AUCTORES
Globalize your Research
case report | DOI: https://doi.org/10.31579/2690-1919/501
Arnas Garibaldi Catania Italy.
*Corresponding Author: Graziella Privitera, Arnas Garibaldi Catania Italy.
Citation: Privitera G, Siciliano E, Vitale A, Bartoloni G, Fornito MC, et al, (2025), Isolated Hepatic Sarcoidosis: Lessons learned from a Case Report, J Clinical Research and Reports, 19(2); DOI:10.31579/2690-1919/501
Copyright: © 2025, Graziella Privitera. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 11 February 2025 | Accepted: 28 February 2025 | Published: 26 March 2025
Keywords: hepatic sarcoidosis; pet-ct scan; liver biopsy
Isolated hepatic localization of sarcoidosis is rare. Several previous reports have described clinical presentation of this disease. We describe the case of a female patient who was referred to the hospital due to fatigue, pruritus and abnormal liver functional test. We performed ultrasound-guided liver biopsy according to the higher accumulation of radiometabolic tracer in PET-CT imaging. Histological report was compatible with hepatic sarcoidosis. We underline the usefulness of using PET-CT scan to guide liver biopsy.
Sarcoidosis is a chronic systemic inflammatory condition which is characterized by noncaseating granulomas of unclear etiology. The liver impairment is variable and can occur in the absence of pulmonary disease. The finding of an isolated localization of hepatic sarcoidosis, however, is rare. Despite its asymptomatic course, between 50% and 60% of patients exhibit granulomas at liver biopsy, emphasizing the importance of the histology in the diagnostic pathway of hepatic sarcoidosis. [1] Liver involvement is characterized by a cholestatic pattern of abnormalities, whereas transaminase elevations is generally less severe and less frequent. Our case report supports the importance of suspect a diagnosis of hepatic sarcoidosis even in the absence of a pulmonary involvement. We aim to underline the essential diagnostic role of the histology and the importance to make guided- liver biopsy with imaging such as PET-CT scan.
A 64-years-old female outpatient presented to the Liver Unit complaining pruritus (1/4 according to verbal rating scale), fatigue and unintentional weight loss (3 kg in 6 months). She had a history of high blood pressure, osteoporosis, adnexectomy at the age of 24-years old, hypercholesterolemia, psoriasis and gastroesophageal reflux disease (GERD – Grade A, Los Angeles classification); she denies smoking, alcohol consumption or intravenous drugs. Family history is insignificant. At presentation the patients was afebrile, normotensive (with blood pressure 120/80 mmHg) and heart rate of 60 bpm. Physical examination was unremarkable.
As shown in Table 1, laboratory workup upon admission revealed increment of gamma-glutamyl transpeptidase (x2.0 UNL normal range) and alkaline phosphatase (x1.3 UNL normal range). The liver ultrasound showed an abnormal pattern of heterogeneous echogenicity of the parenchyma. The spleen was normal in size with homogeneous structure and the presence of an oval hypoechoic lesions with no vascular signals. Vibration controlled transient elastography (VTCE) excluded significant fibrosis (LSM: 4.6 kPa, IQR 7%). Abdominal contrast-CT scan showed multiple hypodense lesions diffuse in all hepatic segments, detectable only in the venous phase.
Further testing revealed absence of autoantibodies, including negative antinuclear antibody (ANA), ANA PBC-specific (anti-gp210, anti-sp100), and anti-mitochondrial antibody (AMA). The sensitivity and specificity of angiotensin-converting enzyme (ACE) are low; our patient had normal level of ACE. We performed an abdomen MRI which showed no changes in the intrahepatic and extrahepatic biliary ducts with no further significant elements for the differential diagnosis.
A liver biopsy was performed with the evidence of granulomatous inflammation inside the portal spaces, damage to the bile canaliculi and the presence of giant cellular epithelioid elements. The leading differential diagnosis for our patient included primary biliary cholangitis and hepatic sarcoidosis. In the suspicion of liver localization of sarcoidosis the patient underwent a chest CT scan and a pneumological consultation, both excluded pulmonary sarcoidosis. We carried on the diagnostic process by performing PET-CT scan with 18 FDG (figure1) which described non-homogeneous distribution of radiopharmaceutical contrast in the liver, with some areas of hyper-uptake in particular between the second and fourth segments and a focality in the upper third of the spleen, highly suggestive for hepatic and splenic localizations of sarcoidosis. The case was discussed in a multidisciplinary setting suggesting to perform a liver biopsy based on PET-CT images. Therefore, the patient underwent a second ultrasound-guided liver biopsy in the PET-CT marked area. The histological report was conclusive for noncaseous granuloma, highly suggestive of localized sarcoidosis (figure 2). Diagnostic work up excluded alternative infective and immune-mediated causes of granulomatous disease. She was started on prednisone in combination with ursodeoxycholic acid (UDCA), with normalization of GGT and ALP.
| On admission | Reference range | |
| ALP (U/l) | 156 | 50-116 |
| GGT (U/l) | 114 | <55> |
| ALT (U/l) | 12 | 0-55 |
| AST (U/l) | 28 | 5-34 |
| Albumin(g/dl) | 3.99 | 3.5-4.6 |
| TotalBilirubin (mg/dl) | 0.6 | 0.3-1.2 |
| Creatinine (mg/dl) | 0.62 | 0.73-1.18 |
| INR | 1.05 | 0.9-1.1 |
| Haemoglobin (g/dl) | 13.3 | 13.6-17.2 |
| Platelets (10^3 µl) | 222 | 156-373 |
| WhiteBlood Cells (10^3µl) | 5.0 | 4.3-10.3 |
| IgM (mg/dl) | 275 | 40-230 |
| IgG (mg/dl) | 1256 | 700-1600 |
| IgA (mg/dl) | 417 | 70-400 |
| ANA | Negative | |
| AMA | Negative | |
| ASMA | Negative | |
| LKM1 | Negative | |
| ds-DNA | Negative | |
| C- ANCA | Negative | |
| P-ANCA | Negative | |
| CRP (mg/dl) | 0.10 | 0.01-0.5 |
| Ferritin (ng/ml) | 256 | 21-275 |
| ACE (U/L) | 84.3 | 66-114 |
| HBsAg,HBcAb HCV-Ab | Negative Negative |
Table1: Laboratory work at admission
ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; ANA, Anti-Nucleus Antibodies; AMA, Anti-Mitochondria Antibodies; ASMA, Anti-smooth muscle Antibodies; LKM1, Anti- Human Liver-Kidney microsomes Antibodies 1; ds- DNA, Anti-dsDNA Antibodies; ANCA, Anti-neutrophil Cytoplasmic Antibodies; CRP, C-Reactive Protein; ACE, angiotensin converting enzyme; HBsAg, hepatitis B surface antigen; HBcAb, hepatits B core antibody; HCV-Ab, hepatitis C antibody.

Figure 1: PET/CT investigation indicative for findings of high metabolic activity in the liver and spleen.
The whole body PET/CT examination documents area of focal accumulation of the tracer carbohydrate metabolism which is projected to the left lobe of the liver (SUVmax 9.23), in all likelihood biopsy site. The distribution of the radiopharmaceutical in the liver is markedly uneven with some areas of hyperuptake in particular in the S2/S4 site (SUVmax 5.5). Furthermore, a hypersensing focality in the upper third of the spleen is noted (SUVmax 4.89). Some areolae of hyperfixation, in the absence of a clear anatomical counterpart on the CT images of co-registration, project to the iliac wings bilaterally. Hyperfixation at the right sternoclavicular joint. Limited to the resolving power of the method (approximately 5 mm), no further areas of accumulation of the tracer in the remaining body regions investigated.
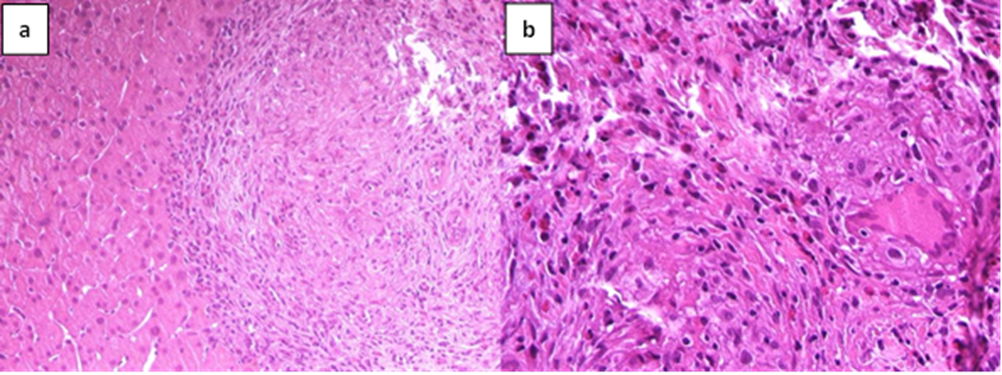
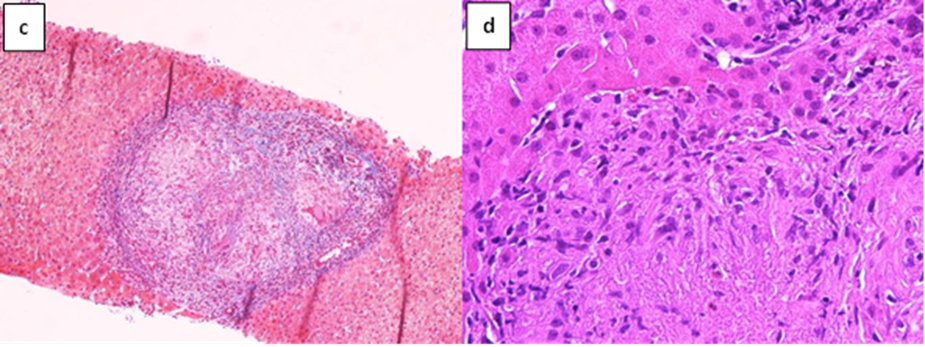
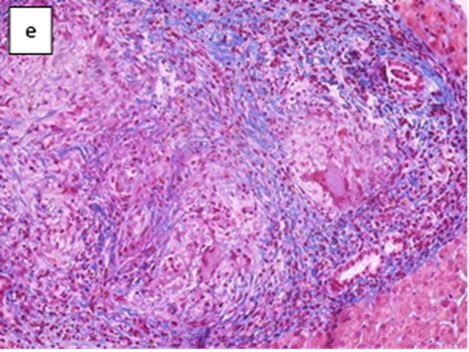
Figure 2: Pathological findings of the liver biopsy.
Sarcoidosis is a rare systemic granulomatous disorder that generally involves lung and hilar lymph nodes leading to a restrictive lung disease and chronic respiratory failure. The histopathological hallmark of sarcoidosis is the presence of non-caseating granulomas, which are clusters of immune cells that form in response to an unknown trigger. Other organs may be involved, such as the gastrointestinal tract, the liver, the spleen and kidneys [1].
While the exact pathogenesis remains unclear, sarcoidosis is considered as an immune-mediated disease. It is believed that various potential agents, such as viruses, pollutants, or allergens, could act as irritant triggers. Up to now, there is no identification of the precise triggering. Genetic factors seem to be important in pathogenesis of the disease, particularly human leukocyte antigen (HLA).
Immune responses through TH1 pathway to putative infectious pathogens may be implicated in the development of granulomas [2].
Liver involvement in sarcoidosis is common but the course of the disease tend to run mild with pulmonary involvement dominating the clinical picture, and hepatic complications remain often under-recognized. Between 3.6 and 30% of patients with systemic sarcoidosis have hepatic involvement at diagnosis, which varies greatly between studies [3]. Clinical presentation of hepatic sarcoidosis may be heterogeneous and spread from asymptomatic or incidental findings to systemic symptoms such as fatigue, pruritus, jaundice, abdominal pain. In the most advanced forms, liver damage could lead to severe portal hypertension (with esophageal varices), cirrhosis (with increased risk of development of HCC) and transplantation [4].
Hepatic sarcoidosis continues to represent a diagnostic challenge due to nonspecific symptoms and overlap with other liver diseases [5]. The diagnosis is usually based on liver biopsy evidence of non-caseating granulomas in the liver, biliary tract and gallbladder, in the presence of a clinical history of systemic sarcoidosis; there are no established diagnostic criteria for this condition.
In line with previous data, our clinical case of hepatic sarcoidosis is characterized by a cholestatic pattern of abnormalities, likely reflecting the infiltrative nature of the disease. Alternative diagnosis must be excluded such as primary biliary cholangitis (PBC). The two granulomatous diseases can be differentiated based on the characteristics of the granuloma, in PBC granulomas tend to be portal based and poorly formed whereas in sarcoidosis granulomas are well formed and associated with presence of giant cells [6]. Pruritus and fatigue are also the most common symptoms of PBC. Additionally, AMA is 90% sensitive and specific of PBC, and both anti-gp210 and anti-sp100 show an over 95% specificity in AMA negative patients. Our patient exhibit AMA and ANA negative. The role of ACE in diagnosing sarcoidosis is limited. Normal ACE levels were detected in our patient reflecting its poor sensitivity for diagnosing hepatic sarcoidosis [7]. Anti- mitochondrial antibody, anti-smooth muscle antibody, anti-liver kidney microsome type 1, and anti- neutrophil cytoplasmic antibody are usually negative. In approximately half of patients ultrasonography and computerized tomography are normal, in the other half imaging abnormality are non-specific (hypodense nodular lesions and hepatomegaly) [8]. Uehara et al. reported the case of a female patient with abnormal liver function test who underwent a gallium scintigraphy with a marked uptake in the liver. The presence of non caseating hepatic granulomas and multinucleated giant cells in the liver biopsy confirmed the diagnosis of sarcoidosis.[9]
According with our clinical presentation, in patients with lack of other organ involvement, biopsy is essential in order to exclude viral, metabolic, autoimmune or neoplastic liver disease. Although biopsy is the gold standard for establishing the diagnosis, other granulomatous diseases of the liver may mimic sarcoidosis supporting the importance of a targeted liver biopsy. Our patient underwent a PET-CT scan which was highly suggestive for hepatic and splenic localization of sarcoidosis, a combined diagnostic approach by performing a PET-marked liver biopsy confirmed our clinical suspicion of hepatic localization of sarcoidosis. A multisystemic approach is required to establish the diagnosis of hepatic sarcoidosis that may resemble primary biliary cholangitis on liver histology.
Oral glucocorticoids, often combined with ursodeoxycholic acid, are the most common first-line treatment [10]. Response to steroid therapy varies between studies. More extensive pulmonary involvement appears to predict persistent symptoms and liver enzyme elevations after steroids [8,11].
After three months of steroid therapy in combination with UDCA our patient has normalized ALP and GGT levels, but a longer follow up will be necessary to evaluate the course of the disease.
We report a rare case of isolated localization of hepatic and splenic sarcoidosis in patient with initial clinical suspicion of PBC.
Diagnosis of hepatic sarcoidosis is challenging as diagnostic criteria are lacking and guidelines with recommendations for diagnosis and follow-up of suspected patients are not available. A multidisciplinary approach is crucial for a reasonable suspect and timely diagnosis, as early treatment can significantly improve the course of the disease.