphate (IP) code. MLKL oligomerization dictates the kinetics and threshold of necroptotic cell death. Although mitochondrial damage and ROS production are not directly involved in the establishment of necroptotic cell death, RIPK3 has downstream effects on mitochondria, promoting aerobic respiration and mitochondrial ROS production. The study explores the role of MLKL in TNF-induced necroptosis [24], a process that compromises cellular integrity. MLKL opens calcium or sodium ion channels, allowing cell swelling and rupture [25,26]. It forms pores in the plasma membrane through interactions with negatively charged phosphatidylinositol phosphates [27,28,29]. The MLKLD139V mutant gene alters the two-helix brace structure, causing lethal inflammation in homozygous mice [30]. MLKL oligomerization also influences necroptotic cell death kinetics and threshold.The necroptotic pathway is a complex process regulated by various factors, including RIPK1's recruitment to signaling complex I, TRAF2 and TRAF5, and RIPK1 [31-36]. Ubiquitylation of RIPK1 and RIPK3 prevents cell death and is essential for NFκB-dependent induction of proinflammatory genes [37]. Low extracellular pH can inhibit RIPK1's kinase activity, preventing cell death [38]. The "death signal" is triggered by CYLD deubiquitylation of TRAF2 and RIPK1, leading to the formation of the ripoptosome [39-43]. The mitochondrial protein Smac promotes necroptosis by triggering proteasomal degradation of cIAP1/2 and XIA [44]P, leading to the development of Smac mimetics as therapeutic tools against cancer and HIV-infected cells [45-47].The immune system has evolved a way to bypass inhibition upstream of RIPK3 as a rapid countermeasure to block viral spread[46,47]. The murine cytomegalovirus (MCMV) DNA genome activates the DNA-dependent activator of IFN regulatory factor (DAI), which binds directly to RIPK3 through its RHIM domain, virtually bypassing RIPK1[48,49]. Recently, Lim et al. proposed a more ambivalent role for DAI in regulating necroptosis in macrophages, demonstrating that DAI protects autophagy-incompetent Atg16l1−/− cells from necroptosis. Depending on the context, DAI [50] acts as either an activator or a suppressor of necroptosis. The exact regulation of these opposite functions of DAI remains to be elucidated. RIPK1 plays a crucial prosurvival role during embryonic development, acting as a "sponge" preventing the activation of RIPK3/MLKL signaling by TRIF or DAI [51-53]. A more extensive study of the regulatory processes involved in the induction of necroptosis is necessary to fully solve this puzzle. In fact, uncontrolled necroptosis or RIPK3-dependent apoptosis causes Ripk animals to die shortly after birth. It is assumed that RIPK1-RHIM typically functions as a "sponge," blocking TRIF or DAI from activating the RIPK3/MLKL signalling pathway, which is currently known to be an effector of the lethality of Ripk1 deletion. Taken together, these findings highlight the critical function of the RHIM domain interaction in both initiating and controlling necroptosis. The cellular environment and the upstream pathway that is triggered appear to have an impact on the RHIM-containing protein that pulls the "RIPK3 trigger." To properly unravel this mystery, a more thorough investigation of the regulatory mechanisms underlying the induction of necroptosis is required. The regulation mechanism of necroptosis is further complicated by the existence of orthologs of MLKL in humans and mice.

Figure 4: Mechanism of Necrosis [ 58]
In figure, Necroptosis is triggered downstream of death domain receptors (e.g., TNFR and Fas) and Toll-like receptor (TLR)-4 or TLR3. Upon activation, these receptors recruit the adapter proteins FADD, TRADD, and TRIF, which interact with RIPK1 and caspase-8 or -10. First, RIPK1 is ubiquitylated by IAPs, keeping it nonfunctional and enabling proinflammatory downstream activity via NFκB. After detection of a “death signal”, RIPK1 is deubiquitylated by CYLD and can thus recruit RIPK3. The RIPK1/RIP3 complex recruits and phosphorylates MLKL. In the presence of highly phosphorylated inositol phosphate (IP6), phosphorylated MLKL oligomerizes, thus forming the necrosome. MLKL oligomers translocate to phosphatidylinositol phosphate (PIP)-rich patches in the plasma membrane and form large pores. Ultimately, MLKL pores lead to necroptotic cell death by allowing ion influx, cell swelling, and membrane lysis followed by the uncontrollable release of intracellular material. The cytosolic nucleic acid sensors RIG-I and cGAS/STING also contribute to necroptotic cell death, as they induce IFN-I and TNFα and thus promote necroptosis via an autocrine feedback loop. Downstream of TNFR or TLR engagement, active caspase-8 cleaves the cytokine blocker N4BP1, thus promoting an increase in cytokine release. Once activated, RIPK3 phosphorylates the pyruvate
dehydrogenase complex (PDC) in mitochondria and promotes aerobic respiration and mitochondrial ROS production. In the presence of cytosolic DNA released from infecting microbes, DNA-dependent activator of IFN regulatory factor (DAI) recruits RIPK3 and thus bypasses RIPK1 for activation of MLKL and formation of the necrosome complex.
Gangrene is a severe, potentially life-threatening condition resulting from tissue necrosis, often caused by injury, infection, or chronic health issues. Diabetes and long-term smoking increase the risk. [14]
10.1 Gangrenous Necrosis:
a.Wet Gangrene, b.Dry Ganggrene c.Gas Gangrene
10.2 Wet Gangrene:
Occurs in moist tissue like mouth , bowel, lung cervix 2. Venous obstruction 3. Moist Swollen, Dark 4. Bacteria present 5. Bed sores [14]
10.3 Dry Gangrene:
Occurs in Toes & feet due to arteriosclerosis 2. Arterial obstruction 3. Dry, Black 4. No bacteria 5. Trauma

Figure 5: Dry Gangrene [16]
10.4 Gas Gangrene:
Myonecrosis, a medical emergency caused by Clostridium perfringes bacteria, is a bacterial infection that produces gas in gangrene tissue, typically found in muscle or colon.
10.5 Treatment of Gangrene:
Antibiotics alone are not effective because they do not penetrate ischemic muscles sufficiently to be effective. 1. Penicillin is given as an adjuvant treatment to surgery 2. In addition to antibiotics Hyperbaric Oxygen Therapy (HBOD) is used and acts to inhibit the growth of and kill the anaerobic Clostridium perfringens.
11.Consequences of Necrosis:
Acute or Chronic inflammation, Lysis & Absorption, Ulceration & Cavity Formation, Encapsulation, Calcification, Immunological reactions subcellular components released by dead tissue
12.Treatment of Necrosis
There are many causes of necrosis. Treatment of necrosis typically involves 2 distinct processes:
12.1 Debridement:
Referring to the removal of dead tissue by surgical or non-surgical means, is the standard therapy for necrosis. Depending on the severity of the necrosis, this may range from removal of small patches of skin, to complete amputation/cutting of affected limbs or organs
12.2 Chemical removal of necrotic tissue:
It is another option in which, enzymatic debriding agents categorized as – Proteolytic, Fibrinolytic, Collagenases are used to target the various components of dead tissue.
| Necrosis | Gangrene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 1 Difference between Necrosis & Gangrene [54]
In conclusion, necrosis and gangrene are serious conditions that result from the death of tissues in the body. Necrosis refers to the loss of blood supply to a specific area, leading to tissue death, while gangrene is a progressive form of necrosis that can spread to surrounding tissues. Both necrosis and gangrene can have various causes, including infection, trauma, underlying medical conditions, or lifestyle choices such as smoking. The symptoms of these conditions can include pain, discoloration of the affected area, foul-smelling discharge, and the formation of ulcers or sores. In this article, the types of necrosis as well as gangrene have discussed also detail with proper figures Prompt medical attention is crucial in the management of necrosis and gangrene. Treatment options may include surgical removal of the affected tissue, antibiotics to control infection, wound care, and in severe cases, amputation. Prevention is the best approach to avoid necrosis and gangrene. It is important to maintain good hygiene, manage underlying medical conditions, quit smoking, and take prompt action in case of any signs or symptoms that may indicate tissue damage. Overall, necrosis and gangrene are serious conditions that can have severe consequences if left untreated. Early recognition, proper medical intervention, and preventive measures are essential in minimizing the risks and complications associated with these conditions. Necrosis , is the death of body tissue due to insufficient blood flow, caused by injury, radiation, or chemicals. It cannot be reversed and allows cells to recruit a defensive or reparative response to damaged organisms. Untreated necrosis can result in gangrene, requiring surgical removal of necrotic tissue, a procedure known as debridement.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.
I would like to thank Assistant Professor Ishita Zalavadiya for her expert advice and encouragement throughout this article.