AUCTORES
Globalize your Research
case report | DOI: https://doi.org/10.31579/2690-4861/694
Department of Gastroenterology, Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS Foundation trust, Birmingham, UK.
*Corresponding Author: Amal Almahroos, Department of Gastroenterology, Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS Foundation trust, Birmingham, UK.
Citation: Amal Almahroos, Peter Rimmer, Rachel Cooney, (2025), Immune Checkpoint Inhibitor Related Gastritis; a Case Report from a Tertiary Centre, International Journal of Clinical Case Reports and Reviews, 25(4); DOI:10.31579/2690-4861/694
Copyright: © 2025, Amal Almahroos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 21 January 2025 | Accepted: 11 February 2025 | Published: 23 April 2025
Keywords: immune checkpoint inhibitors; severe gastritis; steroids; infliximab
Background
Immune Check point Inhibitor (ICI) use has been growing in various fields of oncology. ICI related side effects are therefore being more commonly seen. ICI related gastritis is a rare side effect that can be a challenging complication to manage.
Case summary
We present a severe case of steroid-refractory gastritis secondary to a combination immunotherapy (ipilimumab and nivolumab) being used to treat metastatic melanoma in a middle-aged gentleman. Despite being treated with intravenous (IV) proton pump inhibitors (PPI) and steroids, the patient did not show the expected response to treatment. Therefore, we have treated him with infliximab. He showed good clinical, endoscopic and histological response after a total of 5 doses of infliximab along with steroid treatment.
Conclusion
It is important to keep ICI related gastritis on the differentials list when patients receiving ICI therapy present with upper gastrointestinal symptoms. Anti-Tumour Necrosis Factor (anti-TNF) is a good option to be considered when patients fail to respond to initial treatment with IV PPI and steroids.
The indications for use of ICI are rapidly expanding and this form of immunotherapy is now being used in multiple cancer types including melanoma, renal cancer, urothelial cancers, some breast cancers, lung cancers and gynaecological malignancies. This growth in use is increasing the number of patients suffering from their associated side effects.[1]
Inhibition of the immune checkpoint pathway helps the immune system to destroy malignant cells but this generalised inhibition can result in autoimmune reactions in different organs. Lower gastrointestinal (GI) side effects have been described amongst the common immune-related Adverse Events (irAE). Immune checkpoint inhibitor gastritis, however, is one of the rare side effects associated with the use of ICIs.[2]
A 44-year-old gentleman who was being treated for metastatic melanoma (Stage IV) was found to have intracranial metastases on planned surveillance. After MDT discussion, his treatment plan included stereotactic radiotherapy plus combined immune therapy with ipilimumab (a Cytotoxic T-Lymphocyte-associated antigen-4 [CTLA-4] inhibitor) and nivolumab (a selective Programmed cell death protein 1 [PD-1] inhibitor).
After the completion of three cycles of combined ICIs, the patient developed severe epigastric pain worse with oral intake, associated with nausea and vomiting. He lost 5.6 kg over two weeks; weighed 75.6 kg on admission. Blood tests showed raised inflammatory markers, CRP of 62 mg/L (0-5 mg/L), and raised amylase of 398 U/L (25-125 U/L). CT abdomen and pelvis showed diffusely thick-walled stomach with mucosal hyperenhancement and mural stratification, no peri-gastric fat stranding or lymphadenopathy. There was no evidence of pancreatitis or colitis.Upper gastrointestinal endoscopic examination showed an inflamed Gastro-Oesophageal Junction (GOJ) and pan-gastritis with diffuse inflammation, oedematous and friable gastric mucosa (image 1). Biopsies obtained from GOJ showed nonspecific inflammation, whereas samples obtained from the stomach showed features of ulceration (inflamed denuded granulation tissue).
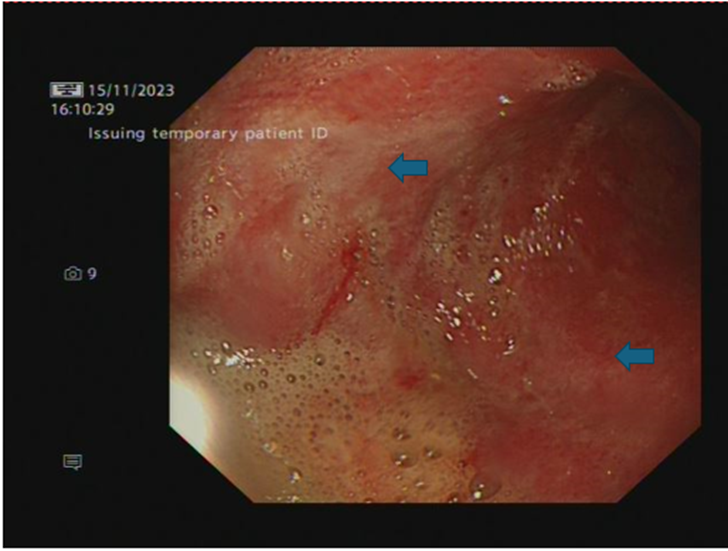
Image 1: OGD - Stomach lower body (pan-gastritis with diffuse inflammation, oedematous and friable gastric mucosa)
Along with high dose protein pump inhibitor (PPI), the patient was commenced on high dose intravenous (IV) steroids (methylprednisolone 1mg/kg). There was no improvement in his symptoms by day 4, therefore methylprednisolone was increased to 2mg/kg/dayand infliximab commenced (5mg/kg). He started to improve, and he started tolerating oral intake. After 14 days of IV methylprednisolone, he was stepped down to tapering dose oral Prednisolone. A second infliximab dose was given following discharge (two weeks from the first dose).
Despite the initial improvements, abdominal pain persisted as steroids were tapered. Nausea, with consequent reduced oral intake, remained an issue. He was re-admitted for further management. His blood tests showed high inflammatory markers with a CRP of 81 mg/L (0-5 mg/L)
and a higher amylase reaching 1060 U/L (25-125 U/L). The patient continued to lose weight; he has lost another 10kg over four weeks (adding up to a total of 15kg since the start
of the illness). A repeat CT scan showed ongoing gastritis with no evidence of pancreatitis. A repeat OGD showed worsening features with oesophagitis, severe suppurative gastritis, and moderate atrophic duodenitis (images 2a-b). Microscopically, there was lymphocytic oesophagitis with patchy parakeratosis and acanthosis, spongiosis and prominent lymphocytic exocytosis. Gastric biopsies displayed extensive ulceration undermined by inflamed granulation tissue admixed with active chronic inflammation. Furthermore, duodenal biopsies showed focally attenuated surface epithelium, partial villous atrophy with intraepithelial lymphocytosis, crypt hyperplasia and lymphocytic exocytosis. The histopathological features described above, on both oesophageal and duodenal biopsies, were most likely immunotherapy related.
He was re-commenced on IV steroids at a lower dose (Methylprednisolone 40 mg once a day). He also received two further doses of infliximab at a higher dose 10mg/kg along with NJ feeding for nutrition, sucralfate and PPI. The patient improved on the above treatment; he was gradually restarted on oral feeding and discharged with tapering course of oral steroids. Fifth infliximab dose (5mg / kg) given as an outpatient in view of ongoing active inflammation on a repeat OGD (Image 3).
Over a few months the patient was able to go back to normal diet with no abdominal pain and he started gaining weight. A repeat OGD confirmed a much-improved appearance with very mild antral gastritis which could represent bile reflux (Image 4). Biopsy has also confirmed features of chronic gastritis.
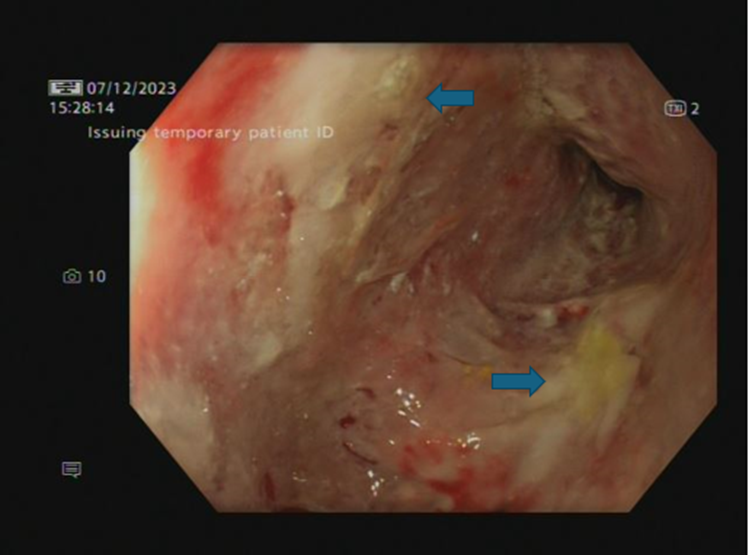
Image 2(a)- OGD - Stomach lower body (severe suppurative gastritis).
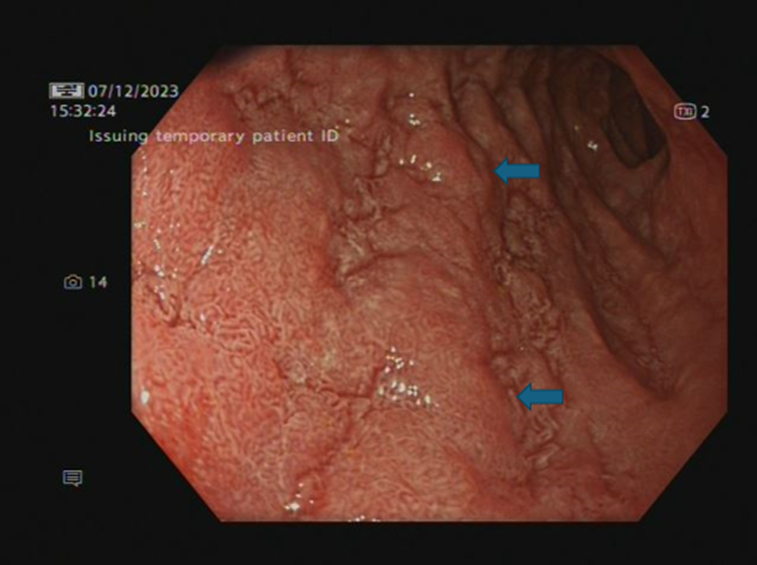
Image 2 (b): Second part of duodenum (moderate atrophic duodenitis)
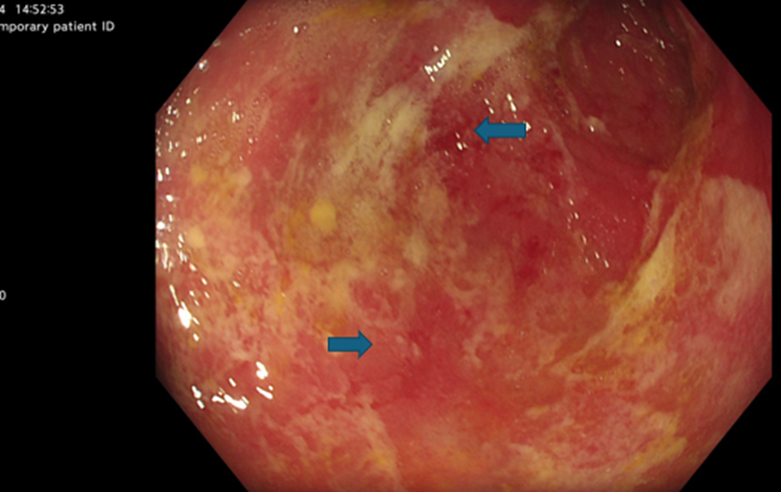
Image 3: OGD post 4th dose infliximab stomach middle body (ongoing active inflammation)
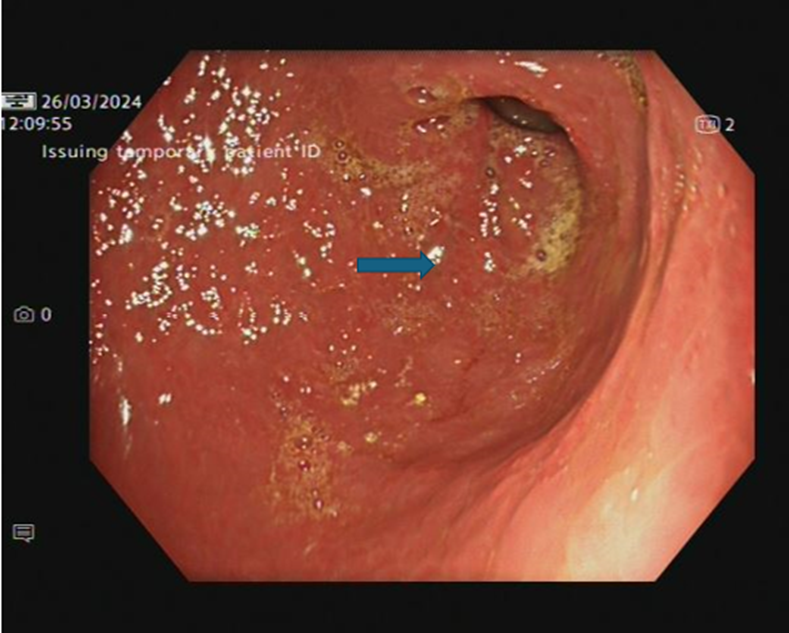
Image 4: OGD- Stomach Antrum post 5th dose of infliximab (nicely healed mucosa, mild antral gastritis- arrow)
In view of significant improvement on clinical, endoscopic and histological grounds as well as patient’s strong wish to continue ICI treatment acknowledging the risk of side-effect recurrence, single agent immunotherapy (nivolumab) was restarted under close monitoring.
He has not had a symptomatic recurrence of immune-related gastritis on this regimen
Our case report highlights the importance of increasing awareness about the rare side effects associated with ICIs, which will likely be seen more commonly seen as the indications for ICIs use keep expanding in the coming years. The overall incidence of gastrointestinal irAE is estimated to be 6-8%, with gastritis contributing to 0.35-1.46% only.[2] There are two main groups of ICIs. The first group targeting PD-1, such as nivolumab and the other group targeting CTLA-4 like ipilimumab. Combination therapy (of anti CTLA-4 and antiPD-1/PD-L1, like the case presented above) and the use of higher doses are associated with a higher incidence of GI-irAE compared to monotherapy and the use of lower doses.[2]
ICIs related gastritis presents most commonly with nausea, vomiting, abdominal pain +/- melena.[3] Other less common symptoms reported in the literature include bloating, dyspepsia, and discomfort.[2] A retrospective study in Cleveland clinic in the USA, found that patients completed a median number of four ICI infusions before the onset of gastritis symptoms.[3] This was very similar to our case report, as our patient became clinically symptomatic following the completion of three cycles on immunotherapy.
Diagnosis can be confirmed using upper endoscopic examination as well as histological assessment. Common endoscopic features include erythema, oedema and friability.[3] Patients could also have signs of mucosal ulcerations and erosions.[3] Most of the previously mentioned endoscopic findings were described at different endoscopic assessments in our case. Histopathological aspects of ICI related gastritis described across literature included active gastritis, chronic active gastritis and ulcerations.[2,3] Although CT scan of the abdomen is usually normal in these cases, our patient had marked features of gastritis on the CT scan indicating that his gastritis was a severe form, which could have contributed to the challenge in the management.
ICI-related gastritis typically responds to acid suppression therapy and/ or steroids.[3] Having said that our patient was resistant to a combination of high dose acid suppression with PPI in addition to sucralfate and intravenous steroids. We therefore needed biologic therapy, infliximab in our case, to suppress the inflammation caused by ICIs.
Although the mechanism behind checkpoint inhibitors related gastritis is poorly understood, it is thought that the resulting imbalance leads to increased cytokine production including tumour necrosis factor (TNF). This in turn results in T cell activation, destruction of normal cells and pro-inflammatory effects.[2] This may explain why patients who fail to respond to steroids, improve with anti TNF therapy.
The risk of recurrence of gastritis following rechallenge with ICIs is still not known given the rarity of this adverse event. Some retrospective data describe the recurrence rate of the same irAEs upon ICI rechallenge to be 28.8%, with some irAE (like colitis) having a higher recurrence rate compared to others.[4] This highlights the importance of close observation of patients who need to be rechallenged with ICIs. Moreover, assessing patients’ complete recovery endoscopically prior to re-initiating the ICI is an important step.[2]
Immune-checkpoint inhibitor related gastritis is a rare but important side effect that we will encounter more frequently as ICI indications continue to expand. Patients presenting with UGI symptoms while on ICI should be investigated for possible gastritis. If present, gastritis in this instance is best managed using a multidisciplinary approach including oncologists as well as gastroenterologists. Most patients respond well to high dose IV steroids and PPI, however, those resistant to steroid management should be considered for immunosuppression for example with an anti-TNF therapy. Assessment of mucosal healing endoscopically is advisable in patients who are being considered for ICI rechallenge.
Verbal informed consent gained 02/09/2024 (documented on patient’s records)