AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2641-5194/049
1 Marmara University School of Medicine, Department of General Surgery, Turkey.
2 Marmara University Ministry of Health Education and Research Hospital, Turkey.
*Corresponding Author: Ömer GÜNAL, Professor of General Surgery, Marmara University Department of General Surgery. İstanbul, Turkey.
Citation: Gunal O., Şahan C., Cayoren H. Erdim A. (2022) Is Abdominal Drain Usage after Bariatric/Metabolic Surgery Fading Out? J. Gastroenterology Pancreatology and Hepatobilary Disorders 6(3) DOI:10.31579/2641-5194/049
Copyright: © 2022, Ömer GÜNAL,This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 05 January 2022 | Accepted: 15 February 2022 | Published: 02 July 2022
Keywords: abdominal surgeries; bariatric surgery; intraabdominal fluid
As in other abdominal surgeries, leakage and hemorrhage are challenging issues for bariatric surgeons. Routine abdominal drainage has also been commonly used to restrain the morbidity associated with gastrointestinal leakage [GL] by allowing for earlier detection and treatment. Abdominal drainage is believed to be able to allow for timely detection of hemorrhage and leakage of anastomoses. Drains may also be helpful to prevent the unexpected intraabdominal fluid collections vulnerable to microbial contamination. By virtue of these benefits, drains are the mainstay of abdominal surgeries. However, the drains may add some morbidities to the patient surveillance.
As in other abdominal surgeries, leakage and hemorrhage are challenging issues for bariatric surgeons. Routine abdominal drainage has also been commonly used to restrain the morbidity associated with gastrointestinal leakage [GL] by allowing for earlier detection and treatment [1]. Abdominal drainage is believed to be able to allow for timely detection of hemorrhage and leakage of anastomoses [3, 5]. Drains may also be helpful to prevent the unexpected intrabdominal fluid collections vulnerable to microbial contamination. By virtue of these benefits, drains are the mainstay of abdominal surgeries. However, the drains may add some morbidities to the patient surveillance [4]. A variety of drain-related problems have been reported in various types of abdominal surgeries such as small bowel obstruction [5], duodenal perforation [6], and even fallopian tube herniation [7]. Leaving a drain in the peritoneal cavity during an elongated period may increase the infection rate. This led us to revise the use of routine drains after uneventful laparoscopic bariatric/metabolic surgical procedures. This study aims to evaluate our changing attitude for abdominal drainage following bariatric/metabolic surgery, and if there is any benefit of intraabdominal drain placement in three types of bariatric surgeries which are mainly performed.
Patient population
Our inclusion criteria included all patients undergoing bariatric surgery between January 1st, 2013, and October 28th, 2020. Eligibility criteria included patients over the age of 18, who have undergone primary or revisional bariatric surgery. Exclusion criteria were patients over 75 years of age and patients with incomplete data. Our institutional review board deemed this study exempt.
Outcome Measure
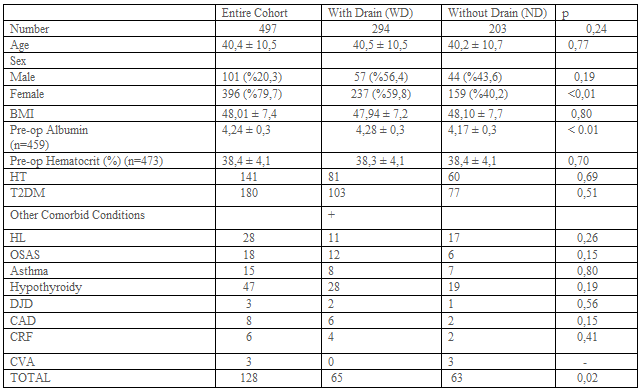
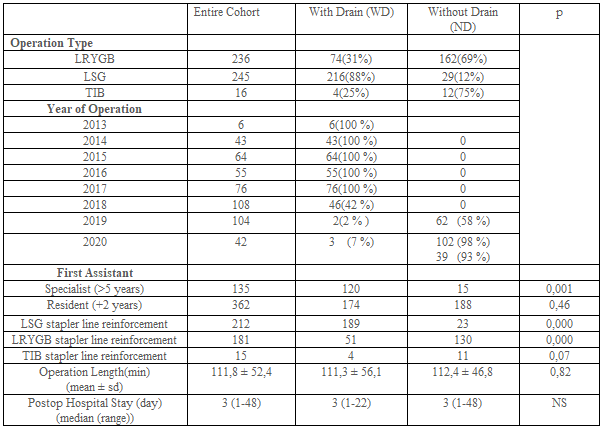
The preoperative characteristics of patients with and without drainage were examined. These included demographics and preoperative comorbidities (Table-1). The main outcome measures were postoperative complications, hospital stay, and length of operation. Operative variables including type of operation, staple line reinforcement, the performance of anastomosis leakage tests such as provocative testing or swallow study were also evaluated (Table-2).
TIB: Transit Ileal Bipartition,
ALT: Anastomosis Leakage Test,
PT: Provocative Test,
SS: Swallow Study,
POL: Positive per-operative Leakage,
MIS: Minimal Invasive Surgery,
RN: Registered Nurse First Assistant,
PGY: Post Graduate Year,
HT: Hypertension,
T2DM: Type 2 Diabetes Mellitus,
HL: Hyperlipidemia, OSAS: Obstructive Sleep Apnea Syndrome,
DJD: Degenerative Joint Disease,
CAD: Coronary Artery Disease,
CRF: Chronic Renal Failure, CVA: Past Cerebro Vascular Accident.
ALT: Anastomosis Leakage Test,
PT: Provocative Test,
SS: Swallow Study,
POL: Positive per-operative Leakage.
WD: Patients with drain,
ND: Patients with no drain placement.
*likelihood ratio.
TIB: Transit Ileal Bipartition,
LSG: Laparoscopic Sleeve Gastrectomy
LRYGB: Laparoscopic Roux-en-Y Gastric Bypass.
ALT: Anastomosis Leakage Test,
PT: Provocative Test,
SS: Swallow Study,
POL: Positive per-operative Leakage,
NS: Nonsignificant
Laparoscopic Sleeve Gastrectomy (LSG)
The patients were placed in a French position with their legs apart. After installation of pneumoperitoneum, five trocars (two 15 mm, one 10 mm, and two 5 mm) were inserted into the abdominal cavity. Gastric resection was performed over a calibration tube of 38 French from pylorus to esophagogastric junction. The specimen was extracted through the left 15 mm trocar site within a specimen retrieval bag. A seromuscular running suture is arbitrarily used to cover the stapling line to reduce bleeding.
Laparoscopic Roux en Y Gastric Bypass (LRYGB)
Following the patient placement in French position with legs apart, six trocars (10 mm for the camera, three 12-15 mm for working, one 5 mm for retractor, one 5 mm for working) technique has been used. Lesser sac has been entered through the hole made over the edge of the lesser curve, 6-7 cm distal to the gastroesophageal junction. Following the transverse transection of the stomach wall, with a 60 mm linear cutter stapler, longitudinal transection of the stomach over the 38 French orogastric tube was completed at the gastroesophageal junction on the esophagus. Thereby, a gastric pouch of 20-30 ml volume was constructed without leaving any fundus. An isoperistaltic gastrojejunal anastomosis was constructed at the anterior or posterior gastric wall approximately 50-80 cm distal to the Treitz ligament. A biliopancreatic segment has been prepared simultaneously with gastrojejunal anastomosis accomplishment. Alimentary segment length was adjusted between 120-180 cm according to the patient’s weight.
Laparoscopic Transit Ileal Bipartition (LTIB)
The operation was performed adhering to the technique as described by Sergio Santoro [8]. Pneumoperitoneum is established with a supraumbilical 10 mm trocar. Six trocars more are positioned, including three 12-mm trocars (1 in the midline 3 to 5 cm below the umbilicus, 3 cm left lateral to the midline, and 2 others in the upper left and right quadrant) and three 5-mm trocars (1 in the epigastrium for the liver retractor and 2 at each lateral flank). Sleeve gastrectomy was performed with the technique described above but leaving approximately 6 cm antrum. A seromuscular running suture is sometimes used to cover the stapling line to reduce bleeding. After the SG, the ileocecal transition is located. The point at 250 cm from the ileocecal valve is then located, and an anastomosis was created between the ileum and antrum. During the performance of gastroileal anastomosis (GIA), simultaneously formed afferent intestinal segment was anastomosed to ileal segment 70-100 cm proximal to the ileocecal valve.
Leakage Test
The leakage test was done both at the time of surgery and after the surgery as a “provocative test” or “swallow study” respectively. Provocative testing is defined as “insufflation of air through an endoscope or a nasogastric tube with the anastomosis under saline to look for bubbles” or “the instillation of methylene blue under pressure” [8]. A postoperative swallow study was done on the postoperative first day by drinking patients 100 ml radiopaque mixed water.
Patient Outcomes
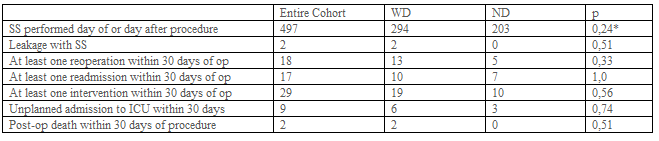
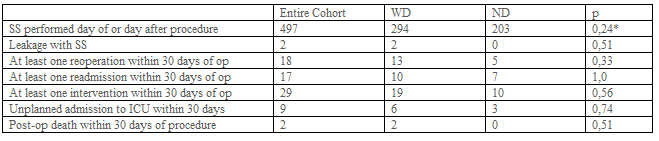
Hospital stay was considered as the time until patient discharge. Postoperative 30-day outcomes for complications were also examined: death/mortality, morbidity (defined as any complication occurring within 30 days after the operation), readmission, reoperation, reintervention, unplanned admission to the intensive care unit (ICU), deep postoperative skin, and soft tissue infection (SSI), organ space infection, progressive renal insufficiency, pulmonary embolism (PE), sepsis, transfusion, acute renal failure, wound disruption, and stroke/cerebral vascular accident, trocar site hernia (Table-3).
Quantitative variables were summarized using means and standard deviations (SD). Continuous variables were compared by using the independent sample t-test while categorical variables were compared using chi-square and Fisher exact test. All analyses were carried out using SPSS V24.
A total of 550 patients were retrospectively evaluated. 32 Patient records can not be obtained. 21 Patients have missing data (e.g. Operation time, hospital stay, complications, etc.) (Figure-1). 497 Patients were included in the study. Patient demographics and preoperative comorbidities are shown in Table-1. While 294 patients had abdominal drains, 203 patients did not have a drain. 236, 245, 16 patients have undergone RYGB, Sleeve, and TIB operations, respectively. Drain usage tendency during 8 years of our surgical practice is presented in Table 2. All patients had a postoperative swallow study. 21,9% of patients had an intraoperative provocative test. The first assistant characteristic showed us drain placement was more in the specialist group (p<0>0,05). Postoperative hospital stay and operation times were similar between the drain (WD) and without a drain (ND) (Table-2). Mean postoperative drain withdrawal time was 3±1 days. Postoperative 30 days follow-up results of the patients were presented in Table-3. Patients' postoperative complications were listed in Table-4.
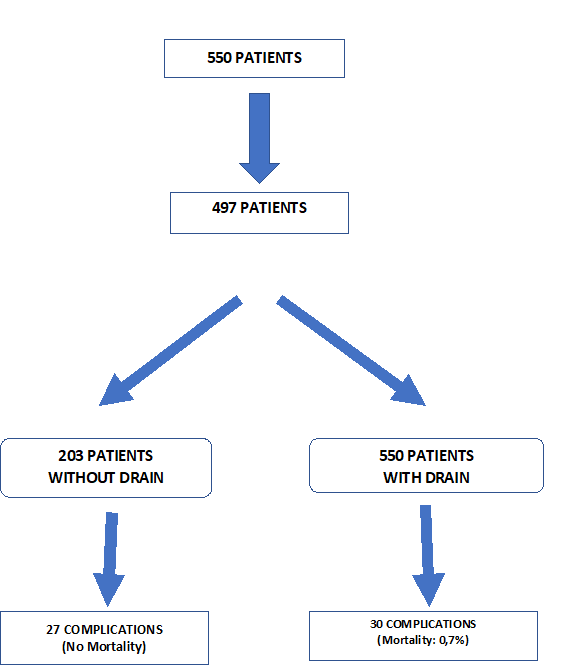
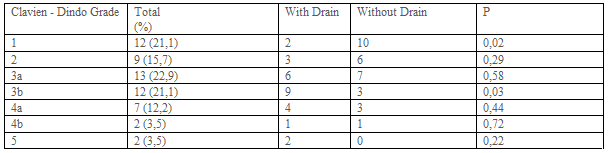
Complications occurred in 57 patients in postoperative 30 days. 105 complications were observed. Three patients had 4, 9 patients had 3, 21 patients 2, and 24 patients had 1 complication (Table 4). Most frequently seen postoperative Clavien-Dindo complication grade was the 3. Clavien-Dindo grade 1 complications were more seen in patients without a drain (Table-5).
The question we wanted to answer was if it is really necessary to get drains placed even in selected patients after bariatric surgery. Clapp et al. promulgated that patients with higher BMI, serious comorbidities, higher ASA status, and previous foregut surgery, and patients undergoing revisions or conversions were more likely to have a drain placed [9]. They also suggested that patients that had longer operative times and positive provocative anastomotic testing were more likely to have a drain placed as well as patients that were converted to open. However, in our series, we did not assure a preventive effect of the drain in any case. We did not find a difference between groups concerning BMI, comorbidities, postoperative complications, operative times, and hospital stay (Table 1-4). We did not notice a likeliness to have a drain in the provocative testing group. Despite the 11 positive perioperative leakage tests, we did not prefer to put a drain in 10 of them. Because we have reinforced the stapler or leakage line in these patients (Table-2). Therefore, we suggest not to place a drain if a surgeon detects a positive POL and repair it at the time of surgery. In our series, we preferred to put drain all of the patients during the first 5 years. Then we decided to decrease drain placement in bariatric surgery when we noticed that leaving a drain in what so ever the reason did not affect the outcome of the patient.
Doumouras et al found that the use of routine abdominal drainage increased the rate of reoperation and odds of the leak. They concluded that it may also result in increased overall morbidity [2]. In concordance with this finding, the reoperation number was increased in patients with a drain in our series (13 in WD vs 5 in ND), (p=0.33), (Table-3). Postoperative morbidity was not statistically different between the two groups (27 in WD vs 30 in ND p=0.06) (Table-4). We supposed that drain usage does not cause such an increased rate of reoperation and odds of the leak in patients with drain. Although drain placement was applied in more patients with detrimental follow-up results, this difference was not statistically significant (p>0.05), (Table- 3). This means that drain placement was not necessarily needed.
Yurcisin et al. suggested the practice of placing a juxta-anastomotic drain. They promulgate that this may allow non-operative management of leaks in stable patients, but this is only done currently in cases in which there is an intraoperative concern about the anastomosis (10). To the best of our knowledge, we used abdominal drains for early detection and management of leaks and hemorrhages and the prevention of fluid collections. However, none of the leaks was treated non-operatively with the help of drains in our series. We chose the early surgical intervention if we had any clinical suspicion of the leak without adhering to drain content and volume. Ribeiro et al. found a false positive value of 87 % for the drain amylase level. They suggest that whenever, there is any suspicion of leaks, early surgical intervention is mandatory. These false-positive values may divert the clinician to false exploration in case of clinical findings absence. Although the value of radiologic findings alone is disputable, to assess the diagnostic accuracy of a test for detection of leaks, the presence of clinical suggestive signs (tachycardia, fever, hypotension, and/or abdominal pain) associated with confirmation through tomographic and/or intraoperative findings was considered the gold standard. Even with a negative radio-opaque upper gastrointestinal series, continued suspicion of the leak, non-improvement of signs/symptoms, or instability should prompt re-exploration [11]. We treated all our strongly suspected patients with laparoscopy and laparotomy depending on the physical signs and symptoms, even though there was not any evidence of radiologic or drainage findings.
Gundogan et al. demonstrated that routine abdominal drainage following laparoscopic RYGB negatively affected patient comfort by increasing their pain [12]. According to them, leaks occurring within the postoperative first 3 days are usually due to technical defects overlooked during surgery. Intraoperative methylene blue testing can help to diagnose and fix these mistakes. After 3 days, the unremoved drain will not help detect late leaks. We decided to remove the drain by drainage amount. When the drainage amount decreased to less than 50 ml, the drain was withdrawn. However, we sometimes removed the drain even though the drainage was not less than 50 ml if the drainage was serous-anginous. Drain withdrawal time was 3±1days in our patients.
Alizadeh et al promulgated that interventions such as intraoperative provocative tests and surgical drain placement were associated with a higher risk for leaks [13]. They found a higher rate of gastrointestinal leak in patients with versus without a provocative test (0.8% vs. 0.4%, respectively, P<0 N=32,650).>
Intraoperative drain placement during a primary bariatric surgery has the goal of either prevention or early detection of postoperative complications. Gray et al. considered whether drain placement was selected for patients at particularly high risk of complications based on preoperative risk factors. They suggested that if this was the case, then increased odds of complications after drain placement would be difficult to separate from the patients' baseline increased preoperative risk [1]. However, steroid use and smoking, both of which are commonly accepted risk factors for complications, were not associated with drain placement (1). We have not been able to find a comorbidity difference between WD and ND. We did not consider the patient comorbidities in drain placement determination.
According to our subjective observations about the effect of drain placement on surgical outcomes, we have ended up with drain placement since 2018. Although we placed intraabdominal drain routinely in all patients until 2018, we started to place a drain in only selected cases in 2018 (42%) (Table-2). Later on, drain placement was decreased to 3.4% (5/146), we have hardly used abdominal drains in our last two years.
We also evaluated the effect of the first assistant experience in determining the drain placement. Interestingly, drain placement was shown to be statistically increased in operations assisted by a surgical specialist (WD 120 vs ND 15) (Table-2). That seems to be due to the specialist attitude concerning drain placement, which could change the primary surgeon's decision.
Neither operation time, nor hospital stay did not differ between WD and ND groups (Table-2). Morbidity was also not different in groups WD and ND (Table-4).
Data in literature, concerning postoperative abdominal drainage following bariatric surgery, seems to advocate the selective use of abdominal drains. In conjunction with supporting these data set, our results showed that drain placement to the abdominal cavity after bariatric surgery does not have any effect on the outcome of the patient. Early detection of postoperative leakage and hemorrhage is mostly achieved by clinical vigilance and physical examination. Abdominal drain placement seems not to avail to surgical practice apart from allaying surgeon’s anxiety.